Abstract
Flavone derivatives and other phytochemicals were found to bind to three subtypes of adenosine receptors in the micromolar range. Affinity was determined in radioligand binding assays at rat brain A1 and A2A receptors using [3H]-N6-PIA ([3H]-(R)-N 6-phenylisopropyladenosine) and [3H]CGS21680 ([3H]-2-[[4-(2-carboxyethyl)phenyl]ethylamino]-5′-(N-ethylcarbamoyl)adenosine), respectively. Affinity was determined at cloned human and rat brain A3 receptors using [125I]-AB-MECA [N6-(4-amino-3-iodobenzyl)adenosine-5′-(N-methyluronamide)]. A structure-activity analysis indicated that the hydroxyl groups of naturally occurring flavones are not essential for affinity at adenosine receptors. Galangin, 14, displayed Ki values of 1 μM at both rat A1 and A2A receptors and 3 μM at human A3 receptors. Methylation but not acetylation of the hydroxyl groups of galangin enhanced A3 affinity. Pentamethylmorin, 20, appeared to bind with 14–17-fold selectivity for human A3 receptors vs rat A1 and A2A receptors, with a Ki value of 2.65 μM. Two flavone derivatives (14 and 15) showed 14-fold greater affinity at human vs rat A3 receptors. Reduction of the 2,3-olefinic bond, as in (±)-dihydroquercetin, or glycosidation, as in robinin, greatly diminished affinity. An isoflavone, genistein, also bound only very weakly at A3 receptors. α-Naphthoflavone had greater receptor affinity (0.79 μM at A1 receptors) than the β-isomer. Other natural products of plant origin, including oxogalanthine lactam, hematoxylin, and arborinine were found to bind to A1 adenosine receptors with Ki values of 3–13 μM. These findings indicate that the flavones, flavonols, flavanones, and other phytochemicals may provide leads for the development of novel adenosine antagonists. The unexpected finding of considerable affinity of flavones at both rat and human A3 receptors may explain some of the previously observed biological effects of these compounds.
Introduction
Adenosine receptors mediate many of the physiological actions of adenosine in the periphery1,2 and in the central nervous system.3 There are four subtypes of adenosine receptors: A1, A2A, A2B, and A3.4 Activation of A1 receptors results in the bradycardiac,5 cerebroprotective,3 and antilipolytic effects of adenosine.7 Activation of A2A receptors results in the hypotensive and antiplatelet aggregatory effects of adenosine.8 There are not yet any selective ligands with which to characterize physiological effects of selective activation of A2B receptors. Several years ago a new subtype, A3 receptors, was discovered9 while screening cDNA libraries for protein sequences resembling the G-protein-coupled receptors through the use of PCR (polymerase chain reaction). Activation of this receptor results in hypotension and promotion of release of inflammatory mediators from mast cells.10 We have introduced the first selective agonist ligands for the A3 subtype, including the agonist IB-MECA (N6-(3-iodobenzyl)adenosine-5′-(N-methyluronamide).11,12 In general adenosine antagonists are sought as renal protective,6 cognition enhancing,3 cerebroprotective,3 antiasthmatic,13 and antiinflammatory agents.13
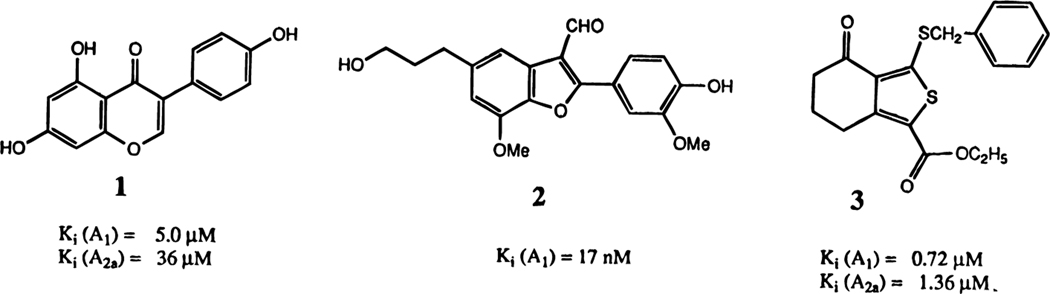
Xanthines are the classical adenosine antagonists;2 however, it is highly desirable to identify additional classes of antagonists. One reason for the current search for non-xanthine antagonists is that most xanthines bind only very weakly to A3 receptors,14 for which no selective antagonists have yet been reported. A number of classes of non-xanthine adenosine antagonists (Figure 1) have already been reported. These include the nitrogen heterocycles11 triazoloquinazolines,16 9-methyladenines,17 pyrazolotriazolepyrimidines,18 and triazolotriazines.19 Many of the non-xanthine antagonists are relatively nonselective,11,20 although selectivity for A1 receptors17 or A2 receptors18,19 has been achieved. Several non-nitrogen heterocycles have also been reported to bind to adenosine receptors (Figure 1), including the protein tyrosine kinase inhibitor genistein (5,7-dihydroxy-4′-hydroxyisoflavone), 1,21 a natural product benzofurancarbaldehyde derivative 5-(3-hydroxypropyl)-7-methoxy-2-(3′-methoxy-4′-hydroxyphenyl)-3-benzo[b]furancarbaldehyde, 2,22 and the recently reported tetrahydrobenzothiophenes, e.g. BTH4, 3.23
Figure 1.
Structures of non-xanthine and non-nitrogen-containing derivatives studied previously as adenosine receptor antagonists.
A broad screening effort of phytochemicals in our laboratory, similar to that recently reported,20 turned the focus to the flavones, members of the larger class of flavonoids, as a result of their relatively high affinity at adenosine receptors. Several of these derivatives even displayed significant affinity for A3 receptors. Flavonoids are phenolic natural products derived largely from fruits, vegetables, bark, and flowers and are consumed in large doses in the human diet.24 They have biological properties ranging from vascular protective and antioxidative properties25 to antiinflammatory26 and antineoplastic.27 Since a related compound, genistein, 1, an isoflavone derivative, was shown to be a competitive adenosine antagonist at A1 receptors in FRTL (thyroid) cells,21 we investigated the interactions of flavonoids in general as leads for developing novel adenosine antagonists.
