Abstract
We report that in Jurkat T cells or freshly isolated T lymphocytes, physiological concentrations of high– molecular weight sulfated polysaccharides such as heparin, heparan sulfate, and dextran sulfate significantly increased the percentage of cell death induced by Fas IgM agonistic antibody. The phenomenon was caspase dependent and P53 independent and correlated with an increased accessibility of cell surface Fas receptors. We also observed that the Fas IgM agonistic antibody–dependent formation of sodium dodecyl sulfate (SDS)–resistant large structures containing Fas receptor was decreased in the presence of heparin-like agents. In contrast, the different agents had no effect when cell death was triggered by FasL, the natural ligand of Fas that does not generate SDS-resistant forms of Fas. Interestingly, the synergistic effect of heparin-like agents toward Fas IgM agonistic antibody–mediated cell death abolished Hsp27 antiapoptotic activity but did not alter much the protection generated by Bcl-2 expression.
INTRODUCTION
Fas (APO-1/CD95) is a receptor belonging to the tumor necrosis factor R (TNF-R) superfamily, which transduces a death pathway that regulates cell populations during development and maintains immune system homeostasis (Itoh et al 1991; Nagata and Golstein 1995; Nagata 1997). This pathway is also implicated in several pathologies such as autoimmune diseases, cancers, neurodegenerative diseases, acquired immune deficiency syndrome, and virally induced hepatitis. The interaction between Fas receptor and Fas ligand (FasL) or an agonistic anti-Fas antibody recruits several signaling proteins, such as the adaptor FADD (Fas-associated death domain protein) (Chinnaiyan et al 1995) and procaspase-8, which form the death-inducing signaling complex (DISC) (Kischkel et al 1995) that activates procaspase-8. Two Fas signaling pathways have been described (Scaffidi et al 1998). In type I cells, caspase-8 activates directly procaspase-3 and initiates the caspases cascade. In type II cells, DISC formation is less intense, and caspase-8 preferentially cleaves the cytoplasmic protein Bid. This results in a truncated form of Bid (tBid), which is able to induce cytochrome c release. This apoptogenic agent triggers the formation of the apoptosome complex constituted of several oligomers of the adaptor protein Apaf-1 and procaspase-9 (Zou et al 1999). Activated through this pathway, caspase-9 then activates procaspase-3 and, subsequently, the caspases cascade. This mitochondrial amplification loop occurs in both cell types but is essential only in the case of type II cells. Different polypeptides regulate the Fas apoptotic pathway. c-FLIP can act as an inhibitor of the recruitment and conversion of procaspase-8 in active caspase-8 (Muzio et al 1996; Thome and Tschopp 2001), and members of the Bcl-2 family modulate the mitochondrial pathway in type II cells (Sun et al 2002). Inhibitors of apoptosis are caspase inhibitors (Bratton et al 2001) that are negatively regulated by Smac/DIABLO, an apoptogenic polypeptide released from the mitochondria (Verhagen et al 2000). Among other negative modulators efficient in type II cells is Hsp27 (Mehlen et al 1996; Bruey et al 2000; Charette et al 2000; Pandey et al 2000; Paul et al 2002; Rane et al 2003). If chemical agents are considered, one can cite sodium butyrate, which enhances the sensitivity of colon carcinoma cell lines to Fas-mediated apoptosis (Bonnotte et al 1998).
Heparin, heparan sulfate, and dextran sulfate belong to the glycosaminoglycan family of macromolecules, which also includes chondroitin sulfate, dermatan sulfate, and keratan sulfate. These molecules are linear polysaccharides consisting of repeating disaccharide unit backbones onto which are superimposed specific modification patterns, such as sulfate groups. Glycosaminoglycans can interact with specific proteins, particularly at the cell surface, and function as a new class of multifunctional cell regulators (Turnbull et al 2001). Apart from their anticoagulant action (Bjork and Lindahl 1982), heparin-like molecules have many other biological properties, such as their potential anti-inflammatory and immunomodulatory effects (Gorski et al 1991; Tyrell et al 1995). In addition, heparin, dextran sulfate, and several other sulfated polysaccharides can induce complex transmembrane signalings such as those resulting in an intracellular rise in free cytosolic Ca2+ ions (Tellam and Parish 1987).
Death receptor resistance is a common phenomenon observed, for example, in cancer cells, which occurs often in spite of the presence of the corresponding death receptor (Owen-Schaub et al 1994; Owen-Schaub 2002). These cells then escape the immune clearance because both cytotoxic T cells and natural killer (NK) cells express FasL and use the Fas-FasL system to kill the target cells (Hanabuchi et al 1994; Kagi et al 1994; Lowin et al 1994; Arase et al 1995). Another example of death receptor resistance is observed in pathologies such as the autoimmune lymphoproliferative syndrome, which is characterized by a deficiency in T cell apoptosis linked to Fas dysfunction (Rifkin and Marshak-Rothstein 1999; Ricci-Vitiani et al 2000; Fleisher et al 2001; Goldman et al 2002). Hence, agents that restore or stimulate T cell sensitivity to Fas-mediated apoptosis might improve current therapeutic strategies.
Here, we provide evidence that heparin-like molecules stimulate low levels of Fas-mediated apoptosis in T lymphocytes exposed to Fas agonistic antibody. This stimulation decreased Fas receptor aggregation and abolished Hsp27 protective activity.
MATERIALS AND METHODS
Reagents
Heparin (ammonium salt from porcine intestinal mucosa), heparan sulfate (fast-moving fraction, sodium salt from porcine intestinal mucosa), dextran sulfate (sodium salt, average molecular weight of 500 000), chondroitin sulfate A (sodium salt from bovine trachea), etoposide, staurosporine, actinomycin D, propidium iodide (PI), crystal violet, apoferritin, thyroglobulin, and blue dextran were purchased from Sigma–Aldrich (St-Quentin-Fallavier, France). Fragmine™ (daltéparine sodique, 1 intravenous dose: 2500 IU of heparin) is a registered trademark product of Pharmacia and Upjohn (Saint-Quentin-en-Yvelines, France). Fraxiparine™ (nadroparine calcique, 1 intravenous dose: 2850 IU of heparin) is a registered trademark product of Sanofi Winthrop (Gentilly, France). Anti-human Fas antibody (clone CH11) was obtained from Upstate Biotechnology (Lake Placid, NY, USA). Anti-human Fas death domain antibody (clone 3D5) was from Alexis Biochemicals (Qbiogen, Illkirch, France). Anti–Bcl-2 and anti-FLIP antibodies as well as rabbit IgG directed against anti-mouse IgM antibodies were from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Anti-Hsp27 antibody was from Stressgen (Victoria, BC, Canada). z-VAD-fmk was from BIOMOL Research Laboratories Inc (Plymouth Meeting, PA, USA). IETD-fmk was from MBL (Medical and Biological Laboratories Co LTT, Nagoya, Japan). TNFα was from PeproTech Inc (Rocky Hill, NJ, USA). Soluble human recombinant superFasL was from Alexis Biochemicals (San Diego, CA, USA). Recombinant TNF-related apoptosis–inducing ligand (TRAIL) was from PeproTech. Annexin-V-FLUOS was from Roche Diagnostics (Meylan, France).
Cell lines
H9 and Jurkat human T lymphocytes were obtained from American Type Culture Collection (Rockville, MD, USA). These cells, as well as human peripheral T lymphocytes, were grown in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal calf serum (FCS) and 50 U/mL penistreptomycin (InVitrogen, Cergy Pontoise, France). The human hepatic HepG2 cell line was grown in Iscove modified, Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS and 50 U/ mL penistreptomycin. HeLa, HT1080, and L929-APO cells were grown in DMEM supplemented with 10% FCS, 50 U/mL penistreptomycin, and 1 μg/mL fungizone (InVitrogen). Jurkat T cells overexpressing Hsp27 or Bcl-2 were obtained after transfection with either pCIneohsp27 or pCIneobcl2 vector. These vectors contain the entire coding sequence of either human Hsp27 or Bcl-2 gene under the control of cytomegalovirus promoter and a neomycin gene selection. pCIneohsp27 was constructed using an EcoRI-EcoRI deoxyribonucleic acid (DNA) fragment of plasmid psvhsp27 (Mehlen et al 1995), which was inserted in the EcoRI site of pCIneo vector (Promega, Charbonnieres, France). pCIneobcl2 was constructed using an EcoRI-EcoRI DNA fragment of plasmid pCDNA3-hbcl2 (Paul and Arrigo 2000), which was inserted in the EcoRI site of pCIneo vector. Control cells resistant to neomycin were obtained by transfecting plain pCIneo vector. The transfection experiments were performed using Superfect transfection reagent (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions. Ten micrograms of DNA were combined with 60 μL of Superfect in 300 μL of RPMI medium devoid of serum and antibiotics. The mixture was then completed to 2 mL with RPMI containing serum and penistreptomycin and added to 8 mL of a Jurkat cell culture at 106 cells/mL. Polyclonal pools of transfected cells were selected with 500 μg/mL of G418 (Invitrogen). Murine L929-APO fibrosarcoma expressing the human Fas gene under the control of CMV constitutive promoter has already been described (Schulze-Osthoff et al 1994; Mehlen et al 1996). All these cells were grown at 37°C in the presence of 5% CO2.
