Abstract
The objective of this study was to determine the consequences for HPA axis functioning among healthy full-term newborns of prenatal treatment with the synthetic glucocorticoid (GC), betamethasone, which is the routine treatment for threatened preterm delivery. Ninety full-term infants were recruited into two study groups (30 betamethasone treated; 60 comparison group matched for GA at birth and sex). The cortisol and behavioral response to the painful stress of a heel-stick blood draw was assessed 24 hr after birth. Full-term infants exposed to prenatal betamethasone displayed a larger cortisol response to the heel-stick procedure, despite no differences in baseline levels. Further, within the recommended window of betamethasone administration (24–34 gestational weeks), infants exposed to betamethasone earlier in gestation displayed the largest cortisol response to the heel-stick. These data add to accumulating evidence that prenatal exposure to elevated GCs programs the development of the HPA axis.
Keywords: betamethasone, HPA axis, cortisol, glucocorticoids, stress, infant, prenatal, full-term
INTRODUCTION
Accumulating evidence demonstrates that developmental trajectories that are established during the prenatal period have lifelong consequences for health outcomes including heart disease, diabetes, and obesity (Barker, 1998). Prenatal exposure to glucocorticoids (GCs) is one putative mechanism for these persisting effects on later health outcomes. The role of GCs in shaping developmental outcomes is an important issue because prenatal treatment with synthetic GCs, administered to accelerate fetal lung maturation, is the standard of care for women in preterm labor (NIH Consensus Statement for the use of Antenatal Steroids, 1995). Treatment with GCs decreases mortality, respiratory distress syndrome and intraventricular hemorrhage among infants born preterm (Crowley, 1995). Because the clinical diagnosis of PTL is imprecise and because preterm delivery may be prevented with clinical interventions, many women diagnosed as being in PTL deliver at term. Further, there is evidence that the exposure of full-term infants to prenatal GCs is on the rise (Polyakov et al., 2007). The long-term effects of prenatal GC treatment on health and development remain poorly understood; an issue that may be particularly relevant for full-term infants who do not receive commensurate medical benefit from treatment.
Synthetic GCs, such as betamethasone, easily cross the placenta and are not metabolized by placental enzymes that regulate endogenous maternal GCs (i.e., cortisol). Whereas this enzyme partially protects the fetus from endogenous maternal cortisol, betamethasone is not metabolized by this enzyme and acts directly on the developing fetus (Albiston, Obeyesekere, Smith, & Krozowski, 1994; Kajantie et al., 2004). Prenatal GC treatment exerts persisting influences on HPA axis functioning among rodents, sheep, and nonhuman primates (Kapoor, Petropoulos, & Matthews, 2008; Sloboda, Moss, Gurrin, Newnham, & Challis, 2002; Uno et al., 1994). Less is known about the consequences of prenatal GC treatment for humans. Several studies have shown that among preterm infants exposed to antenatal GCs, baseline cortisol levels are suppressed during the first postnatal week and then return to normal levels (Ballard, Gluckman, Liggens, Kaplan, & Grumbach, 1980; Dorr, Versmold, Sippell, Bidlingmaier, & Knorr, 1986; Parker, Atkinson, Owen, & Andrews, 1996). Further, in studies of preterm neonates and a combined sample of preterm and term neonates, prenatal treatment with synthetic GCs suppresses the cortisol response to stress (Ashwood et al., 2006; Davis et al., 2004, 2006; Schaffer, Luzi, Burkhardt, Rauh, & Beinder, 2009). The effect of prenatal GCs on HPA axis regulation among exclusively healthy full-term infants has not been examined. By evaluating infants born at term gestation, we can determine the role prenatal GC treatment plays in shaping the development of the HPA axis independent of the effects of preterm delivery and associated illness and exposure to medical procedures (Grunau et al., 2005). The cortisol response to the painful stress of a heel-stick blood draw was evaluated among full-term infants treated with a single course of the GC betamethasone and a matched comparison group of infants born at term gestation.
METHODS
Participants
The Institutional Review Boards for protection of human subjects at the University of California, Irvine approved the study protocol. Mother–infant pairs were recruited into a longitudinal study designed to examine the consequences of prenatal GC treatment on infant development. Written and informed consent was obtained from mothers prior to study enrollment. Inclusion criteria were birth at term (gestational age at birth >37 weeks), singleton status, absence of health complications requiring medical treatment or a prolonged hospital stay, and admission to the well baby/normal newborn nursery. Exclusion criteria were postnatal steroid administration, chromosomal or other congenital anomalies (e.g., trisomy 21), congenital infections, major neonatal illness (e.g., sepsis), and small for gestational age as well as maternal disorders during pregnancy requiring corticosteroid treatment or thyroid medication, adrenal illness or other endocrine problems, and evidence of smoking during the prenatal period (based on maternal report) or evidence of maternal substance use during pregnancy (e.g., alcohol). Eighty-five percent of the women approached agreed to participate in this study.