Results
Affinity at Adenosine Receptors. Ki values at A1 and A2A receptors were determined in radioligand binding assays in brain membranes vs [3H]PIA or [3H]CGS 21680, respectively.31,32 Affinity at human brain A3 receptors expressed in HEK-293 cells43 and at rat brain A3 receptors stably expressed in Chinese hamster ovary (CHO) cells30 was determined using [125I]-AB-MECA [N6-(4-amino-3-iodobenzyl)adenosine-5′-(N-methyluronamide)].30 A typical saturation curve for this radioligand at human A3 receptors is shown in Figure 2. A Kd value of 0.59 nM was obtained, and the Bmax was 0.159 pmol/mg of protein. Comparable curves at rat A3 receptors are shown in ref 30, in which the Kd value was determined to be 1.48 nM. Thus, [125I]AB-MECA is of slightly greater affinity at human vs rat A3 receptors. In contrast to the rat receptor, binding at human A3 receptors is optimal at 25 °C.
Figure 2.
(A) Representative saturation curve for binding of [125I]AB-MECA [N6-(4-amino-3-iodobenzyl)adenosine-5′-(N-methyluronamide)]30 at human brain A3 receptors expressed in HEK-293 cells43 at 37 °C, showing nonspecific (squares) and specific (circles) binding. (B) Corresponding Scatchard analysis. A Kd value ± SEM of 0.59 ± 0.02 nM was obtained (Bmax 0.157 ± 0.08 pmol/mg of protein).
Flavone and its hydroxylated derivatives (compounds 4-21) bound to rat A1 and A2A adenosine receptors in the low micromolar range (Table 1). A structure–activity analysis indicated that the hydroxyl groups of naturally occurring flavones are not essential for affinity at adenosine receptors, since unsubstituted flavone, 4, displayed Ki values of 3.3 and 3.8 μM at A1 and A2A receptors, respectively. In general, there was a tendency toward lack of selectivity among the flavones between A1 and A2A receptors, except for compound 9, which had A1 selectivity of 8-fold. The flavonol galangin (2-phenyl-3,5,7-trihydroxy-4H-1-benzopyran-4-one), 14, displayed the highest affinity at A1 and A2A receptors (≤1 μM). Tetramethylscutallarein, 11, circimaritin,28b 13, galangin, 14, its trimethyl derivative, 15, and tetramethylkaempferol, 17, also had relatively high affinity among the analogues at the A1 subtype (0.8–1.3 μM).
Table 1.
Affinities of Flavone Analogues Determined in Radioligand Binding Assays at A1, A2A, and A3 Receptorsa-e

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
|
Ki (μM) or % inhibition at the indicated concn (M) |
||||||
| compound | R1 | R2 | R3 | (rA1)a | (rA2Δ)b | (hA3)c |
|
| ||||||
| 4 (flavone) | H | H | H | 3.28 ± 0.92 | 3.45 ± 1.16 | 16.9 ± 3.8 |
| 5 | 5-OH | H | H | 2.17 ± 0.06 | 6.20 ± 1.24 | 47 ± 12% (10−4) |
| 6 | 7-OH | H | H | 3.03 ± 0.49 | 2.68 ± 0.68 | 41 ± 7% (10−4) |
| 7 | 7-OH | 3′,4′-(MeO)2 | H | 18.8 ± 3.6 | 35.4 ± 7.3 | 32 ± 4% (10−4) |
| 8 (apigenin) | 5,7-(OH)2 | 4′-OH | H | 3.00 ± 0.29 | 7.58 ± 1.23 | 63 ± 1% (10−5) |
| 9 | 5-OH-7-Me | 4′-OMe | H | 3.40 ± 0.35 | 28.0 ± 7.35 | 6.70 ± 1.78 |
| 10 | 5-OH-7-MeO | 4′-OMe | H | d (10−4) | d (10−4) | 69 ± 7% (10−5) |
| 11 (tetramethylscutallarein) | 5,6,7-(Me)3 | 4′-Me | H | 1.29 ± 0.08 | nd | 4.48 ± 0.14 |
| 12 (hispidulin) | 5,7-(OH)2-6-MeO | 4′-OH | H | 1.61 ± 0.29 | 6.48 ± 0.65 | 63 ± 2% (10−5) |
| 13 (cirsimaritin) | 5-OH-6,7-(MeO)2 | 4′-OH | H | 1.20 ± 0.36 | 3.00 ± 0.70 | 1.72 ± 0.19 |
| 14 (galangin) | 5,7-(OH)2 | H | OH | 0.863 ± 0.092 | 0.966 ± 0.164 | 3.15 ± 0.85 |
| 15 | 5,7-(Me)2O | H | OMe | 0.509 ± 0.049 | 6.45 ± 1.48 | 1.21 ± 0.30 |
| 16 | 5,7-(AcO)2 | H | OAc | 11.6 ± 4.4 | 56.5 ± 9.5 | 17.5 ± 2.0 |
| 17 (tetramethylkaempferol) | 5,7-(MeO)2 | 4′-OMe | OMe | 1.07 ± 0.56 | nd | 3.37 ± 1.83 |
| 18 (rhamnetin) | 7-OMe | 3′,4′-(OH)2 | OH | nd | nd | 1.38 ± 0.18 |
| 19 (quercetin) | 5,7-(OH)2 | 3′,4′-(OH)2 | OH | 2.47 ± 0.64 | 6.99 ± 0.89 | see text |
| 20 (pentamethylmorin) | 5,7-(MeO)2 | 2′,4′-(OMe)2 | OMe | 27.6 ± 7.5 | 46.7 ± 2.7 | 2.65 ± 0.72 |
| 21 (hexamethylmyricetin) | 5,7-(MeO)2 | 3′,4′,5′-(OMe)3 | OMe | d (10−6) | nd | 16.2 ± 2.2 |
Displacement of specific [3H]PIA binding in rat brain membranes, expressed as Ki ± SEM in μM (n = 3–5).
