Abstract
Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) is indispensable for viral DNA replication and episome maintenance in latency. Four promoters, Cp, Wp, Qp, and Fp, are known to drive EBNA1 expression. Here we show that the TATA-less Qp is constitutively active in a variety of EBV-positive [EBV(+)] tumors and cell lines, irrespective of the activities of other EBNA1 promoters, the type of viral latency, and the cell type. The transcription of highly regulated promoters such as the EBV Cp is known to be directly regulated by CpG methylation. To characterize the role of CpG methylation in the regulation of the constitutively active Qp, we performed bisulfite genomic sequencing and functional analyses using a methylation cassette transcriptional reporter assay. Twenty consecutive CpG sites (16 proximal to the Qp initiation site and 4 upstream of the adjacent Fp initiation site) were studied by bisulfite sequencing of DNA extracted from EBV(+) tumors and cell lines. Eighteen EBV(+) tumors of lymphoid (B, T, and NK cell) or epithelial origin and five Burkitt’s lymphoma cell lines were studied. The 16 CpG sites proximal to Qp were virtually all unmethylated, but the 4 CpG sites upstream of the Fp initiation site were variably methylated. The methylation cassette assay showed that in vitro methylation of the Qp cassette (−172 to +32) resulted in strong repression of Qp activity in transient transfection. Thus, Qp is susceptible to repression by methylation but was found to be consistently hypomethylated and expressed in all tumors and tumor-derived cell lines studied.
Methylation of the cytosine residue of CpG dinucleotides in vertebrate DNA is an important mechanism of promoter regulation and genetic imprinting (4). Approximately 70% of all CpG sites in the human genome are methylated. Overall, there is an inverse relationship between the methylation status and the transcriptional activity of a promoter. Methylation of CpG sites in a promoter may cause changes in chromatin that interfere with the binding of general and sequence-specific transcription factors (25, 60). Moreover, methylation-specific transcription repressors such as MeCP1 and MeCP2 may compete with transcription factors for binding sites and result in transcriptional repression (6, 7). Transcriptional silencing of tumor suppressor genes such as p15 and p16 by aberrant methylation has been implicated in tumorigenesis (3, 14).
CpG methylation is also important in the gene regulation of Epstein-Barr virus (EBV), a ubiquitous human gammaherpesvirus implicated in the pathogenesis of a variety of malignancies. Among six viral latent nuclear proteins, EBV nuclear antigen 1 (EBNA1) is the only one constitutively expressed in all types of latent infection (26). EBNA1 binds to the viral latent origin of replication (oriP) and is required for viral DNA replication and episomal maintenance (2, 20, 65). EBNA1 transcripts are known to originate from four different promoters (Fig. 1A). Following the initial infection of B lymphocytes, EBNA1 transcripts originate from the W promoter (Wp). Shortly thereafter, a promoter switch occurs as the C promoter (Cp) is activated and Wp is silenced (55). EBNA1 transcripts from either promoter include exons derived from the internal repeat region (W exons), Y and U exons, and the coding K exon (Fig. 1A). In other forms of latency (24, 46), first recognized in tumors and tumor-derived cell lines, EBNA1 transcripts contain a Q-U-K splice (Fig. 1A) and Wp and Cp are silent (47, 54, 56). Although initially identified as a promoter located at the junction of the F and Q fragments (47, 54), subsequent studies demonstrated that there were actually two adjacent promoters located on either side of the F-Q junction. The promoter located in the F fragment, Fp, is a lytic promoter driving EBNA1 and perhaps other transcripts (28, 51). The promoter located at the beginning of the Q fragment, Qp, is a TATA-less promoter that drives EBNA1 transcription in latency I (40, 50, 63). Positive and negative regulatory elements have been identified in the Fp and Qp regions (37, 48, 58). Recent investigations have suggested that interferon response factors (IRFs) are involved in the regulation of Qp transcription (38, 39, 49, 67).
FIG. 1.
(A) Promoter usage and splicing pattern of EBNA1 transcripts in different types of EBV latency. The diagram at the top shows the structure of the EBV genome, locations of the four different EBNA1 promoters, and coding exons (shadowed boxes) of EBNAs. Four promoters can be used to drive the EBNA1 transcripts: lytic Fp produces FUK transcript with the coding exon (K) for EBNA1 and also transcripts of unknown structure without splicing to the K exon (FUU′); Qp, immediately downstream of Fp, is used for EBNA1 transcription in latency I and II and EBV(+) tumors; latent Cp and Wp are active in latency III. Dark bars represent the RT-PCR primers used to detect these transcripts. (B) RT-PCR for transcripts (FUU′, FUK, QUK, and YUK) initiated from different EBNA1 promoters in EBV(+) tumors and cell lines.
An inverse relationship between methylation and transcriptional activity has been demonstrated for several EBV promoters (Cp, Wp, and promoters for LMP1 and BHRF1) (16, 23, 33, 41, 44). In latency III cell lines expressing the full spectrum of EBV latency proteins (e.g., lymphoblastoid cell lines [LCL] like B95-8), there is little if any methylation of the viral genome (1, 36). EBER promoters are hypomethylated and constitutively active in all settings except in oral hairy leukoplakia in patients with AIDS (19, 35). However, Cp, Wp, and LMP1 promoters are methylated and silent in more restricted forms of latency and in tumors (1, 22, 23, 44, 45). The LMP1 promoter is unmethylated and active in latency II tumors (9, 11–13, 15, 34). Methylation of the EBNA2 response region of Cp abolishes the binding of a transcription factor CBF2 and silences the promoter (44). In addition, 5-azacytidine treatment results in demethylation and transcriptional activation of the Cp (30, 44).