The study sample comprised 90 full-term infants born at the University of California, Irvine Medical Center and recruited into two groups. The GC group included 30 infants (20 female and 10 male) whose mothers received a single course of betamethasone (two doses of 12 mg/dose, 24 hr apart). In this cohort the first dose of betamethasone was given between 25 and 34 weeks’ GA [mean GA at administration=29.5 (2.9) weeks] and was between 28 and 105 days prior to delivery [mean days=60.5 (22.0)]. The primary indication for prenatal GC administration was PTL (87%). Preterm labor was defined based on the diagnosis by the attending obstetrician and indicated in the medical records. Preterm labor was diagnosed by the attending obstetrician based on the following factors: cervical change over time, bloody show, cervical effacement and/or dilation, and rupture of membranes. The remaining 13% received GC treatment for the following indications: placenta previa and prolonged premature rupture of membranes. The comparison group consisted of 60 normal full-term infants without prenatal GC treatment or preterm labor (40 female) matched by GA at birth and sex to the prenatal GC-treated group. The GC group was consecutively recruited. Infants in the comparison groups were infants born concurrently with the target group who met the inclusion criteria for the comparison group. To create a more stable characterization of neonatal cortisol regulation among unexposed infants, two no treatment infants were matched with each infant in the target group.
Procedure
Infants were evaluated at the time of their routine neonatal screen; a heel-stick blood draw administered by a neonatal nurse between 15 and 48 hr after birth (median=25 hr). None of the infants participating in this protocol had experienced a blood draw prior to this assessment. To obtain a resting baseline measurement, infants were monitored continuously by a research assistant for 1-hr prior to the assessment to ensure that they were not handled or fed. The assessment, performed by the research assistant, began with baseline measurements. During the 10-min baseline period, behavioral state was recorded at 20-s intervals. Baseline salivary cortisol was then assessed, followed by a clinically indicated heel-stick blood draw performed by a neonatal nurse. For this protocol, the nurse cleaned the heel with an alcohol swab, applied an automated lancet, and then repeatedly squeezed the heel until a sufficient blood sample was obtained. The median length of heel-stick was 4 min. Behavioral assessments continued during the heel-stick procedure and during the 5 min immediately following the heel-stick (recovery). To capture the peak cortisol response to the heel-stick, salivary cortisol samples were taken at 20 and 40 min after the start of the heel-stick (Gunnar, Hertsgaard, Larson, & Rigatuso, 1992). During the study period, infants were not fed or handled beyond the blood draw protocol. Both the nurse and the research assistant were blind to infant study group.
Measures
Infant salivary cortisol assessment.
Saliva was obtained (without any stimulant) by placing a swab in the infant’s mouth. Saliva samples can be collected in this manner without disturbing or waking the infant. Salivary cortisol reflects the unbound or active fraction of cortisol and is highly correlated with plasma cortisol in premature and full-term newborns and adults (Calixto, Martinez, Jorge, Moreira, & Martinelli, 2002; Kirschbaum & Hellhammer, 1989). The swab was then placed in a saliva extraction tube (Roche Diagnostics, Indianapolis, IN). Saliva samples were spun and stored at −20°C until assayed. Thawed samples were centrifuged at 3,000 rpm for 15 min before assay. Salivary cortisol levels were determined by a competitive luminescence immunoassay (LIA; IBL-America, Minneapolis, MN) with a detection limit of .005 μg/dl. The intra- and inter-assay coefficients of variance were 5.5% and 7.6%, respectively. All of the samples from an infant were included in the same assay batch to eliminate within subject inter-assay variance. Each batch contained subjects from the GC group and the comparison group. Samples were assayed in duplicate and averaged.
Infant behavioral responses to stress.
Behavioral state was assessed using a modified version of a coding system designed for the assessment of neonatal behavioral regulation (Als, 1998). This scheme was used to categorize each infant’s state on a scale of 1–6: quiet sleep, active sleep, quiet awakeness/drowsy, awake and alert, awake and fussy, and crying. Behavior was coded in real-time in 20-s epochs. Codes recorded represented the highest level of state or activity noted during each 20-s epoch. For 15% of the infants, two observers independently coded behavioral state. Percent agreement for state codes was 85.1%. Coders were blinded to infant study group.
Demographic and Clinical Data.
Maternal and infant medical records were reviewed to assess prenatal medical history and birth outcome. Data collected include birth characteristics (e.g., gestational age at delivery, birth weight, and Apgar scores), pregnancy characteristics (e.g., parity, delivery method, and pregnancy complications), and demographic data (e.g., maternal race/ethnicity and maternal age).