Phase contrast analysis of cell morphology
Jurkat cells either kept untreated or treated with CH11 antibody in the presence or absence of high–molecular weight sulfated polysaccharides were analyzed using a ZEISS Axiovert 25 microscope (200×) equipped with a phase contrast filter. Phase contrast microphotography of the cells was recorded using an Olympus digital camera (CAMEDIA C-3040 ZOOM).
Peripheral T lymphocyte purification and activation
Peripheral blood mononuclear cells (PBMC), purified from human blood (Etablissement Français du Sang, Lyon, France) by ficoll gradient centrifugation, were rinsed twice in phosphate-buffered saline (PBS) containing 2 mM ethylenediamine-tetraacetic acid (EDTA) and resuspended in RPMI medium at a concentration of 2.107 cells/mL. PBMC were then incubated for 20 minutes with a cocktail of anti-CD19 (BD Biosciences, Le Pont de Claix, France), anti-CD14 (BD Biosciences), anti-CD16 (BD Biosciences), anti-CD56 (anti-NKH1, Beckman Coulter/Immunotech, Roissy CDG, France), anti-CD35 (Beckman Coulter/Immunotech), and anti–glycophorin A (Beckman Coulter/Immunotech) antibodies. After 3 rinses in PBS containing 2 mM EDTA and 2% FCS, magnetic beads coupled to goat anti-mouse IgG (Dynabeads Pan, Dynal Biotech, Compiègne, France) were added to the cell suspension (2 × 109 beads in 5 mL with a ratio of 5 beads per cell). After 20 minutes at 4°C the cell suspension was run 3 consecutive times over the magnetic column provided by the manufacturer. The fractions not retained on the column were pooled, spun 10 minutes at 1400 rpm, and resuspended in PBS containing 2 mM EDTA and 2% FCS. A new batch of magnetic beads was then added to the cell suspension (1.6 × 109 beads in 4 mL; ratio of 4 beads per cell). After 20 minutes at 4°C the cell suspension was again loaded over the magnetic column. The fractions that were not retained on the column were pooled. The cells were then frozen and kept in liquid nitrogen (−80°C) in dimethyl sulfoxide–FCS. For activation, T cells were thawed in RPMI medium containing 10% FCS and rinsed twice before being seeded at 106 cells/ mL and grown for 5 days in RPMI medium supplemented with 10% FCS, 5 μg/mL phytohemagglutinin (PHA-P from Phaseolus vulgaris red kidney bean, Sigma–Aldrich), and 20 IU/mL of interleukin 2 (R&D Systems, Lille, France). Activated T cells were then induced in apoptosis by treatment with CH11 antibody (1 μg/mL). The purification and use of these human cells was done according to the regulations of the French Ministry of Health.
Annexin-V-FLUOS and PI cell staining
Cells were either kept untreated or exposed to apoptotic treatments. Cells in suspension such as Jurkat were only rinsed in PBS, whereas adherent cells (ie, HepG2 and HeLa) were trypsinized and then rinsed in PBS. After centrifugation each cell pellet was suspended in 200 μL reaction buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid–NaOH, pH 7.4, 140 mM NaCl, 5 mM CaCl2) and 2 μL of Annexin-V-FLUOS (Roche Diagnostics). After 10 minutes in the dark at room temperature, cells were rinsed with reaction buffer and suspended in 500 μL reaction buffer containing 10 μg/mL of PI (Sigma–Aldrich). A dual-color analysis (FL1 filter for Annexin-V-FLUOS and FL3 filter for PI) using a 10 000-cell population was performed on a FACScalibur flow cytometer (BD Biosciences). Results are presented as the percentage of cell death that corresponds to the percentage of Annexin-V/PI positive cells.
Crystal violet staining
Crystal violet staining was used to analyze the resistance of adherent cells (such as HT 1080 and L929-APO) to death induction. In this case, cells (104/well) were grown in 96-well plates for 24 hours before being analyzed. After incubation with apoptosis-inducing agents, the cells were rinsed twice with PBS, and the remaining viable cells were stained for 15 minutes with 0.5% crystal violet in 20% methanol. Microtiter plates were rinsed and dried. A solution containing 0.1 M sodium citrate, pH 4.2, and 50% methanol was then added to induce the solubilization of the stained cells. The absorbance of each well was read at 550 nm with an MR500 MicroElisa reader (Dynatech Laboratories, Chantilly, France). Crystal violet staining index was defined as the relative absorbance of treated vs untreated control cells.
Caspase activation measurement
For the determination of DEVD-AFC–dependent procaspases-3–like and IETD-AFC–dependent procaspase-8– like activities, 106 cells were harvested and subsequently washed twice in ice-cold PBS, pH 7.4. They were then spun at 200 × g for 5 minutes, and the dry cell pellets were stored at −80°C. The determination of DEVD-AFC activity was done using the Apo-Alert CPP32 fluorometric assay kit (Clontech, Montigny les Bretonneux, France). For procaspase-8, IETD-AFC activity was determined using the Apo-Alert FLICE fluorometric assay kit (Clontech). Excitation was at 400 nm and emission at 505 nm in a Victor Wallach cytofluorometer (EG&G Instruments, Evry, France).
Cell surface Fas receptor staining
Cells (5 × 105) were rinsed in PBS, spun, and suspended in 100 μL of a solution containing 1% bovine serum albumin (BSA) in PBS. Five micrograms per milliliter of CH11 anti-Fas antibody was added to the solution, and the cells were incubated with gentle agitation for 45 minutes at 4°C. The cells were then rinsed in PBS and resuspended in 100 μL PBS, 1% BSA, before being exposed for 45 minutes on ice to a goat anti-mouse IgM (μ) antibody (diluted 1:100) conjugated to the fluorochrome R-phycoerythrin (RPE) (Caltag Laboratories, Burlingame, CA, USA). After a last PBS rinse, 104 cells were analyzed by flow cytometry with a FACScalibur flow cytometer (BD Biosciences). RPE fluorescence was read through an FL2 filter. In each case, control was prepared by incubating 5 × 105 cells with only the secondary antibody. The fluorescence measured in this control, which represents the fluorescence background of the cells, was adjusted to the same value to enable comparison of the effects induced by the different treatments of the cells exposed to CH11 antibody.