Qp is a TATA-less promoter, structurally similar to housekeeping gene promoters (50). TATA-less promoters tend to be regulated by upstream CG-rich regions (5), and methylation is one of the mechanisms. A 5-kb region which includes both Qp and Fp has been reported to be hypomethylated (53). In the study reported here, we analyzed EBNA1 promoter usage in a variety of EBV-associated tumors and tumor-derived cell lines, characterized CpG methylation in the Qp and Fp regions by genomic sequencing, and used the methylation cassette assay to evaluate the effect of methylation on the transcriptional activity of Qp.
MATERIALS AND METHODS
Cell lines, 5-azacytidine treatment, and tumor samples.
Rael, Akata, and Chep are EBV-positive [EBV(+)] type I Burkitt’s lymphoma (BL) cell lines. Wan is a type I/II EBV(+) BL cell line. Wewak, Namalwa, Raji, and AG876 are type III EBV(+) BL cell lines. B95-8 is an EBV-immortalized LCL from marmoset B cells. IB4 is a B95-8 EBV-transformed human B-cell line (LCL). CA46 is an EBV-negative [EBV(−)] BL cell line. BJAB is an EBV(−) B-lymphoma cell line. Cell lines were maintained at 37°C in RPMI 1640 supplemented with 10% fetal bovine serum, 1 mM glutamine, and 100 U of penicillin and streptomycin per ml. 5-Azacytidine (Sigma) was used at 1 μM (final concentration) to treat the Rael cell line (to generate Rael-AzaC cells) (44).
Endemic BL (eBL) specimens from Ghana, Africa, posttransplant lymphoproliferative disease (PTLD) specimens from the United States, nasopharyngeal carcinoma (NPC) specimens from Taiwan, and Hodgkin’s disease (HD) specimens from the United States have been previously described (45, 59). DNA and RNA of three cases of nasal lymphoma (NL) (two of NK cell origin [NK1 and -2] and one of T-cell origin [NTL]) have been studied previously (13).
Plasmid DNA.
Qp sequences (−172 to +32 relative to the Qp start site, coordinates 62250 to 62453 in the B95-8 genome) were amplified from B95-8 DNA by PCR and cloned into the pCR2.1 vector (Invitrogen) (Fig. 2A). The Qp fragment was then isolated and cloned into the HindIII and XbaI sites of a chloramphenicol acetyltransferase (CAT) reporter plasmid, pCAT-enhancer (Promega). The sequence and orientation of the insert in the recombinant plasmid pQpCAT were verified by dideoxy sequencing (U.S. Biochemical).
FIG. 2.
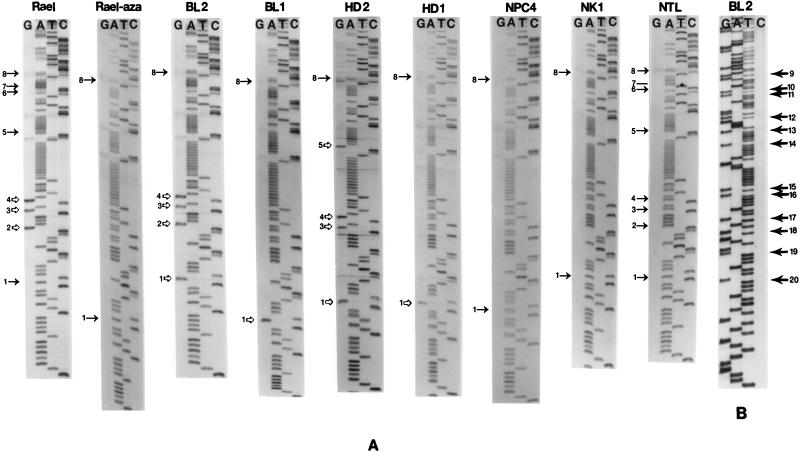
(A and B) Sequence and structure of Qp. (A) Sequence of the region (EBV coordinates 62179 to 62481) analyzed by bisulfite sequencing. The BamHI site between the F and Q fragments is shown. The transcription initiation sites of Qp (EBV coordinate 62422) and Fp (EBV coordinate 62231) are indicated by bent arrows in panel B. The 20 CpG sites are shown by asterisks in panel A and numbers preceded by # in panel B. The region from CpG sites 8 to 15 correspond to the minimal region required for Qp function (37, 39, 63). The CAAT and TATA boxes for Fp and an inverted CCAAT box probably for Qp are labeled. Two putative binding sites for Sp1 and two low-affinity binding sites for EBNA1 are indicated with boxes. (C) Summary of bisulfite genomic sequencing data for Qp in EBV(+) tumors and cell lines. M, methylated CpG site; −, unmethylated CpG site; @, CpG site abolished by mutation (CpG→TpG).
RT-PCR for EBNA1 transcripts.