Data Transformation and Analysis Plan.
Summary scores were created to characterize infant cortisol and behavioral responses to the heel-stick. For infant cortisol, delta scores were calculated by subtracting baseline levels from the two posttest cortisol values taken at 20 min (response) and 40 min (recovery) after the start of the heel-stick. Behavioral state composite scores were calculated for each of the three phases. Baseline was defined as the average behavioral state score during the 10-min period prior to the heel-stick procedure. The heel-stick response score was defined as the average behavioral state score during the first minute of the heel-stick procedure. This interval was selected to ensure that all infants had complete data despite variability in length of heel-stick. Recovery was defined as the average behavioral state score during the 5-min period following the end of the heel-stick. Cortisol and behavioral summary scores were normally distributed as indicated by a nonsignificant Kolmogorov–Smirnov (K–S) test (p’s>.12). Paired t-tests were implemented to determine if cortisol or average behavioral state changed across the three assessment intervals (baseline, heel-stick response, and recovery).
Preliminary analyses were performed to identify factors (e.g., race/ethnicity and duration of heel-stick) that might influence infant cortisol or behavioral regulation. As shown in Table 1, the prenatal GC treatment and matched comparison group did not differ significantly in gestational age at birth, Apgar scores, mode of delivery, maternal age, race/ethnicity, parity, type of medical insurance as a proxy for socioeconomic status (SES), and GA at first prenatal visit (p’s>.2). As reported previously, infants in the GC group had a lower birth weight and a smaller head circumference as compared to infants in the no treatment comparison group (p<.01) (Davis et al., 2009). Birth weight was entered as a covariate in subsequent analyses evaluating the effect of prenatal GC treatment on infant cortisol regulation. Additionally, as shown in Table 1, aspects of the heel-stick event including postnatal age at assessment, length of the heel-stick procedure, and time of day did not significantly differ between the two groups (p’s>.3) and were not significantly associated with infant cortisol or behavior (p’s>.2). Importantly, baseline cortisol and behavioral state did not significantly differ between the two study groups (p’s>.2).
Table 1.
Demographic and Clinical Data for the Study Groups
| GC group, n=30 | Matched comparison group, n=60 | |
|---|---|---|
| GA PTL diagnosed (weeks) | 29.7 (.6)a | N/A |
| Gestational age at birth (weeks) | 38.0 (.2) | 38.3 (.1) |
| Sex | 20f, 10m | 40f, 20m |
| Weight (g) | 2,942 (549)* | 3,346 (524) |
| Head circumference (cm) | 33.3 (1.8)* | 34.4 (1.3) |
| Five-minute Apgar | 9.0 (.2) | 8.9 (.2) |
| Mode of delivery | 53% vaginal | 58% vaginal |
| Parity | ||
| 1 | 27% | 28% |
| 2 | 37% | 37% |
| 3 or more | 36% | 35% |
| Race/ethnicity | ||
| Hispanic | 70% | 50% |
| Caucasian | 17% | 30% |
| Other | 13% | 19% |
| Maternal age at delivery (years) | 28.9 (.7) | 28.7 (1.1) |
| GA at first prenatal visit (weeks) | 12.8 (.9) | 10.8 (1.1) |
| Insurance type | ||
| Commercial HMO | 23% | 17% |
| Commercial PPO | 19% | 10% |
| Government sponsored | 50% | 73% |
| Other | 8% | 0% |
| Postnatal age at heel-stick (hr) | 25.4 (.57) | 25.0 (.61) |
| Length of the heel-stick (min) | 5.0 (4.1) | 4.3 (3.5) |
| Time of day heel-stick (hr) | 13:40 (6:32) | 13:53 (5:41) |
Means are presented with SD in parentheses.
indicates p<.01.
Note. Group differences were assessed using ANOVA for continuous variables and chi-square for categorical variables. Infants in theGCgroup had a lower birth weight and smaller head circumference (p<.01) as compared to infants in the comparison group. None of the other demographic or clinical variables were significantly different between the two study groups.
Preterm labor was the primary indication for GCs in 87% of these participants.
To evaluate group differences (prenatal GC treatment vs. no treatment comparison group) in infant cortisol and behavioral responses to the heel-stick procedure, ANCOVA models controlling for birth weight were implemented. Pearson correlations were used to determine whether gestational age at GC administration was associated with the infant cortisol response score or behavioral state during the heel-stick or recovery period.