Displacement of specific [3H]CGS 21680 binding in rat striatal membranes, expressed as Ki ± SEM in μM (n = 3–6).
Displacement of specific [125I]AB-MECA binding at human A3 receptors expressed in HEK-293 cells, in membranes, expressed as Ki ± SEM in μM (n = 2–3), or as a percentage of specific binding displaced at the specified concentration (M).
Displacement of <10% of specific binding at the specified concentration (M). nd = not determined.
Typical inhibition curves for displacement of [125I]AB-MECA binding at human A3 receptors are shown in Figure 3A. The Ki values of many of the flavone derivatives at human A3 adenosine receptors was in the range of 1–10 μM, with the most potent being the trimethyl derivative of galangin, 15, rhamnetin, 18, and circimaritin, 13. Thus, methylation of the hydroxyl groups of galangin enhanced affinity at A3 receptors, while acetylation of the hydroxyl groups of galangin (e.g. 16) reduced affinity at all receptor subtypes. Unlike at A1 and A2A receptors, at A3 receptors affinity of flavone derivatives was often (e.g. 13-15, 17-19) enhanced by the presence of multiple hydroxyl and/or methoxy substitution. Pentamethylmorin, 20, appeared to bind with some A3 receptor selectivity (10- and 18-fold for human A3 vs rat A1 and A2A receptors, respectively), with a Ki value of 2.65 μM. Substitution at the 3-position was not required (e.g. 13) to achieve micromolar affinity at adenosine receptors. The more selective A3 ligands tended to contain multiple substitutions of the flavone, especially methoxy, of the phenyl ring, as in 7 and 20. In these compounds, affinity was diminished only at A1 and A2A receptors, thus enhancing selectivity for A3 receptors.
Figure 3.
(A) Representative competition curves for inhibition of binding of [125I]AB-MECA [N6-(4-amino-3-iodobenzyl)-adenosine-5′-(N-methyluronamide)] by 15 (triangles), 17 (circles), and 21 (diamonds) at human brain A3 receptors expressed in HEK-293 cells at 25 °C. (B) Effects of quercetin, 19, on the binding of [125I]AB-MECA to membranes from human A3-transfected HEK-293 cells at 25 °C, expressed as percent of control specific binding. Each point represents the average ± SEM of three determinations done in duplicate. (C) Effects of cyanidin, 25, in the absence (b) or presence (●) of 200 μM NECA, on the binding of [125I]AB-MECA to membranes from human A3-transfected HEK-293 cells at 25 °C, expressed as percent of control specific binding. Each point represents the average ± SEM of 3 determinations done in duplicate.
Quercetin, 19, had an unexpected effect on the binding of [125I]AB-MECA binding at human A3 receptors. Rather than inhibiting the specific binding as in Figure 3A, it raised the level of binding of the radioligand. As shown in Figure 3B, at 30 μM quercetin the binding of the A3 receptor radioligand was dramatically enhanced, with >10× the level of control binding in membranes of A3-transfected HEK-293 cells. Curiously, cyanidin chloride, 25, although a very weak displacer in A1 receptor binding (Table 2), also enhanced [125I]ABMECA binding (Figure 3C). Since the additional binding occurred also in the presence of 200 μM NECA (540 ± 110% of control binding at 30 μM 25), it probably does not represent selective binding enhancement at A3 receptors. Instead, cyanidin likely causes enhanced binding of [125I]AB-MECA to a nonreceptor site on the membranes. It is not clear which structural features are necessary for this [125I]AB-MECA effect. Rhamnetin, 18, a flavone also bearing the 3′,4′-dihydroxy groups, did not behave similarly (Table 1).
Table 2.
Affinities of Various Phytochemicals and Related Derivatives Determined in Radioligand Binding Assays at A1, A2A, and A3 Receptorsa-e
| compound | (rA1)a | (rA2Δ)b | (hA3)c |
|---|---|---|---|
| 1 (genistein) | 5.021 | 3621 | 20 ± 2% (10−4) |
| 22 ((±)-dihydroquercetin) | d (10−5) | nd | 34.1 ± 10.1 |
| 23 (sakuranetin) | 8.18 ± 2.53 | 35.6 ± 13.0 | 3.40 ± 0.18 |
| 28 (α-naphthoflavone) | 0.786 ± 0.018 | 1.32 ± 0.43 | 71 ± 2% (10−5) |
| 29 (β-naphthoflavone) | 8.8 ± 3.6 | d (10−4) | 12 ± 1% (10−5) |
| 30 | 7.10 ± 0.10 | 29.9 ± 10.1 | d (10−4) |
| 31 | 7.59 ± 2.14 | 6.98 ± 2.21 | 19 ± 3% (10−4) |
| 32 (oxogalan thine lactam) | 5.55 ± 0.83 | d (10−4) | d (10−4) |
| 33 (acetylhaem anthamine methiodide) | 52.5 ± 3.7 | d (10−4) | d (10−4) |
| 34 | 8.89 ± 2.15 | 84.0 ± 6.9 | 52 ± 6% (10−4) |
| 35 (hematoxylin) | 3.10 ± 0.60 | 28 ± 2% (10−4) | d (10−4) |
| 36 (arborinine) | 12.7 ± 1.2 | 6.47 ± 1.90 | 63 ± 7% (10−4) |
Displacement of specific [3H]PIA binding in rat brain membranes, expressed as Ki ± SEM in μM (n = 3–5). The following compounds at a concentration of 10−5 M displaced less than 10% of the specific binding at A1 receptors: 24 d-(+)-catechin (3,3′,4′,5,7-flavanpentol), 25 cyanidin (3,3′,4′,5,7-pentahydroxy-2-phenylbenzopyrylium chloride), and 27 robinin (kaempferol 3-robinoside 7-rhamnoside).
Displacement of specific [3H]CGS 21680 binding in rat striatal membranes, expressed as Ki ± SEM in μM (n = 3–6). 26 rutin (quercetin 3-rutinoside) at a concentration of 10−4 M displaced less than 10% of the specific binding at A2A receptors.
