Abstract
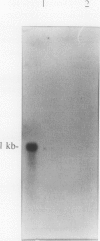
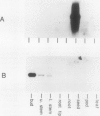
We report a striking example of organ and stage specific gene expression in pea (Pisum sativum L.). We have identified a transcript to a previously isolated cDNA clone, pEA207 (WF Thompson et al. (1983) Planta 158: 487-500) which accumulates in the actively growing bud of the pea plant and is either absent or present at very low levels in the expanded leaves below the bud. The deduced amino acid sequence of pEA207 shows 49% similarity to the phytohemagglutinin lectin sequence of kidney bean (Phaseolus vulgaris) (LM Hoffman, DD Donaldson (1985) EMBO J 4: 883-889) and 37% similarity to that of the major pea seed lectin sequence (TJV Higgins et al. (1983) J Biol Chem 258: 9544-9549). It is also similar to seed lectins from five other legumes. All of the residues directly involved in metal binding by lectins are present in this sequence. We discuss the possibility that pEA207 encodes a sugar binding lectin-like polypeptide associated with the cell walls of actively growing plant cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. W., Reeke G. N., Jr, Cunningham B. A., Edelman G. M. New evidence on the location of the saccharide-binding site of concanavalin A. Nature. 1976 Feb 5;259(5542):406–409. doi: 10.1038/259406a0. [DOI] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit C. M., Meagher R. B. Expression of a gene encoding a glycine-rich protein in petunia. Mol Cell Biol. 1987 Dec;7(12):4273–4279. doi: 10.1128/mcb.7.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Etzler M. E., Macmillan S., Scates S., Gibson D. M., James D. W., Cole D., Thayer S. Subcellular Localizations of Two Dolichos biflorus Lectins. Plant Physiol. 1984 Dec;76(4):871–878. doi: 10.1104/pp.76.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Chandler P. M., Zurawski G., Button S. C., Spencer D. The biosynthesis and primary structure of pea seed lectin. J Biol Chem. 1983 Aug 10;258(15):9544–9549. [PubMed] [Google Scholar]
- Hoffman L. M., Donaldson D. D. Characterization of two Phaseolus vulgaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985 Apr;4(4):883–889. doi: 10.1002/j.1460-2075.1985.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Hemperly J. J., Cunningham B. A. Amino acid sequence and variant forms of favin, a lectin from Vicia faba. J Biol Chem. 1982 Apr 25;257(8):4473–4483. [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W. Three-dimensional structure of favin: saccharide binding-cyclic permutation in leguminous lectins. Science. 1986 Nov 28;234(4780):1108–1111. doi: 10.1126/science.3775378. [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Etzler M. E. Development and Distribution of a Lectin from the Stems and Leaves of Dolichos biflorus. Plant Physiol. 1984 Dec;76(4):879–884. doi: 10.1104/pp.76.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]