RESULTS
Infant Stress Responses
Paired t-tests revealed that cortisol levels were higher at 20 [t(89)=2.7, p<.01] and 40 min [t(89)=2.1, p<.05] postheel-stick as compared to resting baseline levels. Similarly, infants were in a significantly more aroused behavioral state during the heel-stick response [t(89)=8.3, p<.01] and the recovery period [t(89)=2.4, p<.05], as compared to baseline. Infants who displayed a larger cortisol response to the heel-stick procedure at both 20 [r(90)=.27, p<.05] and 40 min [r(90)=.36, p<.05] after the start of the heel-stick stressor were more behaviorally aroused during the recovery period. Infant cortisol was not associated with behavioral state at baseline or during the heel-stick event (p’s>.5).
Prenatal GCs and Infant Cortisol and Behavioral Responses to Stress
As shown in Figure 1, examination of infant cortisol revealed that the GC group had a larger increase in cortisol from baseline to 20 min after the heel-stick as compared to the no treatment comparison group F(1,87)=5.8, p<.05. The magnitude of the increase at 40 min posttreatment did not significantly differ between the two groups, F(1,87)=1.0, p=.33. These data suggest that the GC treatment group displayed a larger increase in cortisol in response to the heel-stick, despite no difference in pretreatment levels. As shown in Figure 2, behavioral state during the responses and recovery periods did not differ between the two groups (p’s>.5).
FIGURE 1.

Delta cortisol levels (change from baseline) with standard error bars at 20 and 40 min after the heel-stick for infants in the comparison group (black) and theGCgroup (gray). Among our cohort of full-term infants, infants produced significantly higher cortisol levels at 20 and 40 min after the heel-stick procedure. However, as shown here, this increase was driven by the infants exposed to prenatal GCs, who displayed a significantly larger cortisol response to the heel-stick procedure as compared to a no treatment comparison group (p<.05). The asterisk indicates significantly larger increase from baseline (i.e., larger delta score) at 20 min after the heel-stick among infants in the GC-treated group as compared to the no treatment comparison group.
FIGURE 2.
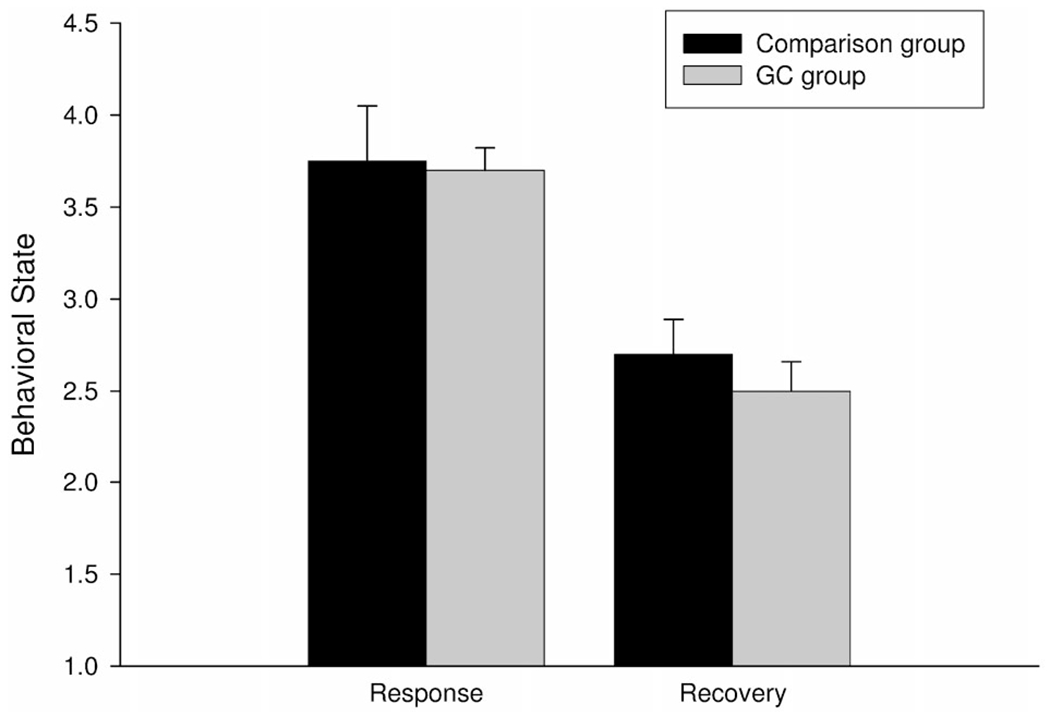
Average behavioral state during the heel-stick and recovery periods with standard error bars is shown for infants in the comparison group (black) and for infants in the GC group (gray). Infants displayed a significantly more aroused behavioral state during the heel-stick and recovery periods as compared to baseline. The GC group and comparison group did not significantly differ in the magnitude of their response.