Reverse transcription-PCR (RT-PCR) primers and internal probes for EBNA1 transcripts (FUK, QUK, and YUK) initiated from different promoters (Fp, Qp, Cp, and Wp) are listed in Table 1. Primers U′ and U2′ were used for the lytic Fp transcript (FUU′) (Fig. 1A). Total RNA was extracted from frozen tissues of EBV(+) tumors and fresh cell lines by using TriZol (Gibco BRL). Random hexamers and the GeneAmp RNA PCR kit (Perkin-Elmer) were used for reverse transcription and PCR. The PCR involved an initial denaturation at 95°C for 3 min, followed by 40 cycles (94°C for 30 s, 55°C for 1 min, and 72°C for 1 min), with a final extension at 72°C for 10 min. RNA from the EBV(−) cell line BJAB was used as negative control. The RT-PCR products were electrophoresed on a 1.8% agarose gel and transferred in 0.4 M NaOH onto a HyBond N(+) membrane. The membrane was hybridized by using a [γ-32P]ATP-labeled internal oligonucleotide probe and the Rapid-Hyb buffer system (Amersham) at 52°C for 2 h. The membrane was then washed and autoradiographed. RT-PCR hybridization signal strength was graded as ++, +, or +/−, corresponding to strong signal after a 1-h exposure, strong signal after an overnight exposure, and weak signal after an overnight exposure, respectively (59).
TABLE 1.
Primers used in RT-PCR for transcripts from different EBNA1 promoters
| Primer | DNA sequence (5′→3′) | Coordinates in B95-8 |
|---|---|---|
| F | GGATCCGGAGGGGACCACTA | 62249–62268 |
| Q | GTGCGCTACCGGATGGCG | 62440–62457 |
| Q′ | GCGGGATAGCGTGCGCTA | 62430–62447 |
| Y3 | TGGCGTGTGACGTGGTGTAA | 48397–48416 |
| U′ | CCTAGTGGCCTTGCAGAATT | 67685–67666 |
| U2′ | CACACATTCGAGATGGGCAA | 67729–67710 |
| K | CATTTCCAGGTCCTGTACCT | 107986–107967 |
| Probe (P) | ATGCCCTGAGACTACTCTCT | 67563–67544 |
Bisulfite genomic sequencing.
Genomic DNA was extracted from tumor tissues or cell pellets with DNAZol (Gibco BRL) or by the conventional proteinase K digestion method. Bisulfite DNA treatment was performed as described previously (17, 44). Briefly, the DNA was digested with EcoRI, denatured with NaOH, precipitated, and incubated with 3.1 M sodium bisulfite (Sigma) at 50°C for 16 h in darkness. After the reaction, DNA was desalted and purified by the Wizard DNA purification system (Promega). The DNA was then treated with 0.3 M NaOH and recovered. The bisulfite-treated DNA was PCR amplified with strand-specific primers (for the bisulfite-converted bottom strand): 5′-AACTAACCTAACTAAAAATAAAAC (corresponding to EBV coordinates 62179 to 62202) and 5′-AATGTAAGGATAGTATGTATTATT (corresponding to EBV coordinates 62481 to 62458). The PCR products were electrophoresed and purified by using Spin-X tubes (Costar). The PCR products were then cloned into the pCR2.1-TA cloning vector (Invitrogen). Four to six colonies were analyzed for each DNA sample. Plasmid DNA was extracted and sequenced.
Methylation cassette assay.
The methylation cassette assay was performed as described previously (42). Briefly, pQpCAT containing the entire Qp was methylated with CpG methylase SssI (New England Biolabs). Completeness of methylation was monitored by digestion of an aliquot with the methylation-sensitive restriction endonuclease HhaI. Methylated and unmethylated plasmid DNAs were then digested overnight with HindIII and XbaI and electrophoresed. Methylated and unmethylated Qp cassette and unmethylated background fragments were recovered by using a QiaexII gel extraction kit (Qiagen). In parallel, 2 μg of Qp cassette (methylated or unmethylated) and unmethylated background fragments were ligated in a 1:1 molar ratio at 16°C for 24 h with 10 U of T4 DNA ligase (Boehringer Mannheim) in a 20-μl volume. The ligation mixture was transfected by the DEAE-dextran method into the Rael and CA46 cell lines. After 2 days, cells were harvested for assay by thin-layer chromatography with 14C-labeled chloramphenicol. Signals were quantitated with a Molecular Dynamics PhosphorImager.
RESULTS
Transcriptional activities of different EBNA1 promoters in EBV(+) tumors and cell lines.
Transcripts (FUK, FUU′, QUK, and YUK) from the four different EBNA1 promoters (Fp, Qp, and Cp/Wp) could usually be amplified and distinguished by RT-PCR using five primers (F, Q, Y3, U′, and K) and a common probe as illustrated in Fig. 1A. In some instances additional primers, Q′ (upstream of Q) and U2′ (downstream of U′), were necessary. Analysis of 16 EBV-associated tumors, including tumors of lymphoid (B, T, and NK cell) or epithelial origin, showed that EBNA1 is consistently driven from Qp (Fig. 1B). Fp- or Cp/Wp-initiated EBNA1 transcripts were only occasionally detected in these tumors and when detected were always weak. Qp-, Fp-, and Cp/Wp-initiated transcripts were readily detected in most EBV(+) BL cell lines. However, type I BL cell lines (Rael, Akata, and Chep) lacked Cp/Wp-initiated EBNA1 transcripts (YUK), and two cell lines with integrated but not episomal viral genomes, Namalwa and IB4 (31), lacked lytic Fp-initiated transcripts (FUU′). These results are summarized in Table 2.