Displacement of specific [125I]AB-MECA binding at human A3 receptors expressed in HEK-293 cells, in membranes, expressed as Ki ± SEM in μM (n ) 2–3), or as a percentage of specific binding displaced at the specified concentration (M). 26 (rutin) at a concentration of 10−4 M displaced 25 ( 3% of the specific binding at A3 receptors.
Displacement of <10% of specific binding at the specified concentration (M). nd = not determined.
At rat A3 receptors the affinity of flavone derivatives, like that of many xanthines,14 was weaker than at human A3 receptors. The Ki values of 14 and 15 were 43.1 and 17.4 μM, respectively, at rat A3 receptors (Figure 4). This represents a human A3 vs rat A3 ratio of 14-fold in both cases. Thus, within the same species (rat) compound 20 is likely nonselective; however, it may indeed prove to be selective when comparison is made between human A3 and A1/A2A subtypes.
Figure 4.
Representative competition curves comparing inhibition of binding of [125I]AB-MECA at human (solid symbols) and at rat (open symbols) A3 receptors. Inhibition by compounds 14 (squares) and 15 (triangles) is shown. Human brain A3 receptors were expressed in HEK-293 cells, and rat brain A3 receptors were expressed in CHO cells. Binding to rat brain A3 receptors was carried out as by the published method.30
Reduction of the 2,3-olefinic bond of a flavonol, as in the trans-dihydroflavonol (±)-dihydroquercetin (Figure 5, Table 2), 22, greatly diminished affinity at adenosine receptors. However a combination of reduction and removal of the 3-hydroxy group, as in the flavanone sakuranetin, 23, restored affinity at A1 and A3 receptors (Table 2). Other stereoisomers were not studied for comparison to 22. Glycosidation, as in the 3-monoglycoside rutin, 26 (derived from quercetin, 19), and the 3,7-diglycoside robinin, 27 (derived from kaempferol), greatly diminished affinity at A1 receptors. Similarly, catechin (a flavan-3-ol), 24, and cyanidin (a flavylium salt), 25, did not appreciably displace binding from A3 receptors. The effects of fused aromatic substitution of the flavone ring system on receptor binding affinity were studied. α-Naphthoflavone, 28, had greater affinity at A1 (0.8 μM), A2A, and A3 receptors than the corresponding β-isomer, 29. The isoflavone, genistein, 1, had very low affinity at human A3 receptors.
Figure 5.
Structures of other flavonoid derivatives studied for affinity at adenosine receptors.
A library of natural products of plant origin and related intermediates was screened for affinity at adenosine receptors. The compounds found to have considerable affinity for at least one subtype of adenosine receptors (Table 2) are shown in Figure 6. Included among the hits are alkaloids of the amaryllidaceae, 30 and 31,34 oxogalanthine lactam, 32,35 acetylhaemanthamine methiodide, 33,36 hematoxylin, 35,37 and arborinine, 36.38 Hematoxylin, 35, a pigment isolated from logwood, was relatively potent at A1 receptors, with a Ki value of 3.1 μM, and thus was highly selective for A1 vs A2A receptors. A synthetic intermediate (2,3-methylenedioxyfluoren-9-one), 34, with a ring system slightly similar to hematoxylin, bound to A1 receptors, with a Ki value of 8.9 μM. Compound 33, was the weakest in binding among these derivatives. Affinities of the non-flavones at A3 adenosine receptors (Table 2) were generally weak. There were several compounds that showed some degree of A1 selectivity. In addition to 35, mentioned above, 32 was specific, and 34 was 10-fold selective for A1 receptors. Only compound 36 was slightly selective for A2A receptors (2-fold).
Figure 6.
Structures of various phytochemicals and related derivatives studied for affinity at adenosine receptors. These compounds were identified through the screening of a natural products library. All were found to have considerable affinity for at least on subtype of adenosine receptors, except for compound 33, which was weaker in binding.
Discussion
The flavonoids are a ubiquitous family of phytochemicals that serve to protect plants from environmental stress. The biological effects of flavonoids in mammals have been studied for over 60 years,39 and currently more than 100 preparations containing flavonoids are marketed in Europe,42 mainly for their action of decreasing the permeability and fragility of peripheral blood vessels.
The biochemical pathways with which flavonoids interact are numerous. Active flavonoids tend to inhibit cell activation at various levels. Many flavonoids, such as quercetin, 19,40 inhibit protein kinase C in the micromolar range. Inhibition by flavonoids of many other mammalian enzymes, including phosphodiesterases46 and transport ATPases, has been studied. At very high concentrations, certain flavonoids, such as quercetin, 19, but not apigenin, 8, have been found to inhibit adenosine deaminase.47 Some flavonoids are carcinogenic and turn on genes or activate cells via fatty acid mobilization. It is also clear that some of the biological effects of flavonoids, such as inhibition of platelet function,41 are not fully explicable by any previously identified mechanism. Quercetin, 19, has been found to act synergisitically with β-adrenergic agonists to promote cAMP accumulation and lipolysis in isolated rat adipocytes.46
We have shown that many flavones have considerable affinity for adenosine receptors. It is an historical coincidence that Albert Szent-Gyorgi,1 the first investigator to detect the biological effects of adenosine, was also one of the first to investigate the biological effects of flavonoids.39 The fact that many of the flavones have micromolar Ki values at adenosine receptors suggests that perhaps these receptors may be important in the spectrum of biological activities reported for flavones. By comparison, caffeine has a Ki value of 29 μM at A1 receptors.2 Middleton40 has commented: “It is estimated that the flavonoids have been persistent in nature for somewhat over one billion years and, therefore, it seems entirely likely that they have been interacting with evolving animal forms/species over the eons”. Purinoceptors are also thought to be among the earliest G-protein-coupled receptors to appear in evolution.45 Thus, just as the biological stimulant properties of the naturally occurring methylxanthines, such as caffeine and theophylline, were known long before their interaction with adenosine was suspected, we raise the possibility that the flavone derivatives are another class of natural products with considerable activity as a result of interaction with adenosine receptors. Moreover, the degree of activation of the receptors by endogenous adenosine may be more dependent on dietary factors than previously realized.