Timing of GC Exposure and Stress Regulation
As shown in Figure 3, infants who received GC treatment at an earlier gestational age displayed a significantly larger cortisol response to the heel-stick blood draw r(30)=−.36, p<.05. Infants receiving GC treatment at an earlier gestational age additionally maintained elevated levels during the recovery period r(30)=−.39, p<.05. Gestational age at the time of GC exposure was not significantly associated with baseline cortisol (p>.3) nor was age at GC administration associated with any demographic or clinical factors (all p’s>.3).
FIGURE 3.

Infants exposed to prenatal GCs during the late 2nd and early 3rd trimester displayed the largest cortisol response (delta 20 min; a) and the highest cortisol levels during recovery (delta 40 min; b).
DISCUSSION
These findings illustrate that prenatal treatment with the synthetic GC, betamethasone, is associated with a more pronounced cortisol response to the painful stress of the heel-stick procedure among healthy infants born at term gestation, despite no difference in baseline levels. Further, within the recommended window of betamethasone administration (24–34 gestational weeks), earlier GC exposure exerts a stronger influence on the fetal HPA axis compared to exposure at later gestational ages. Consistent with work with animal models (Kapoor et al., 2008), these data provide evidence that prenatal GC treatment programs the development of the HPA axis. Confidence in these findings is increased because effects are observed among healthy full-term infants and thus are independent of the consequences of preterm birth.
Based on clear evidence that prenatal GC treatment reduces respiratory distress and intraventricular hemorrhage and increases survival among preterm infants, in 1995, the National Institutes of Health issued a consensus statement recommending that prenatal GC treatment be given to all women at risk of preterm delivery between 24 and 34 weeks of gestation (NIH Consensus Development Conference, 1995). One consequence of the increased use of prenatal GC therapy is that the number of treated infants born at term gestation is amplified. Although it is widely acknowledged that the benefits outweigh the risks among preterm infants, few studies have evaluated the developmental implications of prenatal GC treatment among infants born at term. The only prior studies to include exclusively healthy infants born at term gestation have focused on the effects of GC treatment on fetal growth and have documented adverse consequences including reduced weight and smaller head circumference at birth (Davis et al., 2009; Piazze, Berretta, Cioccio, & Anceschi, 2005).
The current findings additionally suggest that GC treatment alters HPA axis regulation among infants born at term. Among infants born preterm, prenatal exposure to a supraphysiologic dose of GCs suppresses the ability of the HPA axis to respond to stress. Specifically, infants born preterm and exposed to prenatal GC treatment fail to respond to an acute stress with an increase in cortisol (Ashwood et al., 2006; Davis et al., 2004, 2006). In contrast, among full-term infants in the current study, prenatal exposure to elevated GCs upregulates the cortisol response to an acute stressor. The present findings are consistent with evidence that prenatal exposure to endogenous (maternal) cortisol during the third trimester is associated with an amplified cortisol response to stress among healthy full-term neonates (Davis, Glynn, Waffarn, & Sandman, 2010). Further, experimental animal models have shown that prenatal GC treatment given beginning in mid gestation results in increased HPA axis reactivity in response to a stressor in the juvenile animal (de Vries et al., 2007; Sloboda et al., 2002). Although studies with juvenile animals primarily demonstrate upregulation of stress responses, it is worth noting that the relation between prenatal GC exposure and HPA axis functioning is complex and effects may vary based on a number of factors including the developmental stage at assessment and the timing and nature of the exposure (Joels & Baram, 2009; Kapoor et al., 2008).
An intriguing explanation for the differences in the response profiles of term and preterm infants is that the time interval between GC treatment and birth is weeks to months longer among term infants. GC treatment alters maternal and placental physiology (e.g., Challis, Patrick, Richardson, & Tevaarwerk, 1981; Korebrits et al., 1998) and full-term infants are exposed to this altered intrauterine environment for a longer period possibly further potentiating any consequences of GC treatment. Additional contributing factors include (1) Birth outcome: there are well-documented differences between term and preterm infants; (2) Maturity at assessment: preterm infants are tested at a younger postconceptional age. These studies demonstrate that exposure to prenatal GCs alters the trajectory of development of the fetal HPA axis and that the consequences may differ as a function of duration of exposure to a disrupted intrauterine environment, birth outcome, and the level of maturity at assessment.