Analysis of Fas receptor aggregation
Samples were prepared according to Kamitani et al (1997). In brief, 106 Jurkat T cells were treated for the indicated time periods and then washed twice with cold PBS and centrifuged. To prevent protein degradation, cell pellets were snap frozen in liquid nitrogen. Frozen pellets were then suspended and incubated for 1 hour at 45°C in 100 μL of a sample buffer made of 2% sodium dodecyl sulfate (SDS), 0.1 M Tris-HCl, pH 6.8, 20% glycerol, 5% β-mercaptoethanol, and 0.006% bromophenol blue. The samples (100 μL) were then loaded on a 12% polyacrylamide gel for SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane (Protran Amersham, Amersham Biosciences Europe, Orsay, France). The different forms of the Fas receptor were detected with a monoclonal anti-Fas antibody that recognizes the death domain of Fas (clone 3D5, Alexis Biochemicals, Qbiogen, Illkirch, France). Analysis of Fas receptor aggregation was also performed after gel filtration analysis of the cell lysate. In this case, after 2 hours of treatment, 2 × 107 Jurkat cells were rinsed twice in ice-cold PBS before being lysed in 500 μL of TEM buffer (20 mM Tris-HCl, pH 7.4, 20 mM NaCl, 5 mM MgCl2, 0.1 mM EDTA) containing 0.1% Triton X-100, 1 mM Na3VO4, and 1 mM Pefabloc (Merck, Darmstadt, Germany). After 15 minutes of incubation on ice, cells were vortexed for 30 seconds, and the lysates were spun for 10 minutes at 13 000 rpm. Two aliquots of 5 μL of the supernatant were used for protein content determination by the Bradford technique (Bio-Rad Protein Assay, Bio-Rad, Marnes la Coquette, France). Two hundred microliters of supernatant (approximately 500 μg of protein) was loaded onto a Superose 12 gel filtration column (Pharmacia FPLC System, Pharmacia, Uppsala, Sweden). Elution was done in TEM buffer (20 mM Tris-HCl, pH 7.4, 20 mM NaCl, 5 mM MgCl2, 0.1 mM EDTA), and 200-μL fractions were collected. Fraction Nos. 21–52 were analyzed. They were pooled 2 by 2 and boiled after addition of 100 μL of sample buffer (SDS 5%, Tris-HCl 250 mM, pH 6.8, glycerol 50%, β-mercaptoethanol 5%, and bromophenol blue 0.015%). Samples were then separated by SDS-electrophoresis on a 12% polyacrylamide gel, and immunoblots were performed as above.
Gel electrophoresis and immunoblotting
Immunoblots and gel electrophoresis were done as already described (Paul et al 2002). The detection of the immunoblots was performed with the enhanced chemiluminescence (ECL) kit from Amersham Biosciences Europe. Autoradiographs were recorded onto X-Omat LS films (Eastman Kodak Co, Rochester, NY, USA).
RESULTS
Stimulation of Fas-mediated death in Jurkat T cells by high–molecular weight sulfated polysaccharides
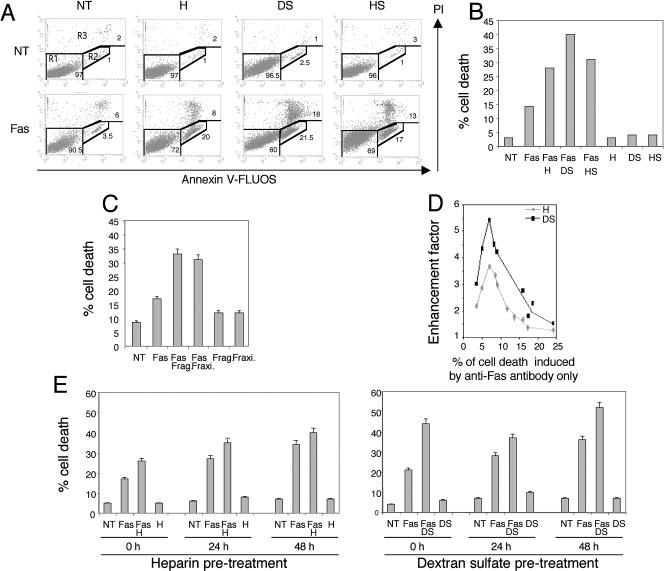
Jurkat T cells were freshly resuspended in 10% serum containing medium and treated for 12 hours with Fas-activating CH11 antibody (100 ng/ml, 106 cells/ml). Under these conditions, and as previously described (Aronis et al 2003), we observed that only approximately 10% of the cells were PI and Annexin-V positive (Fig 1A). A higher percentage of cell death was induced by 100 ng/ mL of CH11 antibody if the cells were grown for a longer time period before the beginning of treatment (up to 40% of cell death after 48 hours of culture in the same medium, see Fig 1E). As seen in Figure 1 A,B, when Jurkat T cells were treated with a combination of CH11 agonistic Fas antibody and 100 μg/mL of heparin, dextran sulfate, or heparan sulfate, an increased number of Annexin-V– and PI-positive cells were noticed. In contrast, treatment with the different sulfated polysaccharides only had no significant effect. Phase contrast analysis of the morphology of the cells confirmed the enhanced killing ability of anti-Fas antibody in the presence of heparin or dextran sulfate (Fig 2). This figure also shows that under our experimental conditions, heparin and dextran sulfate do not significantly promote cell aggregation (Thurn and Underhill 1986) or interfere with the mitotic index of the cells (data not shown). The death-stimulatory effect of sulfated polysaccharides was independent of gene transcription because it was still observed in actinomycin D– treated cells (data not shown). Interestingly, the effect described in Figure 1 A,B was still observed when physiological concentrations of Fraxiparine and Fragmine, trade names of heparin (see the Materials and Methods), were used (Fig 1C). In contrast, when similar experiments were performed with chondroitin sulfate, a sulfated polysaccharide whose molecular weight is lower than that of the sulfated polysaccharides described above, no enhancement in the percentage of Annexin-V– and PI-positive cells was observed (data not shown). We next investigated whether the death-stimulatory effect of sulfated polysaccharides was dependent on the percentage of Annexin-V– and PI-positive cells induced by CH11 antibody only. As seen in Figure 1D, the effect of sulfated polysaccharides was maximal (between 4.2- and 5.5-fold in the case of dextran sulfate) when the percentage of Annexin-V– and PI-positive cells induced by anti-Fas antibody alone was between 6 and 9. At a higher percentage of Annexin-V– and PI-positive cells, the stimulatory effect was gradually lost. This observation was confirmed by phase contrast analysis of the morphology of the cells (data not shown). We then investigated whether a pretreatment of Jurkat T cells with heparin or dextran sulfate could still stimulate a subsequent exposure to agonistic Fas antibody. To do so, Jurkat cells were either kept untreated or treated for 24 hours with 100 μg/mL of heparin or dextran sulfate. The medium was changed, and the cells were treated with CH11 either immediately or after 24 or 48 hours of culture in the absence of the sulfated polysaccharides. As seen in Figure 1E, the killing ability of CH11 antibody treatment alone was greater when cells were allowed to remain 24 or 48 hours in culture. This phenomenon is probably linked to the antagonist action of growth factors toward Fas apoptosis (Holmstrom et al 2000; Tran et al 2001). As seen in Figure 1E, the stimulation of CH11-mediated cell death was still detectable 2 days after the removal of the sulfated polysaccharides from the culture medium. However, the stimulation was less intense than that observed when cells were treated immediately after incubation with the polysaccharides. Whether this decreased stimulation is the result of a gradual loss of the effects induced by the sulfated polysaccharides or of the higher level of cell death induced by Fas antibody alone (or both) (see above, Fig 1D) is not known.
Fig 1.
Sulfated polysaccharides stimulate cell death mediated by Fas agonistic antibody. (A) Sulfated polysaccharides stimulate Fas-mediated propidium iodide (PI)– and Annexin-V–positive cells. Jurkat T cells were resuspended in fresh culture medium and either kept untreated (NT) or treated for 12 hours with 100 ng/mL of agonistic anti-Fas CH11 antibody (Fas) in the presence or absence of 100 μg/mL heparin (H), dextran sulfate (DS), or heparan sulfate (HS). Cells were then stained with Annexin-V-FLUOS and PI, as described in the Materials and Methods, and analyzed by flow cytometry. Annexin-V fluorescence is reported on the x-axis and PI fluorescence on the y-axis. The lower-left area of the graphs (R1) is representative of viable cells (PI- and Annexin-V–negative cells). On the right part of the graphs the R2 region is representative of Annexin-V–positive apoptotic cells, whereas the upper area of the graphs (PI-positive cells, R3 region) represents late-apoptotic or dead cells. (B) Histogram representation of the phenomenon described in (A). Jurkat T cells untreated (NT) or treated with 100 ng/mL CH11 antibody (Fas) in the presence or absence of heparin (H), dextran sulfate (DS), or heparan sulfate (HS). The percentage of cell death, corresponding to the percentage of cells present in the R2 and R3 regions, is represented. (C) Physiological doses of Fragmine™ and Fraxiparine™ stimulate Fas-mediated apoptosis. Jurkat T cells were either kept untreated (NT) or treated for 12 hours with 100 ng/mL of CH11 antibody (Fas) in the presence or absence of 0.5 U/mL of Fragmine™ (Frag) or Fraxiparine™ (Fraxi). This concentration is equivalent to the dilution of about 1 intravenous dose (2500 IU) of these pharmaceutical agents in the blood (5000 mL) of patients. Surviving and dying cells were analyzed as in (A). (D) The initial degree of Fas-induced cell death is critical to observe the stimulation of Fas-induced cell death by sulfated polysaccharides. The same experiment was done with various doses of CH11 antibody. The percentage of Annexin-V–positive cells induced by CH11 alone is reported on the x-axis. The y-axis represents the cell death enhancement factor, calculated as the ratio of the percentage of Annexin-V– and PI-positive cells induced by the cotreatment of CH11 antibody and heparin (H) or dextran sulfate (DS) to the percentage of those induced by the treatment with CH11 only. (E) A pretreatment of Jurkat T cells with heparin or dextran sulfate is efficient enough to stimulate Fas-mediated cell death, even if it occurs 48 hours before the anti-Fas antibody treatment. Jurkat T cells were either kept untreated (NT) or treated for 24 hours with 100 μg/mL heparin (H) or dextran sulfate (DS). The medium was then changed at 0, 24, or 48 hours before the cells were treated for 12 hours with 100 ng/mL CH11 antibody (Fas). The cells were then stained with Annexin-V-FLUOS and PI and analyzed by flow cytometry as described in the Materials and Methods. The percentage of cell death is represented on histogram plots
Fig 2.