TABLE 2.
RT-PCR for transcripts from different EBNA1 promoters in cell lines and tumors
| Cell lines and tumors | RT-PCR signala
|
|||
|---|---|---|---|---|
| Non-EBNA1-coding transcript FUU′ (Fp) | EBNA1-coding transcripts
|
|||
| FUK (Fp) | QUK (Qp) | YUK (Cp/Wp) | ||
| EBV(+) cell lines | ||||
| BL cell lines | ||||
| Rael | + | + | ++ | − |
| Akata | ++ | ++ | ++ | − |
| Chep | + | ++ | ++ | − |
| Wan | ++ | ++ | ++ | ++ |
| Wewak | + | ++ | ++ | ++ |
| Namalwa | − | ++ | ++c | ++ |
| Rael-AzaC | ++ | ++ | ++ | ++ |
| Raji | +b | ++ | ++c | ++ |
| AG876 | ++ | ++ | ++ | ++ |
| LCL | ||||
| IB4 | − | ++ | ++c | ++ |
| B95-8 | ++ | ++ | ++ | ++ |
| EBV(+) tumors | ||||
| eBL | ||||
| BL1 | +/− | − | ++ | − |
| BL2 | − | − | + | − |
| BL6 | − | − | ++ | − |
| BL7 | − | − | ++ | − |
| BL8 | − | − | ++ | − |
| BL9 | − | − | ++c | − |
| BL10 | − | − | ++ | +/− |
| PTLD | ||||
| PTLD1 | +/− | +/− | ++ | − |
| PTLD2 | − | +/− | ++ | − |
| NPC | ||||
| NPC1 | +/− | +/− | ++ | − |
| NPC2 | − | +/− | ++ | − |
| NPC3 | − | − | ++ | NA |
| NPC4 | − | − | ++ | NA |
| NL | ||||
| NK1 | − | − | ++ | − |
| NK2 | − | − | ++ | − |
| NTL | − | − | ++ | − |
Scored as described in Materials and Methods. NA, not available because of lack of RNA.
Positive for U2′ primer but not U′ primer.
Weakly positive or negative for QUK but positive for Q′UK.
Methylation status of Qp.
CpG methylation has been implicated in the silencing of the EBV Cp in BL, HD, and NPC. The results above suggest that Qp is never silent. We carried out bisulfite genomic sequencing to determine whether it is ever methylated. The minimal Qp region and adjacent sequence include 20 CpG sites (Fig. 2A) (37, 39, 50, 63). All 16 CpG sites downstream of the Fp initiation site (which includes the entire Qp) were unmethylated in virtually all EBV(+) tumors and BL cell lines (Fig. 2C and 3). However, the four CpG sites upstream of the initiation site of Fp were variably methylated. In the Rael cell line, which is weak for Fp activity, these four CpG sites were partially methylated. 5-Azacytidine treatment leads to full demethylation of these CpG sites and increased Fp activity. In cell lines with strong lytic Fp activities, these four sites were always unmethylated.
FIG. 3.
Genomic sequencing of Qp for bisulfite-treated DNA from EBV(+) tumors and cell lines. For bisulfite sequencing, the unmethylated C residue (or G on the opposite strand) within a CpG site will be converted to T (or A) in the sequencing gel, while the methylated C residue is not changed. CpG sites 1 to 8 were sequenced from one direction (A), while CpG sites 9 to 20 were sequenced from the other direction (B). Dark arrows indicate unmethylated CpG dinucleotides, and open arrows indicate methylated CpG dinucleotides. The asterisk indicates a mutation in an NTL which abolishes CpG site 7.
Sequence conservation of Qp.
The sequence of Qp is highly conserved among specimens collected from Africa, North America, and Southeast Asia (Hong Kong and Taiwan). Only two instances of divergence from the prototype B95-8 sequence were found: a T-to-C mutation at 62403 in an African BL tumor (BL6) and a C-to-T mutation at 62275 in an Asian NTL isolate which abolishes CpG site 7 (Fig. 2C).
Effect of methylation on the transcriptional activity of Qp.
Qp appears to be consistently hypomethylated in the context of a viral genome that is generally hypermethylated. To evaluate whether the transcriptional activity of Qp is sensitive to methylation, we applied the methylation cassette assay (42). Recombinant plasmid pQpCAT, containing Qp from −172 to +32 (CpG sites 5 to 19) in a CAT reporter plasmid, was created. A cassette corresponding to Qp was excised and methylated in vitro with CpG methylase. The methylated cassette was ligated back into the CAT background, and the ligated mixture was transfected into the EBV(+) and EBV(−) BL cell lines Rael and CA46. In parallel, the unmethylated cassette was ligated back into the CAT background and also transfected. Methylation of the Qp fragment resulted in an average of 40.4- or 70.2-fold reduction in CAT activity in the Rael or CA46 cell line, respectively in three independent transfections (Fig. 4). Thus, the transcription of Qp is strongly inhibited by methylation and Qp is a methylation-sensitive promoter.
FIG. 4.