The findings of the present study contrast with a recent study by Schaffer et al. (2009) demonstrating suppression of cortisol in response to the heel-stick procedure among infants born after 34 gestational weeks. However, there are several plausible explanations for this apparent inconsistency. First, there is growing recognition of significant differences between late preterm infants and full-term infants (Kramer, 2009; van Baar, Vermaas, Knots, de Kleine, & Soons, 2009). Because of the documented consequences of prematurity (even at later GAs) this is a plausible explanation for the discrepancies between the two studies. Second, the inclusion criteria differed between the two studies and thus it is possible that differences in the health status of the infants contributed to the difference in the results. The current study included healthy full-term infants who went to the well baby/normal newborn nursery and excluded any infants who had their hospital stay prolonged for health complications. The prior study by Schaffer and colleagues included infants born after 34 gestational weeks and list exclusion criteria as infants who needed intensive care or invasive procedures or who had congenital malformations. Additional factors that may contribute to differences between the two studies include: (1) Number of prior heel–sticks: this was the first heel-stick for all infants in the current report. The number of prior heel-sticks in the Schaffer study is unknown. (2) Postnatal age at assessment: infants were assessed at a younger postnatal age in the present report.
The current finding suggests that betamethasone treatment during the early second and late third trimester exerts a stronger influence on the infant HPA axis as compared to later treatment. This timing effect is consistent with evidence that the association between endogenous maternal cortisol and infant HPA axis regulation is strongest during the late second and early third trimester (Davis et al., 2010). Because betamethasone is not given for fetal lung maturation prior to 24 gestational weeks, the consequences of GC administration earlier in gestation cannot be determined from the present investigation. It is plausible, but unknown in humans, that earlier administration of synthetic GC treatment would have consequences for fetal HPA axis development. There is evidence from human and nonhuman primate studies that exposure to endogenous maternal GCs and synthetic GC administration early in gestation exerts persisting influences on aspects of development including cognitive functioning and brain development (Davis & Sandman, 2010; Diaz Heijtz, Fuchs, Feldon, Pryce, & Forssberg, 2010; Hirvikoski et al., 2007).
Exposure to excess GCs may influence the development of the fetal HPA axis by modifying GC receptor development in regions such as the paraventricular nucleus of the hypothalamus, hippocampus, and amygdala (Herman & Cullinan, 1997; Levitt, Lindsay, Holmes, & Seckl, 1996). These regions are particularly sensitive to excessive levels of GCs. Findings from animal models suggest that exposure to elevated levels of GCs alters the density of both types of GC receptors (mineralocorticoid and glucocorticoid) in the hippocampus and the amygdala with consequences for HPA axis feedback sensitivity (Kapoor, Dunn, Kostaki, Andrews, & Matthews, 2006). Limited information exists regarding the time course of prenatal development of GC receptors in humans. There is evidence that both types of GC receptors are present in the human hippocampus by 24 gestational weeks (Noorlander, De Graan, Middeldorp, Van Beers, & Visser, 2006) indicating that in the latter half of gestation this region is susceptible to the consequences of excess GCs.
Alterations to neurological systems during fetal development resulting from prenatal exposure to GCs may determine the neonate’s ability to respond physiologically to stressors in the postnatal environment and may establish a trajectory of development associated with increased cortisol reactivity to stress and a greater risk for the development of behavioral inhibition and anxiety. Evidence for these developmental consequences comes from studies suggesting that infants and toddlers who display greater cortisol responses to challenge tend to be more fearful or anxious in response to novel situations (Buss, Davidson, Kalin, & Goldsmith, 2004; Fortunato, Dribin, Granger, & Buss, 2008; Kagan, Snidman, & Arcus, 1998; Stansbury & Gunnar, 1994) and from evidence indicating that prenatal exposure to excess GCs contributes to the development of fearful or anxious temperament (Davis et al., 2007).
Participants were not randomly assigned to treatment and no treatment groups. Thus, we cannot exclude the possibility that differences exist between the two groups not related to betamethasone. It is notable; however, that only healthy full-term infants were included. Thus, the association cannot be attributed to the illness or differences in physiological regulation associated with shortened gestation. It is plausible; however, that prenatal conditions (e.g., the presence of PTL, prenatal maternal stress hormones) that differed between the two groups contributed to the association between GC treatment and cortisol regulation. As illustrated in Table 1, group differences are not observed on a number of clinical and demographic factors. Further, aspects of the heel-stick procedure including duration of the heel-stick and postnatal age at assessment did not differ between the two study groups indicating that the two groups are responding differently to the same challenge. An additional limitation is that proximity to birth may have affected the infant cortisol response to stress. Healthy full-term infants receive only one blood draw approximately 24 hr after birth. Thus, it is not possible to assess the response to heel-sticks at older ages among healthy infants. It is important to note that the two groups did not differ in postnatal age at assessment.