Phase contrast analysis of cell morphology. Jurkat cells were either kept untreated (NT) or treated with 100 ng/mL of anti-Fas CH11 antibody (Fas) combined or not combined with 100 μg/ mL of heparin (Fas + H) or dextran sulfate (Fas + DS). Phase contrast microphotography analysis of the cells was recorded after 12 hours of treatment. The white bar represents approximately 35 μm
Further analysis revealed that the stimulation in the CH11-mediated appearance of Annexin-V– and PI-positive cells was dependent on the concentration of either heparin or dextran sulfate: a maximal effect was observed with a concentration of 100 μg/mL (data not shown). This concentration was further used in this study because it represents a physiological concentration for which the treatment with the polysaccharides only does not induce Annexin-V– and PI-positive cells. It was also observed that the activation of caspase-8–like and caspase-3–like proteases, measured after 6 hours of cotreatment with anti-Fas antibody and heparin or dextran sulfate, was enhanced compared with their activation induced by CH11 antibody only (Fig 3 A,B). Control experiments showed that in the presence of the broad-range caspase inhibitor z-VAD-fmk, CH11 antibody did not generate Annexin-V– and PI-positive cells even if heparin or dextran sulfate was present in the culture medium (Fig 3C). The stimulatory effect of sulfated polysaccharides on Fas-mediated apoptosis is then a caspase-dependent phenomenon.
Fig 3.
Heparin and dextran sulfate in the presence of Fas agonist antibody enhance caspase activation. (A,B) Heparin and dextran sulfate stimulate Fas-mediated caspase-8 and caspase-3 activation. Jurkat T cells were either kept untreated (NT) or treated for 6 hours with 100 ng/mL CH11 antibody (Fas), 100 μg/mL of heparin (H) or dextran sulfate (DS), or both. The activation of caspase-8– and caspase-3–like caspases was measured as described in the Materials and Methods. The caspase activation index corresponds to the ratio between the fluorescence read in treated cell lysates to that read in untreated cell lysates. (C) Effects of z-VAD-fmk caspase inhibitor on cell death stimulation by heparin and dextran sulfate. Jurkat T cells were either kept untreated (NT) or treated for 6 hours with 100 ng/ mL anti-Fas antibody (Fas) combined or not combined with 100 μg/ mL heparin (H) or dextran sulfate (DS) in the presence or absence of 20 μM of the general caspase inhibitor z-VAD-fmk. Cells were then stained with Annexin-V-FLUOS and propidium iodide and analyzed by flow cytometry as described in the Materials and Methods. The percentage of cell death is represented as histogram plots
The stimulatory effect of heparin and dextran sulfate on Fas apoptosis appears cell specific
To test whether the observations described above in the case of Jurkat T cells could be relevant in vivo, freshly isolated peripheral human T lymphocytes were analyzed. As seen in Figure 4 A,B, the enhancement of the killing ability of CH11 Fas-activating antibody by heparin was still observed. However, in this case dextran sulfate was not effective.
Fig 4.
Fas-mediated cell death stimulation by heparin and dextran sulfate is cell line dependent. Human peripheral T lymphocytes; HepG2, HeLa, and HT1080 human cells; as well as murine L929-APO cells were either kept untreated (NT) or treated for 24 hours with anti-CH11 antibody (Fas) combined or not combined with 100 μg/mL of heparin (H) or dextran sulfate (DS). (A) Peripheral T lymphocytes and HepG2 and HeLa cells were treated with different concentrations of CH11 antibody (peripheral lymphocytes: 1000 ng/mL; HepG2: 100 ng/mL; HeLa: 10 and 200 ng/mL in the presence of 10 ng/mL actinomycin D; HT1080: 10, 50, and 500 ng/mL; L929-APO: 2.5, 5, and 20 ng/mL). Cells were then incubated with Annexin-V and propidium iodide and analyzed by flow cytometry as described in the Materials and Methods. The percentage of cell death is represented on histogram plots. (B) Survival of HT108 and L929-APO cells was analyzed by crystal violet staining as described in the Materials and Methods. Crystal violet staining index corresponds to the ratio of the absorbance detected for treated cells to the absorbance detected for untreated cells
In the case of the highly sensitive H9 human T lymphocyte cell line, we did not observe a stimulation of Fas-mediated cell death by heparin or dextran sulfate. However, this result may be taken with caution because of our lack of ability to induce a low percentage of cell death (less than 20%) using CH11 antibody only. In contrast, liver-derived HepG2 cells were reactive and showed enhancement of the killing ability of CH11 by heparin and dextran sulfate. Contrasting with these observations, when the experiments were performed in either HeLa or HT1080 cells, heparin and dextran sulfate counteracted the killing ability of Fas agonistic antibody (Fig 4). At low doses of CH11 antibody (10–50 ng/mL), the presence of these sulfated polysaccharides abolished the low level of spontaneous death observed in untreated cells and even stimulated cell proliferation. These observations clearly indicate that the death-stimulatory effect mediated by sulfated polysaccharides is cell specific and does not depend only on the presence of Fas receptor at the cell surface. To further test this assumption, we analyzed murine L929 fibrosarcoma cells transfected with a vector carrying the human Fas gene under the control of CMV constitutive promoter. These murine L929-APO cells express human Fas at their surface and undergo apoptosis when exposed to CH11 antibody (Mehlen et al 1996). In this case, heparin and dextran sulfate had either no effect (in the case of a weak killing effect induced by CH11 alone) or induced a protective effect (in the case of a strong killing effect induced by CH11 alone). These observations favor the hypothesis of a signal, specific to lymphocytes and hepatoma cells, that is triggered by the sulfated polysaccharides and stimulates a low rate of CH11-induced cell death.
Sulfated polysaccharides are active in the presence of specific cell death inducers such as Fas agonist antibody and TRAIL but do not stimulate FasL
We next investigated whether the putative specific signal generated by the sulfated polysaccharides was effective only if lymphocytes or hepatoma cells were exposed to CH11 agonistic antibody. As seen in Figure 5A, when Jurkat cells were treated for 12 hours with recombinant FasL (25 ng/mL), the percentage of cells undergoing apoptosis was about 13. When FasL incubation was performed in the presence of 100 μg/mL of heparin or dextran sulfate, no increase in the percentage of cells undergoing apoptosis was observed. In contrast, the oligosaccharides decreased the extent of apoptosis induced by FasL. This effect was not due to the fact that FasL apoptosis was already maximal because a similar observation was made in response to 100 ng/mL of FasL, which by itself killed at least 50% of the cells (data not shown). This observation prompted us to analyze cell death mediated by other death receptors. In the case of TNFα, no effect was induced (Fig 5B), whereas in the presence of TRAIL, dextran sulfate (Fig 5C), but not heparin (data not shown), strongly increased the number of cells committed to death. Analysis of other apoptotic inducers revealed that dextran sulfate slightly stimulated the low rate of cell death induced by 0.25 μM etoposide but did not interfere with the more drastic death induced by 1 μM of this agent (Fig 5D). No modulation of the death induced by either 0.05 or 0.1 μM of staurosporine was observed (Fig 5E). Hence, the death enhancement effect mediated by high–molecular weight sulfated polysaccharides appears rather specific to Fas agonistic antibody and TRAIL.