Effect of methylation on the transcriptional activity of Qp as tested by the methylation cassette assay. Representative results of a CAT assay after transient transfection into the Rael and CA46 cell lines, using methylated or unmethylated Qp cassette fragments, are shown. Relative CAT activity values are averages of three independent transfection assays.
DISCUSSION
EBNA1 is indispensable for maintenance of the viral episome (65). This housekeeping function is appropriately driven by a promoter with the features of housekeeping promoters: Qp is a TATA-less promoter and constitutively active in all tissues and cell types investigated. Qp-initiated EBNA1 transcripts were readily detected in EBV(+) tumors and cell lines of various cell types (B cell, T cell, NK cell, and epithelial) and with various patterns of viral gene expression (latency I, II, and III). A similar strategy has previously been applied to distinguish EBNA1 transcripts from different promoters in EBV(+) B-cell lines (52). This report extends those results to tumors of various cellular origins. Previous investigations of tumor tissues have demonstrated QUK transcription (9, 11–13, 15, 34, 57) but did not exclude the possibility that the transcripts originated from Fp since the transcripts from Qp overlap those from Fp. Our RT-PCR results demonstrate that Qp activity is distinct from that of Fp.
While we have detected Qp activity in all settings tested, it has previously been reported that Qp is silent in the Mutu III cell line (47). We do not know whether the Mutu III cell line may reflect a difference in sensitivity of RT-PCR in the assays used or whether the pattern of transcription in the Mutu III cell line differs qualitatively from those of other latency III cell lines examined here in which Qp transcripts were detected (B95-8, Wewak, Namalwa, Rael-AzaC, Raji, and AG876). Although we have universally detected Qp transcripts, mechanisms other than methylation for the down-regulation of Qp have been proposed. Evidence has been presented that EBNA1, through its low-affinity binding sites just downstream of Qp, can suppress Qp transcription and that transcription repressors IRF2 and IRF7, through their binding sites immediately upstream of Qp initiation site, can suppress Qp transcription (38, 48, 53, 63, 67). However, the repression of Qp by EBNA1 is a dosage-dependent effect (63). An aberrant level of EBNA1 driven by exogenous powerful promoters leads to a dramatic repression of Qp activity, even to undetectable levels, while a physiologic level of endogenous EBNA1 only represses Qp moderately (53, 63).
Combined usage of multiple promoters for EBNA1 transcription in EBV(+) BL cell lines with different types of EBV latency was observed. Previously, dual usage of Qp and Cp/Wp has been found in some BL cell lines (61). EBNA1-coding transcripts initiated from the lytic Fp have also been reported for some cell lines (40), a finding confirmed in this study. Although an early report suggested that Cp and Wp were mutually exclusive (64), a recent study showed that this is not always the case (66). We have also detected leader exons from both Cp and Wp in Raji, Wan, Namalwa, and Wewak cell lines (59a), as reported for the B95-8 cell line (62). This promiscuous usage of EBNA1 promoters in cell lines contrasts with the sole use of Qp in EBV-associated tumors. Recently, multiple usage of Qp, Cp, and Wp has also been found in peripheral blood mononuclear cells from patients with infectious mononucleosis (27, 62).
What mechanism is involved in keeping Qp, a TATA-less promoter residing within a CG-rich region, constitutively active? Transcription of TATA-less promoters is regulated by upstream CG-rich elements (5). All housekeeping genes have upstream CG-rich elements (18), while CpG methylation is a feasible mechanism of promoter regulation mediated by CG-rich regions. There are numerous examples of de novo methylation of CpG islands in tumors or tumor cell lines which is often associated with the silencing of the associated tumor suppressor genes (3). As for EBV promoters, methylation-mediated transcriptional silence has been well documented for Cp, Wp, and promoters for LMP1 and BHRF1 (1, 16, 23, 33, 36, 41, 44). For Qp, hypomethylation of a 5-kb region that includes both Fp and Qp has previously been inferred from the analysis of methylation-sensitive restriction enzyme sites (53). With genomic sequencing, it is possible to extend the analysis beyond the three CpG sites which happen to correspond to methylation-sensitive restriction sites (CpG sites 8, 10, and 15) to include all sites in the region. This more detailed analysis reveals another level of complexity in that CpG sites in Qp but not Fp are consistently unmethylated in all samples analyzed. A strong correlation between the constitutive activity of Qp and the hypomethylation of CpG sites in this promoter is revealed. Our observation also reinforces the idea that Qp and Fp should be regarded as distinct promoters. Although there is an apparent inverse relationship between the methylation status of the four CpG sites upstream of Fp and Fp expression in the cell lines examined (Table 2 and Fig. 2), a similar relationship was not apparent in tumor specimens. Methylation of one or more of these four CpG sites could be detected in some eBL and HD tumors, but little methylation was detected in NPC and NL although most of these tumors showed little if any Fp expression. This may indicate that CpG methylation is not a critical regulator for Fp expression, or it may indicate methylation is involved in Fp regulation but the critical region of methylation in Fp is not included in the region that we analyzed in this study.