Most curious is the relatively high affinity among many of the flavones and flavonols at A3 receptors. Unlike xanthines and nearly all other classes of non-xanthine adenosine antagonists,14 the affinity of flavone derivatives at the newly cloned A3 receptor is generally in the same range as the affinity at A1 and A2A receptors. The A3 receptors have already been implicated in vascular effects, inflammation, and cancer,44 three areas in which flavonoids are also biologically active.25–27 For example, flavone derivatives such as 8 and 19 have been shown to diminish the release of histamines from mast cells.26 Adenosine agonists, presumably acting via A3 receptors, have been shown to promote such a release.13 Thus, the unexpected finding of considerable affinity of flavone derivatives at both rat and human A3 receptors may explain some of the previously observed biological effects of these compounds. Further pharmacological experiments will be required to determine to which degree these known effects of flavones are related to A3 or other adenosine receptor action.
The structure-activity analysis of the flavonoids indicates that a carbonyl group at the 4-position promotes affinity at adenosine receptors. A comparison of α- and β-naphthoflavone also suggests that to achieve optimal receptor interaction the side of the molecule bearing the carbonyl group not be sterically crowded, as in the less active β-isomer. The various phytochemicals in Figure 5 were selected for their moderate adenosine receptor affinity by screening a library of several hundred phytochemicals. A feature common to all of the more potent adenosine receptors ligands is a phenyl ring that is fused to a five- or six-membered ring, which contains an α-carbonyl group. Compound 33, which is lacking the α-carbonyl group, was the weakest in this series in binding to A1 receptors. This structural pattern, shared also with the flavones, suggests that there is a common pharmacophore among all of these phytochemicals. More detailed structure-activity studies are in progress in our laboratory.
The presence of multiple hydroxyl groups on the flavones tended to increase A3 affinity and either had no effect on or decreased A1 and A2A affinity. The best A3 selectivity was achieved with pentamethylmorin, 20, which contained a 2′-methoxy group on the phenyl ring that may impede free rotation of the ring.
In conclusion, this series of flavone derivatives may provide important leads for the development of potent and selective A3 antagonists, which are as yet unknown.14 The present findings indicate that the chemical modification of flavonoids and other phytochemicals, in general, may lead to the development of novel adenosine antagonists.
Experimental Section
Materials.
Compounds 4, 7-14, 28, and 29 were obtained from Fluka, Ronkonoma, NY, or from Aldrich, St. Louis, MO. Compounds 18 and 22 were obtained from Apin Chemicals, Ltd., Oxon, U.K. Compounds 5, 6, 19, and 23-27 were obtained from K+K Laboratories, Jamaica, NY. Compound 1 was obtained from Sigma, Milwaukee, WI. Compound 32 was from Fisher, New York, NY. Compounds 31-33 and 36 (1-hydroxy-2,3-dimethoxy-10-methyl-9(10H)-acridinone) were obtained from Dr. Henry Fales (NIH) and were synthesized as described.34–36,38
Synthesis.
Proton nuclear magnetic resonance spectroscopy was performed on a Varian GEMINI-300 spectrometer and spectra were taken in DMSO-d6. Electron-impact mass spectrometry was performed with a VG7070F mass spectrometer at 6 kV. Elemental analysis was performed by Atlantic Microlab Inc. (Norcross, GA).
3,5,7-Triacetoxyflavone, 16. (A) Acetylation of Galangin, 14.
Galangin (9.0 mg, 33 μmol) was dissolved in 1 mL of DMF and treated with acetic anhydride (0.2 mL) and 4-(dimethylamino)pyridine (3 mg). After stirring for 10 min, 2 mL of 1 N NaH2PO4 was added. A white precipitate was removed by filtration and recrystallized from methanol/water to yield 11.4 mg of a solid (87%), which was homogeneous by thin layer chromatography (Rf 0.56, silica, chloroform/methanol/acetic acid, 95:4:1). MS: 414 (M + 1 + NH3), 397 (m+1), 355 (M + 1 + NH3 - OAc). Mp: 128–129 °C. NMR: δ 7.6 (3H, Ar), 7.9 (m, 2H, Ar), 7.65 and 7.17 (each, d, 1H, J = 2 Hz, Ar, ortho to AcO), 2.34 (s, 6H, Ac), 2.30 (s, 3H, Ac). CHN analysis.
(B) Methylation of the Flavones.
Methylation of hydroxyflavones (galangin, kaempferol, morin, and myricetin) and mass spectral analysis using ammonia gas ionization were carried out by the general methods reported28 and as below.
2′,3,4′,5,7-Pentabis(methyloxy)flavone, 20.
Morin hydrate (25 mg, 82 μmol, Aldrich) was dissolved in dry acetone (20 mL), in which potassium carbonate (1.0 g) was suspended. Dimethyl sulfate (1.0 mL, 11 mmol) was added, and the mixture was refluxed for 4 h under nitrogen. After cooling in an ice bath, 2 mL of concentrated ammonium hydroxide were added in aliquots followed by 20 mL of water. The solution was extracted with ethyl acetate. The organic layer was dried and evaporated, and the white solid residue was recrystallized from methanol/water to yield 11 mg (36%) of 20, which was homogeneous by thin layer chromatography (Rf 0.32, silica, chloroform/methanol/acetic acid, 95:4:1). MS: 373 (M + 1), 359 (M + 1 - CH2), 343. Mp: 153–156 °C. NMR: δ 7.35 (d, 1H, J = 8 Hz, Ar, 6-Ph), 6.66 (dd, 1H, J = 2, 8 Hz, Ar, 5-Ph), 6.70, 6.62 and 6.48 (each, d, 1H, J = 2 Hz, Ar), 3.84 (s, 9H, Me), 3.80 (s, 3H, Me), 3.61 (s, 3H, Me). CHN analysis.
1-Keto-8,9-dimethyloxy-1,2,3,4-tetrahydrodibenzo[b,d]6-pyrone, 30.