Fig 5.
High–molecular weight sulfated polysaccharides do not stimulate cell death induced by Fas ligand (FasL), tumor necrosis factor alpha (TNFα), or staurosporine, but dextran sulfate is efficient in the case of TNF-related apoptosis–inducing ligand (TRAIL). (A) Heparin and dextran sulfate do not stimulate cell death induced by recombinant superFas-L. Jurkat T cells were either kept untreated (NT) or treated for 24 hours with recombinant FasL (25 ng/mL) combined or not combined with 100 μg/mL heparin (H) or dextran sulfate (DS). Cells were then stained with Annexin-V and propidium iodide (PI) and analyzed by flow cytometry as described in the Materials and Methods. The percentage of cell death is represented on histogram plots. (B,C,D,E) Dextran sulfate does not stimulate cell death induced by TNFα, etoposide, or staurosporine but is effective when cell death is induced by TRAIL. Jurkat T cells were either kept untreated (NT) or treated for 24 hours with dextran sulfate (DS) alone or in the presence of TNFα (50 or 200 U/mL) plus actinomycin D (Act) (10 ng/mL) (B), recombinant TRAIL (100 ng/mL) (C), or etoposide (Eto) (0.25 and 1 μM) (D). Cells were also treated for 16 hours with dextran sulfate (DS) in the presence of staurosporine (St) (0.05 and 0.1 μM) (E). Cells were then stained with Annexin-V and PI and analyzed by flow cytometry as described in the Materials and Methods. The percentage of cell death is represented on histogram plots
The sulfated polysaccharides modulate the detection as well as the aggregation of Fas receptors
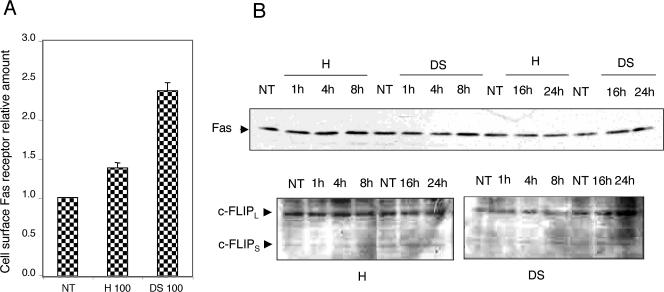
We next analyzed whether the level of Fas receptors was modified when cells were exposed to heparin or dextran sulfate. Interestingly, Figure 6A shows that heparin increased the detection of Fas at the surface of Jurkat T cells by about 40%. The increase was about 2.3-fold after exposure to dextran sulfate. This suggests that 1 step in the mechanism of heparin and dextran sulfate stimulation of Fas-mediated cell death is increasing either the number of Fas receptors at the cell surface or their accessibility to CH11 antibody. However, this is not the only mechanism that is involved because a similar phenomenon was observed in HeLa cells (data not shown) that do not respond to sulfated polysaccharides (see Fig 4). This suggests that additional cell-specific signals activated by the sulfated polysaccharides are required. Further immunoblot analysis revealed that heparin and dextran sulfate did not significantly modify the total cellular content of Fas and c-FLIPs/l (Fig 6B) as well as that of Bcl-2, Bax, Bid, Bcl-xL, caspase-3, P53, Apaf-1, FasL, and FADD (data not shown).
Fig 6.
Sulfated polysaccharides enhance the detection of cell surface Fas receptors but do not alter the total level of this protein as well as that of FLIP. (A) Sulfated polysaccharides enhance the detection of surface Fas receptors. Jurkat T cells were either kept untreated (NT) or treated for 24 hours with 100 μg/mL of heparin (H) or dextran sulfate (DS). The cell surface Fas receptors were then stained as described in the Materials and Methods, and the cells were analyzed by flow cytometry. The cell surface Fas receptor relative amount corresponds to the ratio between the number of Fas receptors detected on treated cells to the number of those detected at the surface of control untreated cells. (B) Sulfated polysaccharides do not modify the level of total cellular Fas and c-FLIPL/S proteins. Jurkat T cells were either kept untreated (NT) or treated for various time periods with 100 μg/mL of heparin (H) or dextran sulfate (DS). Protein samples were then prepared and analyzed by gel electrophoresis, and the immunoblots were probed with either CH11 or anti–c-FLIPs/l antibodies and revealed by enhanced chemiluminescence, as described in the Materials and Methods. Anti-actin antibody staining was used to verify the equal loading of the samples in the gel (data not shown). Autoradiographs of the immunoblots are presented
We next analyzed whether the sulfated polysaccharides induced a modification in Fas receptor aggregation. Indeed, it has already been reported (Kamitani et al 1997) that when cells are treated with Fas agonistic antibody, a fraction of the Fas receptor is detected in immunoblots, probed with an antibody specific to the Fas death domain, as high–molecular weight complexes (>200 kDa). These structures are resistant to SDS and β-mercaptoethanol denaturation. We observed a similar phenomenon in Jurkat T cells treated for more than 30 minutes with CH11 (Fig 7A). These complexes, which are still observed when samples are prepared in 6 M urea (data not shown), are believed to be inactive aggregates of Fas receptors (Lee and Shacter 2001) trapped into ceramide-containing membrane rafts (Cremesti et al 2001; Grassme et al 2001). We also observed the appearance of a clived form of Fas (less than 28 kDa, denoted short form of Fas in the figure). The formation of these high–molecular weight complexes and the appearance of the short form of Fas are caspase independent because they were still observed in cells treated with caspase inhibitors such as z-VAD-fmk or IETD-fmk (Fig 7B). Surprisingly, in the presence of heparin or dextran sulfate, the SDS-resistant Fas aggregates were no longer observed, and only the monomeric and short forms of Fas were detected. The membranes of the immunoblots presented in Figure 7A were stripped and reprobed with anti-FADD and anti–procaspase-8 antibodies to test for the presence of these DISC components at the level of the aggregated form of Fas. No colocalization was detected, indicating that the aggregated form of Fas does not appear to contain DISC components (data not shown). To better understand the nature of the 210-kDa SDS-resistant Fas aggregates, we analyzed the proteins present in cell lysates by gel filtration before SDS-PAGE electrophoresis and immunobloting (see the Materials and Methods). It can be seen in the immunoblots probed with an antibody that recognizes the Fas death domain that in untreated cells a large fraction of Fas (45 kDa) was recovered, with a native size that corresponds to the monomeric form (Fig 8). Some 45-kDa Fas molecules were also detected slightly in the fraction, displaying a native molecular weight larger than 670 kDa. This indicates that some Fas receptors could be preassociated in large aggregates that are sensitive to SDS-mediated dissociation. In cells treated with heparin or dextran sulfate only, no change in the elution profile of Fas was detected (data not shown). In cells treated with CH11 antibody, a small fraction of Fas was still recovered, with a native size corresponding to monomer, whereas the remainder of this protein showed a native molecular weight larger than 670 kDa. This native size appeared higher than that described previously (Kamitani et al 1997). In the corresponding 12% SDS-PAGE a fraction of this large complex was partly dissociated and had an apparent molecular weight of 210 kDa, whereas the remainder of this complex was completely dissociated and recovered as 45-kDa bands (Fig 8). The short form of the Fas (less than 28 kDa) was also recovered in the fractions corresponding to molecular weights higher than 670 kDa. This suggests that this <28-kDa fragment originated from and was part of the high–molecular weight complex formed by Fas in the presence of CH11 agonistic antibody. The antibody also revealed several minor protein bands, but because they did not show any changes in their native size, they were not further characterized.
Fig 7.
Sodium dodecyl sulfate–resistant Fas aggregates formed in response to CH11 agonistic antibody treatment are no longer observed in the presence of heparin or dextran sulfate. Jurkat T cells were either kept untreated (NT) or treated for various time periods (30, 60, or 120 minutes) with 100 ng/mL of CH11 antibody, 100 μg/mL of heparin (H) or dextran sulfate (DS), or both. Control experiments were also performed in which cells were treated for 10, 30, or 60 minutes with 100 μg/mL of either heparin (H) or dextran sulfate (DS). Protein samples were prepared as described in the Materials and Methods and analyzed by electrophoresis in a 12% polyacrylamide gel. An immunoblot analysis was performed, which was probed with a monoclonal antibody specific to the Fas death domain. An autoradiograph of the enhanced chemiluminescence detection is presented. The white arrows indicate the different forms of Fas that are detected. (B) The same analysis was done with Jurkat T cells either kept untreated (NT) or treated for 60 minutes with 100 ng/mL of anti-Fas CH11 antibody in the absence or presence of 20 μM of z-VAD-fmk or IETD-fmk. (C) Similar type of experiment as above, but in this case Jurkat T cells were either kept untreated (NT) or treated for different time periods (10–180 minutes) with 100 ng/mL of recombinant Fas ligand. A control of cells treated for 180 minutes with 100 ng/mL of CH11 antibody is presented. Autoradiography of the immunoblots is presented
Fig 8.