Most of the EBV genome is methylated in tumors and tumor cell lines with restricted patterns of latency. Methylation has also been demonstrated in EBV-infected lymphocytes from healthy individuals (43). Is the absence of CpG methylation in Qp merely a consequence of constitutive transcription, or is maintenance of an unmethylated CpG island necessary for this constitutive expression? We tested the effects of methylation on Qp by using the methylation cassette assay (42). This assay showed that Qp is markedly inhibited by methylation. Our investigation did not address the mechanism by which Qp is excluded from the global methylation of the viral genome in tumors and tumor cell lines. However, inspection of the sequences reveals two potential Sp1 binding sites within Qp and its proximal region (Fig. 2A). Sp1 has been shown to bind to the Sp1 site 1 in vitro (10); however, binding to the putative site 2 has not yet been studied. These putative Sp1 binding sites may provide the key to the protection of Qp region from CpG methylation in that Sp1 sites are known to protect adjacent regions from methylation (8, 29). Moreover, as a ubiquitous transactivator, Sp1 will enhance the transcription of Qp in all cell types (21, 32), keeping Qp as a housekeeping promoter. Nothing is known of the process by which the EBV genome becomes methylated in normal lymphocytes or in tumor tissues. However, our results suggest that the process is highly regulated and that extension of methylation into Qp would interfere with Qp activity and preclude persistence of the viral episome. The high sequence conservation of Qp region among all specimens from Africa, North America, and Asia is consistent with the interpretation that Qp is functionally important for the EBV life cycle.
ACKNOWLEDGMENTS
We thank the staff of the Burkitt’s Tumor Project, University of Ghana, for collecting eBL tumors, F. C. S. Ho and G. Srivastava, University of Hong Kong, for providing the DNA and RNA of NL, and I.-H. Chen, MacKay Memorial Hospital, Taipei, Taiwan, for providing NPC tumors.
Support was provided by grant R01 CA63532 (R.F.A.). R.F.A. is a Leukemia Society Scholar.
REFERENCES
- 1.Altiok E, Minarovits J, Hu L F, Contreras-Brodin B, Klein G, Ernberg I. Host-cell-phenotype-dependent control of the BCR2/BWR1 promoter complex regulates the expression of Epstein-Barr virus nuclear antigens 2-6. Proc Natl Acad Sci USA. 1992;89:905–909. doi: 10.1073/pnas.89.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambinder R F, Shah W A, Rawlins D R, Hayward G S, Hayward S D. Definition of the sequence requirements for binding of the EBNA-1 protein to its palindromic target sites in Epstein-Barr virus DNA. J Virol. 1990;64:2369–2379. doi: 10.1128/jvi.64.5.2369-2379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylin S B, Herman J G, Graff J R, Vertino P M, Issa J P. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 4.Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- 5.Blake M C, Jambou R C, Swick A G, Kahn J W, Azizkhan J C. Transcriptional initiation is controlled by upstream GC-box interaction in a TATAA-less promoter. Mol Cell Biol. 1990;10:6632–6641. doi: 10.1128/mcb.10.12.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyes J, Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991;64:1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- 7.Boyes J, Bird A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 1992;11:327–333. doi: 10.1002/j.1460-2075.1992.tb05055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandeis M, Frank D, Keshet I, Siegfried Z, Mendelsohn M, Nemes A, Temper V, Razin A, Cedar H. Sp1 elements protect a CpG island from de novo methylation. Nature. 1994;371:435–438. doi: 10.1038/371435a0. [DOI] [PubMed] [Google Scholar]
- 9.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1 and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulfone-Paus S, Dempsey L A, Maizels N. Host factors LR1 and Sp1 regulate the Fp promoter of Epstein-Barr virus. Proc Natl Acad Sci USA. 1995;92:8293–8297. doi: 10.1073/pnas.92.18.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busson P, McCoy R, Sadler R, Gilligan K, Tursz T, Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992;66:3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C L, Sadler R H, Walling D M, Su I J, Hsieh H C, Raab-Traub N. Epstein-Barr virus (EBV) gene expression in EBV-positive peripheral T-cell lymphomas. J Virol. 1993;67:6303–6308. doi: 10.1128/jvi.67.10.6303-6308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang A K S, Tao Q, Srivastava G, Ho F C S. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin’s disease. Int J Cancer. 1996;68:285–290. doi: 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Counts J L, Goodman J I. Alterations in DNA methylation may play a variety of roles in carcinogenesis. Cell. 1995;83:13–15. doi: 10.1016/0092-8674(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 15.Deacon E M, Pallesen G, Niedobitek G, Crocker J, Brooks L, Rickinson A B, Young L S. Epstein-Barr virus and Hodgkin’s disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med. 1993;177:339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernberg I, Falk K, Minarovits J, Busson P, Tursz T, Masucci M G, Klein G. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J Gen Virol. 1989;70:2989–3002. doi: 10.1099/0022-1317-70-11-2989. [DOI] [PubMed] [Google Scholar]