CONCLUSION
The beneficial effects of GC treatment for reduced respiratory distress and increased survival among preterm infants are clearly established (Crowley, 1995). Equivalent benefits are not conferred on those infants born at term. Prenatal GC treatment disrupts HPA axis regulation among term infants, especially those exposed earlier in pregnancy. Disruptions in HPA axis functioning may have long-term consequences for increased risk for disease later in life. This is of particular concern for term infants who do not receive commensurate medical benefit. Because PTL is difficult to diagnose, a significant number of women who receive GC treatment subsequently deliver beyond 37 weeks gestation. The potential harm of GC treatment to term infants must be evaluated in the context of the clear benefit to preterm infants. Thus, these data do not suggest that antenatal GC treatment should be withheld, but rather emphasize the importance of improving the accuracy of diagnosis of preterm labor to reduce the exposure of infants subsequently born at term to prenatal GC treatment. Further, our findings emphasize the importance of evaluating long-term consequences of prenatal GC treatment on multiple domains of development. More broadly, these data add to the body of evidence indicating the importance of early exposure to GCs in shaping brain development and emphasize the necessity of considering prenatal influences on individual differences in health and development across the lifespan.
Acknowledgments
This research was supported by NIH R01 HD050662 to E.P.D. and NIH R01 NS41298 to C.A.S. The authors wish to thank the families who participated in this project. The assistance of Megan Blair, Natalie Hernandez, Jocelle Marucut, and Carol Patillo is gratefully acknowledged.
REFERENCES
- Albiston AL, Obeyesekere VR, Smith RE, & Krozowski ZS (1994). Cloning and tissue distribution of the human 11β-hydroxysteroid dehydrogenase type 2 enzyme. Molecular and Cellular Endocrinology, 105(2), R11–R17. [DOI] [PubMed] [Google Scholar]
- Als H (1998). Manual for the naturalistic observation of newborn behavior (preterm and full-term). Boston, MA: The Children’s Hospital. [Google Scholar]
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DJ, Hiller JE, et al. (2006). Neonatal adrenal function after repeat dose prenatal corticosteroids: A randomized controlled trial. American Journal of Obstetrics and Gynecology, 194(3), 861–867. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Gluckman PD, Liggens GC, Kaplan SL, & Grumbach MM (1980). Steroid and growth hormone levels in premature infants after prenatal betamethasone therapy to prevent respiratory distress syndrome. Pediatric Research, 14(2), 122–127. [DOI] [PubMed] [Google Scholar]
- Barker DJP (1998). Mothers, babies and health in later life. Edinburgh: Churchill Livingston. [Google Scholar]
- Buss K, Davidson RJ, Kalin NH, & Goldsmith HH (2004). Context specific freezing. Developmental Psychology, 40, 583–594. [DOI] [PubMed] [Google Scholar]
- Calixto C, Martinez FE, Jorge SM, Moreira AC, & Martinelli CE (2002). Correlation between plasma and salivary cortisol levels in preterm infants. Journal of Pediatrics, 140, 116–118. [DOI] [PubMed] [Google Scholar]
- Challis J, Patrick J, Richardson B, & Tevaarwerk G (1981). Loss of diurnal rhythm in plasma estrone, estradiol, and estriol in women treated with synthetic glucocorticoids at 34 to 35 weeks’ gestation. American Journal of Obstetrics and Gynecology, 139(3), 338–343. [DOI] [PubMed] [Google Scholar]
- Crowley P (1995). Antenatal corticosteroid therapy: A meta-analysis of the randomized trials, 1972–1994. American Journal of Obstetrics & Gynecology, 173, 322–355. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn L, Waffarn F, & Sandman CA (2010). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. DOI: 10.1111/J.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-DeMet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry, 46(6), 737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81(1), 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Cifuentes RF, et al. (2004). Effects of prenatal corticosteroid exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology, 29, 1028–1036. [DOI] [PubMed] [Google Scholar]
- Davis EP, Townsend EL, Gunnar MR, Guiang SF, Lussky RC, Cifuentes RF, et al. (2006). Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. Journal of Perinatology, 26(3), 147–153. [DOI] [PubMed] [Google Scholar]
- Davis EP, Waffarn F, Uy C, Hobel CJ, Glynn LM, & Sandman CA (2009). Effect of prenatal glucocorticoid treatment on size at birth among infants born at term gestation. Journal of Perinatology 11(26), 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, et al. (2007). Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. Journal of Clinical Investigation, 117(4), 1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Fuchs E, Feldon J, Pryce CR, & Forssberg H (2010). Effects of antenatal dexamethasone treatment on glucocorticoid receptor and calcyon gene expression in the prefrontal cortex of neonatal and adult common marmoset monkeys. Behavioral and Brain Functions, 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr HG, Versmold HT, Sippell WG, Bidlingmaier F, & Knorr D (1986). Antenatal betamethasone therapy: Effects on maternal, fetal, and neonatal mineralocorticoids, glucocorticoids, and progestins. Journal of Pediatrics, 108(6), 990–993. [DOI] [PubMed] [Google Scholar]