Analysis of the Fas aggregates by gel filtration. Jurkat T cells were either kept untreated (NT) or treated for 120 minutes with 100 ng/mL of anti-Fas CH11 antibody in the absence or presence of 100 μg/mL of heparin (H) or dextran sulfate (DS). Cells were then lysed as described in the Materials and Methods, and the cell lysates were loaded onto a fast performance liquid chromatography gel filtration column. The protein content of each fraction was then analyzed by electrophresis on a 12% polyacrylamide gel that was electrotransferred and immunoblotted with a monoclonal antibody specific to the Fas death domain. Autoradiographs of the enhanced chemiluminescence (ECL) detections are presented. The white rectangle underlines the completely dissociated monomeric form of Fas, whereas the white ellipsis shows the presence of the sodium dodecyl sulfate–resistant aggregated forms of Fas as well as that of the <28-kDa short form of Fas. Molecular weight markers used were apoferritin (443 kDa), thyroglobulin (670 kDa), and blue dextran (2000 kDa). Autoradiographs of ECL detection are presented
Interestingly, when cells were treated with CH11 antibody in the presence of heparin or dextran sulfate, Fas was still detected with a native molecular weight larger than 670 kDa; however, after SDS–gel electrophoresis these Fas complexes were completely dissociated in 45-kDa protein bands (Fig 8). The short form of Fas was less detectable in these fractions. Hence, heparin and dextran sulfate appear to modulate the Fas complexes formed in the presence of CH11 antibody in such a way that they facilitate their dissociation during SDS-gel sample preparation.
Because no stimulation of Fas-induced cell death by sulfated polysaccharides was observed when cells were treated with FasL (see Fig 5), we tested for the presence of large Fas aggregates in cells exposed to FasL. It can be seen in Figure 7C that FasL treatment of Jurkat T cells did not induce the appearance of the 210-kDa SDS-resistant form of Fas. However, analysis of cell lysates by gel filtration revealed that FasL induced the formation of Fas complexes with native sizes larger than 670 kDa, but these complexes were completely dissociated during SDS-gel sample preparation (data not shown). The Fas complexes formed in the presence of FasL appear therefore to differ from those formed in the presence of CH11 antibody and may resemble those formed by this antibody in the presence of sulfated polysaccharides.
Heparin-like agents abolish Hsp27 protective effects against Fas IgM–induced apoptosis
To understand the mechanisms underlying the stimulatory effect generated by sulfated polysaccharides toward CH11-mediated cell death, we have taken advantage of 2 Jurkat cell lines that were genetically manipulated to constitutively overexpress the antiapoptotic polypeptides Bcl-2 and Hsp27 (see the immunoblots presented in Fig 9A). These 2 proteins use different mechanisms to interfere with the mitochondrial pathway, which is the main apoptotic route triggered by Fas in Jurkat T cells (Scaffidi et al 1998). As already described, Bcl-2 (Sun et al 2002) and Hsp27 (Mehlen et al 1996) interfere with the cell death induced by CH11 agonistic antibody alone (Fig 9B). Interestingly, the cells expressing Bcl-2 or Hsp27 displayed a different behavior toward the synergistic effect induced by sulfated polysaccharides and Fas agonistic antibody. It can be seen in Figure 9B that Bcl-2 overexpression still delayed the death induced by the combination of sulfated polysaccharides and anti-Fas antibody compared with control cells. Nevertheless, the Bcl-2–overexpressing cells appeared still sensitive to the stimulatory effect of heparin or dextran sulfate. In contrast, the protective effect of Hsp27 observed against CH11-mediated apoptosis was almost completely lost in the presence of sulfated polysaccharides. Hence, the sulfated polysaccharides may interfere with some pathways upstream of mitochondria that are protected by Hsp27 (Paul et al 2002).
Fig 9.
Different effects mediated by Bcl-2 or Hsp27 overexpression on the sensitivity of Jurkat cells exposed to Fas agonistic antibody in the presence of heparin or dextran sulfate. (A) Western blot analysis of Bcl-2 or Hsp27 expression in polyclonal populations of Jurkat cells stably transfected with either pCIneobcl2 or pCIneohsp27 vectors. Immunoblots were probed with either anti–Bcl-2 or Hsp27 antibodies and revealed by enhanced chemiluminescence, as described in the Materials and Methods. Analysis of a control population of Jurkat T cells stably transfected with plain pCIneo vector is presented. (B) Cell death analysis. Polyclonal populations of stably transfected Jurkat T cells, overexpressing either Bcl-2 or Hsp27, and mock transfected cells were either kept untreated (NT) or treated for 16 hours with 100 ng/mL anti-Fas antibody (Fas) combined or not combined with 100 μg/mL heparin (H) or dextran sulfate (DS). Cells were then stained with Annexin-V and propidium iodide and analyzed by flow cytometry as described in the Materials and Methods. The percentage of cell death is represented as histogram plots.
DISCUSSION
The results presented here demonstrate that several well-known high–molecular weight sulfated polysaccharides such as heparin-like molecules stimulate CH11-mediated, but not FasL-mediated, cell death. As shown in our study, the stimulatory effect mediated by these agents was specific to T lymphocytes and hepatoma cells committed to a low level of Fas apoptosis. Moreover, even if they were removed from the growth medium, these sulfated polysaccharides were still able to induce a stimulatory effect that lasted for at least 48 hours. Whether this memory effect results from the sticking of the polyanions at the cell surface is not known. Interestingly, stimulation of CH11-mediated death was observed in Jurkat cells incubated with physiological concentrations of commercial preparations of heparin (Fraxiparine™ or Fragmine™) commonly used in the clinic. This suggests that a daily injection of 1 dose of heparin (about 2500 IU) in patients could stimulate their T lymphocytes (and probably also hepatic cells) to exogenous Fas agonistic antibody–mediated death. However, more work will be needed to analyze how heparin influences T cell apoptosis in an in vivo format and to test whether this observation could have therapeutic applications.
From a mechanistic point of view our results suggest that the caspase-dependent cell death–stimulatory effect of heparin and dextran sulfate is not correlated with changes in the cellular content of proteins known to regulate the cell death machinery. For example, no downregulation of c-FLIPS/L is observed in contrast to what is observed in the sensitization to Fas-mediated apoptosis by actinomycin D, cisplatin, or doxorubicin (Fulda et al 2000; Kinoshita et al 2000; Nitobe et al 2003). Moreover, heparin and dextran sulfate were active in a P53-mutated genetic environment and downregulated the antiapoptotic activity of Hsp27. In cells other than T lymphocytes and hepatoma, these agents either had no effect or even induced a protection against CH11-induced cell death. They were also rather ineffective when other cell death inducers were considered, excepted in the case of TRAIL. Hence, our findings are in agreement with earlier studies showing that, depending on the cell context, heparin-like molecules can either protect (Ishikawa and Kitamura 1999) or stimulate (Erduran et al 1999) apoptosis or inflammatory angiogenesis by local stimulation of Fas (Biancone et al 1997).