Compound 30 was synthesized by Dr. Henry Fales by a modification of a literature procedure.34 6-Bromo-3,4-dimethoxybenzoic acid (6-bromoveratric acid, 2.0 g, 7.7 mmol, Spectrum Chem. Corp., New Brunswick, NJ) was dissolved in 50% EtOH/H2O (100 mL), and treated with resorcinol (0.85 g, 7.7 mmol), 50 mg of copper powder, 50 mg of cupric acetate, and sodium hydroxide (0.31 g, 7.7 mmol). The mixture was heated to reflux overnight. The reaction mixture was extracted once with ether, and insolubles solids were removed by filtration. The aqueous layer was acidified with 1 N HCl to form the lactone. The precipitate was removed by filtration and dried. In order to remove unreacted 6-bromoveratric acid from this solid containing the product, it was suspended and partially dissolved in cold saturated aqueous NaHCO3. The remaining insoluble solids were collected by filtration and the washings discarded. The dried residue was recrystallized from EtOH to give 0.74 g of the pure product (35% yield). NMR (CDCl3) δ 8.68 and 7.65 (each s, 1H, Ar), 4.06 and 3.99 (each s, 3H, Me), 2.95 and 2.67 (each t, 2H, CH2), 2.18 (m, 2H, CH2). Mp: 236–237 °C. CHN analysis.
2,3-(Methylenedioxy)fluoren-9-one, 34, was synthesized by Dr. H. Fales (NIH) by the following method: Gaseous HF was condensed by collecting in a Teflon vial placed in a dry ice/acetone bath. 6-Phenylpiperonylic acid48 (100 mg, 0.41 mmol) was added to HF (2 mL) and the solution let stand overnight. The HF was evaporated leaving the pure fluorenone derivative in quantitative yield (92 mg). Mp: 156–157 °C. CHN analysis.
Pharmacology. Radioligand Binding Studies.
The radioligand binding data for the novel compounds were determined as described previously.12,23
Membranes prepared from HEK-293 cells stably expressing the human A3 receptor were obtained from Receptor Biology, Inc. (Baltimore MD). CHO cells stably expressing the rat A3 adenosine receptor were grown in F-12 medium containing 10% FBS and penicillin/streptomycin (100 units/mL and 100 μg/mL, respectively) at 37 °C in a 5% CO2 atmosphere, and membrane homogenates were prepared as reported.29 Binding of [125I]-N6-(4-amino-3-iodobenzyl)adenosine-5′-(N-methyluronamide) ([125I]AB-MECA) to rat A3 receptors in stably transfected CHO cell membranes (generous gift of Prof. Gary L. Stiles, Duke University, Durham, NC) was performed as described.30 Assays at human A3 receptors were performed in a Mg2+-free buffer containing 50 mM Tris and 1 mM EDTA, at pH 8.0. The glass tubes contained 100 μL of the membrane suspension (0.3 mg of protein/mL, stored at −80 °C in the same buffer), 50 μL of [125I]AB-MECA (final concentration 0.3 nM), and 50 μL of a solution of the proposed antagonist. All nonradioactive compounds were initially dissolved in DMSO and diluted with buffer to the final concentration, where the amount of DMSO never exceeded 1%. Duplicate incubations were carried out for 1 h at 37 or 25 °C (the latter temperature provided roughly twice the level of specific binding for the human, but not rat, A3 receptor) and were terminated by rapid filtration over Whatman GF/B filters, using a Brandell cell harvester (Brandell, Gaithersburg, MD). The tubes were rinsed three times with 3 mL of buffer each. The radioactivity on the filters was determined with a Beckman 5500B γ-counter. Nonspecific binding was determined in the presence of 100 μM N6-phenylisopropyladenosine ((R)-PIA).
Binding of [3H]-(R)-PIA to A1 receptors from rat cortical membranes and of [3H]CGS 21680 to A2A receptors from rat striatal membranes was performed as described previously.31,32
Adenosine deaminase (3 units/mL) was present during the preparation of the brain membranes, in which an incubation of 30 min at 30 °C is carried out, and during the incubation with the radioligands. At least five different concentrations spanning 3 orders of magnitude, adjusted appropriately for the IC50 of each compound, were used. IC50 values, computer-generated using the nonlinear regression method implemented in the InPlot program (Graph-PAD, San Diego, CA), were converted to apparent Ki values using Kd values of 1.0 and 14 nM for [3H]PIA and [3H]CGS 21680 binding, respectively, applying the Cheng-Prusoff equation.33
Acknowledgment.
We thank Dr. Henry Fales of the Laboratory of Chemistry, NHLBI (Bethesda, MD), and Dr. David Kingston of Virginia Polytech (Blacksburg, VA) for providing samples of many of the compounds used in this study and for helpful discussions. We thank Gilead Sciences (Foster City, CA) and the Cystic Fibrosis Foundation for financial support. We thank Dr. Pat Towers of Amersham and Dr. Garth Brown of DuPont NEN for the synthesis of [125I]I-AB-MECA.