- Fortunato CK, Dribin AE, Granger DA, & Buss KA (2008). Salivary alpha-amylase and cortisol in toddlers: Differential relations to affective behavior. Developmental Psychobiology, 50, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, et al. (2005). Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain, 113(3), 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Hertsgaard L, Larson M, & Rigatuso J (1992). Cortisol and behavioral responses to repeated stressors in the human newborn. Developmental Psychobiology, 24(7), 487–505. [DOI] [PubMed] [Google Scholar]
- Herman JP, & Cullinan WE (1997). Neurocircuitry of stress: Central control of the hypothalamio-pituitary-adrenocortical axis. Trends in Neuroscience, 20(2), 78–84. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Wedell A, et al. (2007). Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. Journal of Clinical Endocrinology and Metabolism, 92(2), 542–548. [DOI] [PubMed] [Google Scholar]
- Joels M, & Baram TZ (2009). The neuro-symphony of stress. Nature Reviews Neuroscience, 10(6), 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N, & Arcus D (1998). Childhood derivatives of high and low reactivity in infancy. Child Development, 69(6), 1483–1493. [PubMed] [Google Scholar]
- Kajantie E, Raivio T, Janne OA, Hovi P, Dunkel L, & Andersson S (2004). Circulating glucocorticoid bioactivity in the preterm newborn after antenatal betamethasone treatment. Journal of Clinical Endocrinology and Metabolism, 89(8), 3999–4003. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, & Matthews SG (2006). Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. Journal of Physiology, 572(Pt 1), 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Petropoulos S, & Matthews SG (2008). Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Research Reviews, 57, 586–595. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22(1), 150–169. [DOI] [PubMed] [Google Scholar]
- Korebrits C, Yu DH, Ramirez MM, Marinoni E, Bocking AD, & Challis JR (1998). Antenatal glucocorticoid administration increases corticotrophin-releasing hormone in maternal plasma. British Journal of Obstetrics and Gynecology, 105(5), 556–561. [DOI] [PubMed] [Google Scholar]
- Kramer MS (2009). Late preterm birth: Appreciable risks, rising incidence. Journal of Pediatric, 154(2), 159–160. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, & Seckl JR (1996). Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology, 64(6), 412–418. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Conference. (1995). Effects of corticosteroids for fetal maturation and perinatal outcomes. American Journal of Obstetrics and Gynecology, 173, 253–344. [Google Scholar]
- NIH Consensus Statement for the use of Antenatal Steroids. (1995). Effects of corticosteroids for fetal maturation and perinatal outcomes. American Journal of Obstetrics and Gynecology, 173, 253–344. [Google Scholar]
- Noorlander CW, De Graan PN, Middeldorp J, Van Beers JJ, & Visser GH (2006). Ontogeny of hippocampal corticosteroid receptors: Effects of antenatal glucocorticoids in human and mouse. Journal of Comparative Neurology, 499(6), 924–932. [DOI] [PubMed] [Google Scholar]
- Parker CR, Atkinson MW, Owen J, & Andrews WW (1996). Dynamics of the fetal adrenal, cholesterol, and apolipoprotein B responses to antenatal betamethasone therapy. American Journal of Obstetrics and Gynecology, 174(2), 562–565. [DOI] [PubMed] [Google Scholar]
- Piazze J, Berretta AR, Cioccio D, & Anceschi M (2005). Neonatal length and cranial circumference are reduced in human pregnancies at term after antepartum administration of betamethasone. Journal of Perinatal Medicine, 33(5), 463–464. [DOI] [PubMed] [Google Scholar]
- Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, & Wallace EM (2007). Patterns of antenatal corticosteroid prescribing 1998–2004. Australian and New Zealand Journal of Obstetics and Gynaecology, 47(1), 42–45. [DOI] [PubMed] [Google Scholar]
- Schaffer L, Luzi F, Burkhardt T, Rauh M, & Beinder E (2009). Antenatal betamethasone administration alters stress physiology in healthy neonates. Obstetrica si Ginecologia, 113(5), 1082–1088. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, & Challis JR (2002). The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. Journal of Endocrinology, 172(1), 71–81. [DOI] [PubMed] [Google Scholar]
- Stansbury K, & Gunnar MR (1994). Adrenocortical activity and emotion regulation. Monogragraphs of the Society for Research on Child Development, 59, 108–134. [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. (1994). Neurotoxicity of glucocorticoids in the primate brain. Hormone and Behavior, 28, 336–348. [DOI] [PubMed] [Google Scholar]
- van Baar AL, Vermaas J, Knots E, de Kleine MJ, & Soons P (2009). Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics, 124(1), 251–257. [DOI] [PubMed] [Google Scholar]