Several important effects are induced by sulfated polysaccharides, which could play a role in their death-stimulatory effect. First, in the presence of sulfated polysaccharides a higher number of CH11 antibodies recognize Fas receptor without altering the cellular content of this receptor. This may result in (1) an increased number of Fas receptors on the cell surface or (2) increased recognition of a constant number of Fas receptors by CH11. Concerning the second possibility, we have observed that CH11 IgM antibody displayed an increased apparent native size in the presence of sulfated polysaccharides (data not shown). Whether this phenomenon increases CH11 ability to recognizes Fas will merit further attention. The increased accessibility of Fas receptors may also depend on a modification of their distribution at the cell surface or on their molecular structure. In this respect, we have observed that in the presence of heparin or dextran sulfate the SDS-resistant aggregation of Fas induced by CH11 was lost. Hence, heparin and dextran sulfate probably facilitate the dissociation of Fas aggregates in the presence of SDS and β-mercaptoethanol. These large aggregates are supposed to be localized in ceramide-rich membrane rafts that are resistant to detergents (Rothberg et al 1990) and may arise during endocytosis of Fas bound to agonistic antibodies. By gel filtration experiments, we showed that these Fas aggregates had molecular weights larger than 670 kDa. Upon analysis in SDS-gel these structures were partly dissociated in monomers of Fas and showed the presence of a processed form of the receptor (less than 28 kDa). In the presence of heparin or dextran sulfate these large structures were completely dissociated after SDS-gel analysis. Because high–molecular weight sulfated polysaccharides cannot enter the cell, their effect on Fas aggregates results from their presence at the cell surface and could be related to their ability to remove cell surface lipoproteins (Favre et al 2001). Our results suggest that the SDS-resistant Fas aggregates are less efficient at inducing cell death than the dissociable form of these aggregates. This conclusion is in perfect agreement with the report of Lee and Shacter (2001), who showed that the sensitization of Jurkat T cells to Fas-mediated apoptosis by the serine protease inhibitor TLCK (N-tosyl-l-lysine chloromethyl ketone), cycloheximide, or protein kinase C inhibitors correlated with a drastic decrease in the detection of SDS-resistant Fas aggregates. These authors also suggested that the SDS-resistant Fas aggregates are not required for transduction of the death signal and represent a negative regulation of this phenomenon, leading to a protective mechanism to limit apoptosis induction.
When cells were treated with recombinant FasL instead of CH11 antibody, no SDS-resistant Fas aggregates were observed. This may be related to the fact that FasL and Fas agonistic antibodies act differently to activate the Fas receptor (Fadeel et al 1998; Huang et al 1999; Bando et al 2002; Caricchio et al 2002). Interestingly, under these conditions heparin and dextran sulfate did not stimulate Fas apoptosis. Hence, a major event mediated by sulfated oligosaccharides appears to be the inhibition of the formation of SDS-resistant Fas aggregates triggered by CH11 antibody. Future experiments will have to test whether membrane-bound FasL–induced apoptosis correlates or not with the formation of Fas aggregates that are sensitive to heparin. We also observed that sulfated polysaccharides lose their stimulatory effect when the percentage of cell death induction by CH11 antibody only was above 20–30%. The mechanism regulating this phenomenon is not known. One hypothesis could be that the inhibition mediated by Fas aggregates loses its importance under intense conditions of apoptosis.
A second important point in the mechanism of action of sulfated polysaccharides is that their effect seems to be lymphocyte and hepatoma specific. This specific signal may be related to the fact that heparin, dextran sulfate, and several other sulfated polysaccharides recognize lymphocyte- or hepatoma-specific cell surface receptors (Parish et al 1984; Parish and Snowden 1985; Thurn and Underhill 1986), resulting in modifications of cell-to-cell adhesion (Thurn and Underhill 1986) and in complex transmembrane signalings. Among these signals, one can cite the intracellular rise in free Ca2+ ions (Tellam and Parish 1987). This particular signaling pathway is interesting because it requires sulfated polysaccharides of high molecular weight. For example, the low–molecular weight polysaccharide chondroitin sulfate, which was inactive in our study, is also unable to stimulate this calcium-related pathway (Tellam and Parish 1987). Further work will be needed to test the importance of this signaling pathway in the sensitization of T lymphocytes to Fas-dependent apoptosis by sulfated oligosaccharides.
We have observed that the stimulation of Fas IgM agonistic antibody–mediated cell death by sulfated polysaccharides abolished Hsp27 antiapoptotic activity. In contrast, the protection generated by Bcl-2 expression was less altered. Hsp27 interferes with apoptosis both upstream and downstream of mitochondria (Bruey et al 2000; Pandey et al 2000; Samali et al 2001; Paul et al 2002), whereas Bcl-2 action occurs essentially at the level of this organelle (Sun et al 2002). Hence, the different effects inducted by Bcl-2 and Hsp27 expression suggest that sulfated polysaccharides interfere with Hsp27 protective activities located upstream of mitochondria. In this respect, we have recently described an apoptotic signaling pathway linking cytoskeleton damages to mitochondria that is negatively regulated by the ability of Hsp27 to protect F-actin network integrity (Paul et al 2002). Moreover, several recent studies point to the link that exists between Fas and F-actin. For example, it has been reported that in murine B lymphoma A20 cells, F-actin is required for early apoptosis signaling induced by anti-Fas antibody but not by FasL (Bando et al 2002). Another study showed that Fas cell membrane polarization, through an ezrin-mediated association with the actin cytoskeleton, was a key mechanism in rendering human T lymphocytes susceptible to Fas-mediated apoptosis (Parlato et al 2000). It has also been reported that apoptosis induced by disruption of the actin cytoskeleton is mediated by the activation of Fas (Kulms et al 2002). Whether F-actin is necessary for the formation of SDS-resistant large structures of Fas is not yet known. Further work will also have to test the role of Hsp27 in this process. Taken together, these studies suggest that the modulation of F-actin integrity by Hsp27 (Mounier and Arrigo 2002) is an important parameter that regulates the protective activity of Hsp27 against early apoptosis signaling induced by anti-Fas antibody but not by FasL or a combination of heparin-like molecules and anti-Fas antibody.
Acknowledgments
We wish to thank Dominique Guillet and Marie-France Grasset for excellent technical assistance. This work was supported by a research grant from Goemar Inc, St-Malo, France, and by grant 4602 from the Association pour la Recherche sur le Cancer as well as by the Région Rhône-Alpes (Thématique Cancer). F.M. was supported by a PhD training grant from the French Ministry of Science and Technology (Allocation de Recherche Couplée). M.M. was supported by a PhD fellowship from the Ligue contre le Cancer.