Glossary
- [125I]AB-MECA
[125I]-N6-(4-amino-3-iodobenzyl)adenosine-5′-(N-methyluronamide)
- ADA
adenosine deaminase
- BTH4
ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydro-benzo[c]thiophen-1-carboxylate
- CGS21680
2-[[4-(2-carboxyethyl)phenyl]ethylamino]-5′-(N-ethylcarbamoyl)adenosine
- CHO
Chinese hamster ovary
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- K i
equilibrium inhibition constant
- (R)-PIA
(R)-N6-phenylisopropyladenosine
- Tris
tris(hydroxymethyl)aminomethane
References
- (1).Drury AN; Szent-Györgyi A The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929, 68, 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Jacobson KA; van Galen PJM; Williams M Adenosine receptors - pharmacology, structure activity relationships, and therapeutic potential. J. Med. Chem. 1992, 35, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).von Lubitz DKJE; Jacobson KA Behavioral effects of adenosine receptor stimulation. In Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology, Bellardinelli L, Pelleg A, Eds.; Kluwer: Norwell, 1995; pp 489–498. [Google Scholar]
- (4).Jacobson MA Cloning and expression of human adenosine receptor subtypes. In Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology; Bellardinelli L, Pelleg A, Eds.; Kluwer: Norwell, 1995; pp 5–13. [Google Scholar]
- (5).Sidi A; Wesley R; Barrett R; Rush W; Belardinelli L Cardiovascular effects of a non-xanthine-selective antagonist of the A1 adenosine receptor in the anaesthetised pig: pharmacological and therapeutic implications. Cardiovasc. Res. 1994, 28, 621–628. [DOI] [PubMed] [Google Scholar]
- (6).Suzuki F; Shimada J; Mizumoto H; Karasawa A; Kubo K; Nonaka H; Ishii A; Kawakita T Adenosine-A1 antagonists. 2. Structure-activity relationships on diuretic activities and protective effects against acute renal failure. J. Med. Chem. 1992, 35, 3066–3075. [DOI] [PubMed] [Google Scholar]
- (7).Jacobson KA; Nikodijević O; Ji X-D; Berkich DA; Eveleth D; Dean RL; Hiramatsu KI; Kassell NF; van Galen PJM; Lee KS; Bartus R; Daly JW; LaNoue KF; Maillard M Synthesis and biological activity of N6-p-sulfophenylalkyland derivatives of adenosine: Water soluble and peripherally selective adenosine agonists. J. Med. Chem. 1992, 35, 4143–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hutchison AJ; Williams M; Jesus R. d.; Yokoyama R; Oei HH; Ghai GR; Webb RL; Zoganas HC; Stone GA; Jarvis MF 2-(Arylalkylamino)adenosin-5′-uronamides: a new class of highly selective adenosine A2 receptor ligands. J. Med. Chem. 1990, 33, 1919–1924. [DOI] [PubMed] [Google Scholar]
- (9).Zhou QY; Li CY; Olah ME; Johnson RA; Stiles GL; Civelli O Molecular cloning and characterization of an adenosine receptor - The A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 7432–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hannon JP; Pfannkuche HJ; Fozard JR A role for mast-cells in adenosine A(3) receptor-mediated hypotension in the rat. Br. J. Pharmacol. 1995, 115, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Jacobson KA; Nikodijević O; Shi D; Gallo-Rodriguez C; Olah ME; Stiles GL; Daly JW A role for central A3-adenosine receptors: Mediation of behavioral depressant effects. FEBS Lett. 1993, 336, 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gallo-Rodriguez C; Ji XD; Melman N; Siegman BD; Sanders LH; Orlina J; Pu QL; Olah ME; van Galen PJM; Stiles GL; Jacobson KA Structure-activity relationships at A3-adenosine receptors. J. Med. Chem. 1994, 37, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Beaven MA; Ramkumar V; Ali H Adenosine-A(3) receptors in mast-cells. Trends Pharmacol. Sci. 1994, 15, 13–14. [DOI] [PubMed] [Google Scholar]
- (14).Kim HO; Ji XD; Melman N; Olah ME; Stiles GL; Jacobson KA Structure-activity relationships of 1,3-dialkylxanthine derivatives at rat A3 adenosine receptors. J. Med. Chem. 1994, 37, 3373–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Daly JW; Hong O; Padgett WL; Shamim MT; Jacobson KA; Ukena D Non-xanthine heterocycles: activity as antagonists of A1- and A2-adenosine receptors. Biochem. Pharmacol. 1988, 37, 655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Francis JE; Cash WD; Psychoyos S; Ghai G; Wenk P; Friedmann RC; Atkins C; Warren V; Furness P; Hyun JL Structure-activity profile of a series of novel triazoloquinazoline adenosine antagonists. J. Med. Chem. 1988, 31, 1014–1020. [DOI] [PubMed] [Google Scholar]
- (17).Thompson RD; Secunda S; Daly JW; Olsson RA N6,9-Disubstituted Adenines - Potent, Selective Antagonists at the A1-Adenosine Receptor. J. Med. Chem. 1991, 34, 2877–2882. [DOI] [PubMed] [Google Scholar]
- (18).Baraldi PG; Manfredini S; Simoni D; Zappaterra L; Zocchi C; Dionisotti S; Ongini E Synthesis of new pyrazolo[4,3–e]1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c] pyrimidine displaying potent and selective activity as A2A adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 1994, 4, 2539–2544. [Google Scholar]
- (19).Poucher SM; Keddie JR; Singh P; Stoggall SM; Caulkett PWR; Jones G; Collis MG The in-vitro pharmacology of ZM241385, a potent, nonxanthine, A(2a) selective adenosine receptor antagonist. Br. J. Pharmacol. 1995, 115, 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Siddiqi SM; Ji X. d.; Melman N; Olah ME; Jain R; Evans P; Glashofer M; Padgett WL; Cohen LA; Daly JW; Stiles GL; Jacobson KA A survey of non-xanthnine derivatives as adenosine receptor ligands. Nucleosides Nucleotides 1996, 15, 693–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Okajima F; Akbar M; Abdul Majid M; Sho K; Tomura H; Kondo Y Genistein, an inhibitor of protein tyrosine kinase, is also a competitive antagonist for P1-purinergic (adenosine) receptor in FRTL-5 thyroid cells. Biochem. Biophys. Res. Commun. 1994, 203, 1488–1495. [DOI] [PubMed] [Google Scholar]