- 17.Frommer M, McDonald L E, Millar D S, Collis C M, Watt F, Grigg G W, Molloy P L, Paul C L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 19.Gilligan K, Rajadurai P, Resnick L, Raab-Traub N. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc Natl Acad Sci USA. 1990;87:8790–8794. doi: 10.1073/pnas.87.22.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldsmith K, Bendell L, Frappier L. Identification of EBNA1 amino acid sequences required for the interaction of the functional elements of the Epstein-Barr virus latent origin of DNA replication. J Virol. 1993;67:3418–3426. doi: 10.1128/jvi.67.6.3418-3426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holler M, Westin G, Jiricny J, Schaffner W. Sp1 transcription factor binds DNA and activates transcription even when the binding site is CpG methylated. Genes Dev. 1988;2:1127–1135. doi: 10.1101/gad.2.9.1127. [DOI] [PubMed] [Google Scholar]
- 22.Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA. 1994;91:9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansson A, Masucci M, Rymo L. Methylation of discrete sites within the enhancer region regulates the activity of the Epstein-Barr virus BamHI W promoter in Burkitt lymphoma lines. J Virol. 1992;66:62–69. doi: 10.1128/jvi.66.1.62-69.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr B M, Lear A L, Rowe M, Croom-Carter D, Young L S, Rookes S M, Gallimore P H, Rickinson A B. Three transcriptionally distinct forms of Epstein-Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology. 1992;187:189–201. doi: 10.1016/0042-6822(92)90307-b. [DOI] [PubMed] [Google Scholar]
- 25.Keshet I, Lieman-Hurwitz J, Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986;44:535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- 26.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Raven; 1996. pp. 2343–2396. [Google Scholar]
- 27.Laytragoon-Lewin N, Chen F, Avila-Carino J, Klein G, Mellstedt H. Epstein-Barr virus (EBV) gene expression in lymphoid B cells during acute infectious mononucleosis (IM) and clonality of the directly growing cell lines. Int J Cancer. 1997;71:345–349. doi: 10.1002/(sici)1097-0215(19970502)71:3<345::aid-ijc6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Lear A L, Rowe M, Kurilla M G, Lee S, Henderson S, Kieff E, Rickinson A B. The Epstein-Barr virus (EBV) nuclear antigen 1 BamHI F promoter is activated on entry of EBV-transformed B cells into lytic cycle. J Virol. 1992;66:7461–7468. doi: 10.1128/jvi.66.12.7461-7468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macleod D, Charlton J, Mullins J, Bird A P. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 1994;8:2282–2292. doi: 10.1101/gad.8.19.2282. [DOI] [PubMed] [Google Scholar]
- 30.Masucci M G, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, Klein G. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt’s lymphoma line Rael. J Virol. 1989;63:3135–3141. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuo T, Heller M, Petti L, O’Shiro E, Kieff E. Persistence of the entire Epstein-Barr virus genome integrated into human lymphocyte DNA. Science. 1984;226:1322–1325. doi: 10.1126/science.6095452. [DOI] [PubMed] [Google Scholar]
- 32.McKnight S, Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986;46:795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- 33.Minarovits J, Li-Fu H, Minarovits-Kormuta S, Klein G, Ernberg I. Sequence-specific methylation inhibits the activity of the Epstein-Barr virus LMP 1 and BCR2 enhancer-promoter regions. Virology. 1994;200:661–667. doi: 10.1006/viro.1994.1229. [DOI] [PubMed] [Google Scholar]
- 34.Minarovits J, Hu L-F, Imai S, Harabuchi Y, Kataura A, Minarovits-Kormuta S, Osato T, Klein G. Clonality, expression and methylation patterns of the Epstein-Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol. 1994;75:77–84. doi: 10.1099/0022-1317-75-1-77. [DOI] [PubMed] [Google Scholar]
- 35.Minarovits J, Hu L F, Marcsek Z, Minarovits-Kormuta S, Klein G, Ernberg I. RNA polymerase III-transcribed EBER 1 and 2 transcription units are expressed and hypomethylated in the major Epstein-Barr virus-carrying cell types. J Gen Virol. 1992;73:1687–1692. doi: 10.1099/0022-1317-73-7-1687. [DOI] [PubMed] [Google Scholar]
- 36.Minarovits J, Minarovits-Kormuta S, Ehlin-Henriksson B, Falk K, Klein G, Ernberg I. Host cell phenotype-dependent methylation patterns of Epstein-Barr virus DNA. J Gen Virol. 1991;72:1591–1599. doi: 10.1099/0022-1317-72-7-1591. [DOI] [PubMed] [Google Scholar]
- 37.Nonkwelo C, Daniel Henson E B, Sample J. Characterization of the Epstein-Barr virus Fp promoter. Virology. 1995;206:183–195. doi: 10.1016/s0042-6822(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 38.Nonkwelo C, Ruf I K, Sample J. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nonkwelo C, Ruf I K, Sample J. The Epstein-Barr virus EBNA-1 promoter Qp requires an initiator-like element. J Virol. 1997;71:354–361. doi: 10.1128/jvi.71.1.354-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996;70:623–627. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nonkwelo C B, Long W K. Regulation of Epstein-Barr virus BamHI-H divergent promoter by DNA methylation. Virology. 1993;197:205–215. doi: 10.1006/viro.1993.1581. [DOI] [PubMed] [Google Scholar]
- 42.Robertson K D, Ambinder R F. Mapping promoter regions that are hypersensitive to methylation-mediated inhibition of transcription: application of the methylation cassette assay to the Epstein-Barr virus major latency promoter. J Virol. 1997;71:6445–6454. doi: 10.1128/jvi.71.9.6445-6454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson K D, Ambinder R F. Methylation of Epstein-Barr virus genome in normal lymphocytes. Blood. 1997;90:4480–4484. [PubMed] [Google Scholar]