REFERENCES
- Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235.0022-1007(1995)181<1235:FCBFIN>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronis A, Melendez JA, Golan O, Shilo S, Dicter N, Tirosh O. Potentiation of Fas-mediated apoptosis by attenuated production of mitochondria-derived reactive oxygen species. Cell Death Differ. 2003;10:335–344. doi: 10.1038/sj.cdd.4401150.1350-9047(2003)010<0335:POFABA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bando M, Miyake Y, Shiina M, Wachi M, Nagai K, Kataoka T. Actin cytoskeleton is required for early apoptosis signaling induced by anti-Fas antibody but not Fas ligand in murine B lymphoma A20 cells. Biochem Biophys Res Commun. 2002;290:268–274. doi: 10.1006/bbrc.2001.6199.0006-291X(2002)290<0268:ACIRFE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Biancone L, Martino AD, Orlandi V, Conaldi PG, Toniolo A, Camussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147–152. doi: 10.1084/jem.186.1.147.0022-1007(1997)186<0147:DOIABL>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982;48:161–182. doi: 10.1007/BF00421226.0300-8177(1982)048<0161:MOTAAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bonnotte B, Favre N, and Reveneau S. et al. 1998 Cancer cell sensitization to fas-mediated apoptosis by sodium butyrate. Cell Death Differ. 5:480–487. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Walker G, Srinivasula SM, Sun XM, Butterworth M, Alnemri ES, Cohen GM. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20:998–1009. doi: 10.1093/emboj/20.5.998.0261-4189(2001)020<0998:RAAROC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, and Bonniaud P. et al. 2000 Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Caricchio R, D'Adamio L, Cohen PL. Fas, ceramide and serum withdrawal induce apoptosis via a common pathway in a type II Jurkat cell line. Cell Death Differ. 2002;9:574–580. doi: 10.1038/sj.cdd.4400996.1350-9047(2002)009<0574:FCASWI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000.0270-7306(2000)020<7602:IODABH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3.0092-8674(1995)081<0505:FANDDP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200.0021-9258(2001)276<23954:CEFTCA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Erduran E, Tekelioglu Y, Gedik Y, Yildiran A. Apoptotic effects of heparin on lymphoblasts, neutrophils, and mononuclear cells: results of a preliminary in vitro study. Am J Hematol. 1999;61:90–93. doi: 10.1002/(sici)1096-8652(199906)61:2<90::aid-ajh2>3.0.co;2-9.0361-8609(1999)061<0090:AEOHOL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fadeel B, Lindberg J, Achour A, Chiodi F. A three-dimensional model of the Fas/APO-1 molecule: cross-reactivity of anti-Fas antibodies explained by structural mimicry of antigenic sites. Int Immunol. 1998;10:131–140. doi: 10.1093/intimm/10.2.131.0953-8178(1998)010<0131:ATMOTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Favre D, Berthillon P, Trepo C. Removal of cell-bound lipoproteins: a crucial step for the efficient infection of liver cells with hepatitis C virus in vitro. C R Acad Sci III. 2001;324:1141–1148. doi: 10.1016/s0764-4469(01)01397-x.0764-4469(2001)324<1141:ROCLAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fleisher TA, Straus SE, Bleesing JJ. A genetic disorder of lymphocyte apoptosis involving the fas pathway: the autoimmune lymphoproliferative syndrome. Curr Allergy Asthma Rep. 2001;1:534–540. doi: 10.1007/s11882-001-0062-y. [DOI] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 2000;60:3947–3956.0008-5472(2000)060<3947:MISFCF>2.0.CO;2 [PubMed] [Google Scholar]
- Goldman FD, Vibhakar R, and Puck JM. et al. 2002 Aberrant T-cell antigen receptor-mediated responses in autoimmune lymphoproliferative syndrome. Clin Immunol. 104:31–39. [DOI] [PubMed] [Google Scholar]
- Gorski A, Wasik M, Nowaczyk M, Korczak-Kowalska G. Immunomodulating activity of heparin. FASEB J. 1991;5:2287–2291. doi: 10.1096/fasebj.5.9.1860620.0892-6638(1991)005<2287:IAOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200.0021-9258(2001)276<20589:CSVCMR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hanabuchi S, Koyanagi M, and Kawasaki A. et al. 1994 Fas and its ligand in a general mechanism of T-cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 91:4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom TH, Schmitz I, Soderstrom TS, Poukkula M, Johnson VL, Chow SC, Krammer PH, Eriksson JE. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 2000;19:5418–5428. doi: 10.1093/emboj/19.20.5418.0261-4189(2000)019<5418:ESIATC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DC, Hahne M, and Schroeter M. et al. 1999 Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L). Proc Natl Acad Sci U S A. 96:14871–14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Kitamura M. Inhibition of glomerular cell apoptosis by heparin. Kidney Int. 1999;56:954–963. doi: 10.1046/j.1523-1755.1999.00639.x.0085-2538(1999)056<0954:IOGCAB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, and Ishii A. et al. 1991 The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 66:233–243. [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614.0193-4511(1994)265<0528:FAPPAM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamitani T, Nguyen HP, Yeh ET. Activation-induced aggregation and processing of the human Fas antigen. Detection with cytoplasmic domain-specific antibodies. J Biol Chem. 1997;272:22307–22314. doi: 10.1074/jbc.272.35.22307.0021-9258(1997)272<22307:AAAPOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Yoshikawa H, Shiiki K, Hamada Y, Nakajima Y, Tasaka K. Cisplatin (CDDP) sensitizes human osteosarcoma cell to Fas/CD95-mediated apoptosis by down-regulating FLIP-L expression. Int J Cancer. 2000;88:986–991. doi: 10.1002/1097-0215(20001215)88:6<986::aid-ijc23>3.0.co;2-b.0020-7136(2000)088<0986:CCSHOC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/ CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x.0261-4189(1995)014<5579:CACPFA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulms D, Dussmann H, Poppelmann B, Stander S, Schwarz A, Schwarz T. Apoptosis induced by disruption of the actin cytoskeleton is mediated via activation of CD95 (Fas/APO-1) Cell Death Differ. 2002;9:598–608. doi: 10.1038/sj.cdd.4401002.1350-9047(2002)009<0598:AIBDOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee Y, Shacter E. Fas aggregation does not correlate with Fas-mediated apoptosis. J Immunol. 2001;167:82–89. doi: 10.4049/jimmunol.167.1.82.0022-1767(2001)167<0082:FADNCW>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0.0028-0836(1994)370<0650:CTCIMT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Préville X, Chareyron P, Briolay J, Klemenz R, Arrigo A-P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374.0022-1767(1995)154<0363:CEOHHD>2.0.CO;2 [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510.0021-9258(1996)271<16510:SSPANR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2.1466-1268(2002)007<0167:ACASHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, and Kischkel FC. et al. 1996 FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 85:817–827. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7.0092-8674(1997)088<0355:ABDF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326.0193-4511(1995)267<1449:TFDF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nitobe J, Yamaguchi S, and Okuyama M. et al. 2003 Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res. 57:119–128. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub LB. Fas function and tumor progression: use it and lose it. Cancer Cell. 2002;2:95–96. doi: 10.1016/s1535-6108(02)00099-5.1042-2196(2002)002<0095:FFATPU>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res. 1994;54:1580–1586.0008-5472(1994)054<1580:AONTLO>2.0.CO;2 [PubMed] [Google Scholar]
- Pandey P, Farber R, and Nakazawa A. et al. 2000 Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 19:1975–1981. [DOI] [PubMed] [Google Scholar]
- Parish CR, Rylatt DB, Snowden JM. Demonstration of lymphocyte surface lectins that recognize sulphated polysaccharides. J Cell Sci. 1984;67:145–158. doi: 10.1242/jcs.67.1.145.0021-9533(1984)067<0145:DOLSLT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parish CR, Snowden JM. Lymphocytes express a diverse array of specific receptors for sulfated polysaccharides. Cell Immunol. 1985;91:201–214. doi: 10.1016/0008-8749(85)90044-9.0008-8749(1985)091<0201:LEADAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parlato S, Giammarioli AM, and Logozzi M. et al. 2000 CD95 (APO-1/ Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 19:5123–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Arrigo AP. Comparison of the protective activities generated by two survival proteins: Bcl-2 and Hsp27 in L929 murine fibroblasts exposed to menadione or staurosporine. Exp Gerontol. 2000;35:757–766. doi: 10.1016/s0531-5565(00)00150-9.0531-5565(2000)035<0757:COTPAG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002.0270-7306(2002)022<0816:HAANRO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane MJ, Pan Y, and Singh S. et al. 2003 Heat shock protein 27 controls apoptosis by regulating akt activation. J Biol Chem. 278:27828–27835. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Conticello C, Zeuner A, De Maria R. CD95/ CD95L interactions and their role in autoimmunity. Apoptosis. 2000;5:419–424. doi: 10.1023/a:1009668212375.1360-8185(2000)005<0419:CIATRI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rifkin IR, Marshak-Rothstein A. Fas and Fas ligand in autoimmunity. Curr Dir Autoimmun. 1999;1:31–55. doi: 10.1159/000060496.1422-2132(1999)001<0031:FAFLIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931.0021-9525(1990)111<2931:CCTCOT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Robertson JD, and Peterson E. et al. 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, and Srinivasan A. et al. 1998 Two CD95 (APO-1/ Fas) signaling pathways. EMBO J. 17:1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Krammer PH, Droge W. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994;13:4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x.0261-4189(1994)013<4587:DSVFAT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem. 2002;277:11345–11351. doi: 10.1074/jbc.M109893200.0021-9258(2002)277<11345:BABICA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tellam RL, Parish CR. The effect of sulfated polysaccharides on the free intracellular calcium ion concentration of lymphocytes. Biochim Biophys Acta. 1987;930:55–64. doi: 10.1016/0167-4889(87)90155-8.0006-3002(1987)930<0055:TEOSPO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508.1474-1733(2001)001<0050:ROLPAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thurn AL, Underhill CB. Heparin-induced aggregation of lymphoid cells. J Cell Physiol. 1986;126:352–358. doi: 10.1002/jcp.1041260305.0021-9541(1986)126<0352:HAOLC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tran SE, Holmstrom TH, Ahonen M, Kahari VM, Eriksson JE. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem. 2001;276:16484–16490. doi: 10.1074/jbc.M010384200.0021-9258(2001)276<16484:EOTASF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3.0962-8924(2001)011<0075:HSDADM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tyrell DJ, Kilfeather S, Page CP. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci. 1995;16:198–204. doi: 10.1016/s0165-6147(00)89022-7.0165-6147(1995)016<0198:TUOHBI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, and Pakusch M. et al. 2000 Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 102:43–53. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549.0021-9258(1999)274<11549:AACCMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]