- (22).Yang Z; Hon PM; Chui KY; Xu ZL; Chang HM; Lee CM; Cui YX; Wong HNC; Poon CD; Fung BM Naturally occurring benzofuran - isolation, structure elucidation and total synthesis of 5-(3-hydroxypropyl)-7-methoxy-2-(3′-methoxy-4′-hydroxyphenyl)-3-benzo[b]furancarbaldehyde, a novel adenosine-A1 receptor ligand isolated from Salvia Militorrhiza bunge (Danshen). Tetrahedron Lett. 1991, 32, 2061–2064. [Google Scholar]
- (23).van Rhee AM; Siddiqi SM; Melman N; Shi D; Padgett WL; Daly JW; Jacobson KA Tetrahydrobenzothiophenone derivatives as a novel class of adenosine receptor antagonists. J. Med. Chem. 1996, 39, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Anti-inflammatory Gabor M. and anti-allergic properties of flavonoids. Prog. Clin. Biol. Res. 1986, 213, 471–80. [PubMed] [Google Scholar]
- (25) (a).Hertog MG; Feskens EJ; Hollman PC; Katan MB; Kromhout D Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [DOI] [PubMed] [Google Scholar]; (b) Limasset B; le Doucen C; Dore JC; Ojasoo T; Damon M; Crastes de Paulet A Effects of flavonoids on the release of reactive oxygen species by stimulated human neutrophils. Multivariate analysis of structure-activity relationships (SAR). Biochem. Pharmacol. 1993, 46, 1257–1271. [DOI] [PubMed] [Google Scholar]
- (26).Middleton E and Kandaswami C Effect of flavonoids on immune and inflammatory cell function. Biochem. Pharmacol. 1992, 43, 1167–1179. [DOI] [PubMed] [Google Scholar]
- (27).Critchfield JW; Welsh CJ; Phang JM; Yeh GC Modulation of adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells. Activation of P-glycoprotein as a putative mechanism. Biochem. Pharmacol. 1994, 48, 1437–1445. [DOI] [PubMed] [Google Scholar]
- (28) (a).Kingston DGI; Fales HM Methane chemical ionization mass spectrometry of flavonoids. Tetrahedron 1973, 29, 4083–4086. [Google Scholar]; (b) Rao MM; Kingston DGI; Spittler TD Flavonoids from fluorensia cerua. Phytochemistry 1970, 9, 227–228. [Google Scholar]
- (29).van Galen PJM; van Bergen AH; Gallo-Rodriguez C; Olah ME; IJzerman AP; Stiles GL; Jacobson KA A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Mol. Pharmacol. 1994, 45, 1101–1111. [PMC free article] [PubMed] [Google Scholar]
- (30).Olah ME; Gallo-Rodriguez C; Jacobson KA; Stiles GL [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacol. 1994, 45, 978–982. [PMC free article] [PubMed] [Google Scholar]
- (31).Schwabe U; Trost T Characterization of adenosine receptors in rat brain by (−) [3H]N6-phenylisopropyladenosine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 313, 179–187. [DOI] [PubMed] [Google Scholar]
- (32).Jarvis MF; Schutz R; Hutchison AJ; Do E; Sills MA; Williams M [3H]CGS 21680, an A2 selective adenosine receptor agonist directly labels A2 receptors in rat brain tissue. J. Pharmacol. Exp. Ther. 1989, 251, 888–893. [PubMed] [Google Scholar]
- (33).Cheng YC; Prusoff WH Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzyme reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- (34).Fales HM; Warnhoff EW; Wildman WC Alkaloids of the Amaryllidaceae. VI. The action of oxidizing agents on lycorine and caranine. J. Amer. Chem. Soc. 1955, 77, 5885–5890. [Google Scholar]
- (35).Fales HM; Wildman WC Alkaloids of the Amaryllidaceae. IX. On the structure of galanthine. J. Am. Chem. Soc. 1956, 78, 4151–4153. [Google Scholar]
- (36).Fales HM; Wildman WC Structure of haemanthamine. Chem. Ind. (London) 1958, 561–562. [Google Scholar]
- (37).Craig JC; Naik AR; Pratt R; Johnson E; Bhacca NS Nuclear magnetic resonace spectra and stereochemistry of the antibacterial principle from Haematoxylon braziletto. J. Org. Chem. 1965, 30, 1573–1576. [DOI] [PubMed] [Google Scholar]
- (38) (a).Johne S; Bernasch H; Groger D Biosynthesis of the acridine alkaloid arborinine. Pharmazie 1970, 25, 777–779. [PubMed] [Google Scholar]; (b) Banerjee SK; Chakravarti RN Norarborinine, a demethylation product of arborinine. Bull. Calcutta Sch. Trop. Med. 1965, 13, 60. [PubMed] [Google Scholar]
- (39).Roger CR The nutritional incidence of flavonoids: some physiological and metabolic considerations. Experientia 1988, 44, 725–733. [DOI] [PubMed] [Google Scholar]
- (40).Middleton E Jr. Some biological properties of plant flavonoids. Ann. Allergy 1988, 61, 53–57. [PubMed] [Google Scholar]
- (41).Beretz A; Cazenave JP The effect of flavonoids on blood-vessel wall interactions. Prog. Clin. Biol. Res. 1988, 280, 187–200. [PubMed] [Google Scholar]
- (42).Jaeger A; Walti M; Neftel K Side effects of flavonoids in medical practice. Prog. Clin. Biol. Res 1988, 280, 379–394. [PubMed] [Google Scholar]
- (43).Salvatore CA; Jacobson MA; Taylor HE; Linden J; Johnson RG Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 10365–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Jacobson KA, Kim HO; Siddiqi SM; Olah ME; Stiles G; von Lubitz DKJE A3 adenosine receptors: design of selective ligands and therapeutic prospects. Drugs Future 1995, 20, 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Burnstock G in P2 Purinoceptors: Localization, Function, and Transduction Mechanisms; CIBA Foundation Symposium; 1996, in press. [Google Scholar]
- (46).Kuppusamy UR; Das NP Potentiation of beta-adrenoceptor agonist-mediated lipolysis by quercetin and fisetin in isolated rat adipocytes. Biochem. Pharmacol. 1994, 47, 521–529. [DOI] [PubMed] [Google Scholar]
- (47).Koch HP; Jager W; Groh U; Plank G In vitro inhibition of adenosine deaminase by flavonoids and related compounds. New insight into the mechanism of action of plant phenolics. Methods Find. Exp. Clin. Pharmacol. 1992, 14, 413–417. [PubMed] [Google Scholar]
- (48).Kondo H; Ikeda T, Taga J Annu. Rep. ITSUU Labs. 1952, 3, 65–69; Chem. Abstr. 1953, 7517b. [Google Scholar]