- 44.Robertson K D, Hayward D J, Ling P D, Samid D, Ambinder R F. Transcriptional activation of the EBV latency C promoter following 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol. 1995;15:6150–6159. doi: 10.1128/mcb.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson K D, Manns A, Swinnen L J, Zong J C, Gulley M L, Ambinder R F. CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt’s lymphoma and Hodgkin’s disease. Blood. 1996;88:3129–3136. [PubMed] [Google Scholar]
- 46.Rowe M, Lear A L, Croom-Carter D, Davies A H, Rickinson A B. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66:122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sample J, Brooks L, Sample C, Young L, Rowe M, Gregory C, Rickinson A, Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci USA. 1991;88:6343–6347. doi: 10.1073/pnas.88.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sample J, Henson E B, Sample C. The Epstein-Barr virus nuclear protein 1 promoter active in type I latency is autoregulated. J Virol. 1992;66:4654–4661. doi: 10.1128/jvi.66.8.4654-4661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer B C, Paulson E, Strominger J L, Speck S H. Constitutive activation of Epstein-Barr virus (EBV) nuclear antigen 1 gene transcription by IRF1 and IRF2 during restricted EBV latency. Mol Cell Biol. 1997;17:873–886. doi: 10.1128/mcb.17.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer B C, Strominger J L, Speck S H. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group 1 Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaefer B C, Strominger J L, Speck S H. The Epstein-Barr virus BamHI F promoter is an early lytic promoter: lack of correlation with EBNA 1 gene transcription in group 1 Burkitt’s lymphoma cell lines. J Virol. 1995;69:5039–5047. doi: 10.1128/jvi.69.8.5039-5047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaefer B C, Strominger J L, Speck S H. A simple reverse transcriptase PCR assay to distinguish EBNA1 gene transcripts associated with type I and II latency from those arising during induction of the viral lytic cycle. J Virol. 1996;70:8204–8208. doi: 10.1128/jvi.70.11.8204-8208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaefer B C, Strominger J L, Speck S H. Host-cell-determined methylation of specific Epstein-Barr virus promoters regulates the choice between distinct viral latency programs. Mol Cell Biol. 1997;17:364–377. doi: 10.1128/mcb.17.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaefer B C, Woisetschlaeger M, Strominger J L, Speck S H. Exclusive expression of Epstein-Barr virus nuclear antigen 1 in Burkitt lymphoma arises from a third promoter, distinct from the promoters used in latently infected lymphocytes. Proc Natl Acad Sci USA. 1991;88:6550–6554. doi: 10.1073/pnas.88.15.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlager S, Speck S H, Woisetschlaeger M. Transcription of the Epstein-Barr virus nuclear antigen 1 (EBNA 1) gene occurs before induction of the BCR2 (Cp) EBNA gene promoter during the initial stages of infection of B cells. J Virol. 1996;70:3561–3570. doi: 10.1128/jvi.70.6.3561-3570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith P R, Griffin B E. Transcription of the Epstein-Barr virus gene EBNA-1 from different promoters in nasopharyngeal carcinoma and B-lymphoblastoid cells. J Virol. 1992;66:706–714. doi: 10.1128/jvi.66.2.706-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugiura M, Imai S, Tokunaga M, Koizumi S, Uchizawa M, Okamoto K, Osato T. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer. 1996;74:625–631. doi: 10.1038/bjc.1996.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sung N S, Wilson J, Davenport M, Sista N D, Pagano J S. Reciprocal regulation of the Epstein-Barr virus BamHI-F promoter by EBNA-1 and an E2F transcription factor. Mol Cell Biol. 1994;14:7144–7152. doi: 10.1128/mcb.14.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tao Q, Robertson K D, Manns A, Hildesheim A, Ambinder R F. Epstein-Barr virus (EBV) in endemic Burkitt’s lymphoma: molecular analysis of primary tumor tissue. Blood. 1998;91:1373–1381. [PubMed] [Google Scholar]
- 59a.Tao, Q., et al. Unpublished data.
- 60.Tate P H, Bird A P. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993;3:226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- 61.Taylor K A, Wetzel S, Lyles D S, Pollok B A. Dual EBNA1 promoter usage by Epstein-Barr virus in human B-cell lines expressing unique intermediate cellular phenotypes. J Virol. 1994;68:6421–6431. doi: 10.1128/jvi.68.10.6421-6431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai C N, Liu S T, Chang Y S. Identification of a novel promoter located within the Bam HI Q region of the Epstein-Barr virus genome for the EBNA 1 gene. DNA Cell Biol. 1995;14:767–776. doi: 10.1089/dna.1995.14.767. [DOI] [PubMed] [Google Scholar]
- 64.Woisetschlaeger M, Strominger J L, Speck S H. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc Natl Acad Sci USA. 1989;86:6498–6502. doi: 10.1073/pnas.86.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yates J L, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mannalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 66.Yoo L I, Mooney M, Puglieli M T, Speck S H. B-cell lines immortalized with an Epstein-Barr virus mutant lacking the Cp EBNA2 enhancer are biased toward utilization of the oriP-proximal EBNA gene promoter Wp1. J Virol. 1997;71:9134–9142. doi: 10.1128/jvi.71.12.9134-9142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]