Abstract
Mucosa-associated invariant T (MAIT) cells are a subset of innate-like T lymphocytes known for their ability to respond to MHC-related protein 1 (MR1)-restricted stimuli and select cytokine signals. They are abundant in humans and especially enriched in mucosal layers, common sites of neoplastic transformation. MAIT cells have been found within primary and metastatic tumors. However, whether they promote malignancy or contribute to anticancer immunity is unclear. On the one hand, MAIT cells produce IL-17A in certain locations and under certain circumstances, which could in turn facilitate neoangiogenesis, intratumoral accumulation of immunosuppressive cell populations, and cancer progression. On the other hand, they can express a potent arsenal of cytotoxic effector molecules, NKG2D and IFN-γ, all of which have established roles in cancer immune surveillance. In this review, we highlight MAIT cells’ characteristics as they might pertain to cancer initiation, progression, or control. We discuss recent findings, including our own, that link MAIT cells to cancer, with a focus on colorectal carcinoma, as well as some of the outstanding questions in this active area of research. Finally, we provide a hypothetical picture in which MAIT cells constitute attractive targets in cancer immunotherapy.
Keywords: MAIT cells, Cancer, Tumor-infiltrating leukocytes, IL-17, IFN-γ, CITIM 2017
MAIT cells: a brief overview
Mucosa-associated invariant T (MAIT) cells are innate-like T lymphocytes that express a restricted TCRα with a unique gene rearrangement pattern, namely TRAV1-2-TRAJ33/12/20 (Vα7.2-Jα33/12/20) in humans and TRAV1-TRAJ33 (Vα19-Jα33) in mice [1–3]. MAIT cells’ TCRα chain pairs with one of a few TCRβ chains to form a semi-invariant TCR, which is restricted by MHC-related protein 1 (MR1) [4]. MR1 is highly conserved across mammalian species [5] and best known for its capacity to present vitamin B derivatives of microbial origin to MAIT cells [6].
MAIT cells are positively selected by MR1+CD4+CD8+ thymocytes [7] and undergo further maturation extrathymically before they populate mucosal layers [8]. Human MAIT cells are a prominent T cell population in the gut and in the liver, and maintain a noticeable presence in the peripheral blood, where they comprise 1–10% of all CD3+ cells [9, 10]. MAIT cells are not as plentiful in mice, but are somewhat enriched in the lungs where they account for approximately 3% of all αβ T cells [11].
Human MAIT cells can be immunophenotyped as CD3+Vα7.2+CD161high cells, most of which express CD8αα [12]. There also exist CD4−CD8− and CD4+CD8− subsets. Up until recently, mouse MAIT cells could be traced, almost exclusively, through detection of their invariant TCRα (iTCRα) chain at the nucleic acid level. However, the recent advent of MR1 tetramer reagents has enabled reliable identification of mouse and human MAIT cells [12, 13].
Most MAIT cells exhibit an effector memory-like phenotype [9] and can be readily and rapidly activated via TCR-dependent and-independent mechanisms [14], a property they share with other innate-like T cells such as invariant natural killer T (iNKT) and γδ T cells. The ability of MAIT cells to serve as one of the ‘first responders’ to environmental cues is relatively well characterized in certain infections [15]. Numerous bacterial strains and certain fungi harbor MR1-restricted compounds and can, as such, trigger MAIT cell activation in a TCR-dependent manner. Viruses, on the other hand, do not possess the vitamin B synthesis machinery; yet, they prompt MAIT cell activation in a bystander, cytokine-dependent fashion [16, 17].
When used alone or in various combinations, IL-7, IL-12, IL-15, IL-18, and IFN-α/β can activate MAIT cells in the overt or covert absence of a TCR signal [10, 14, 16–20]. Even in the presence of TCR triggering, inflammatory cytokines may be the main driving force for MAIT cell activation. We recently demonstrated that once exposed to staphylococcal enterotoxin B (SEB), a powerful superantigen that binds select TCR Vβ families to quickly provoke inflammatory cytokine production, MAIT cells elicit a robust response that is dominated by IL-12/IL-18 receptor signaling [21]. MAIT cells express CD212 (IL-12Rβ1) and CD218a (IL-18Rα) on their surface constitutively [14], which allows them to respond to these cytokines under both helpful and harmful inflammatory conditions. Optimal stimulation of MAIT cells arms them with a myriad of cytotoxic effector molecules, such as perforin and several granzymes (GZMs), and confers upon them the ability to unleash ‘fire and fury’ against MR1+ infected cells [22–24] and perhaps other target cells. It also enables them to secrete immunomodulatory cytokines of their own, including IL-2, IL-17A, IFN-γ, TNF-α, and IL-10.
MAIT cells can display a T helper (TH)1-type, TH17-type or a mixed cytokine profile depending largely on their stimulation mode and means, local tissue imprinting and the cytokine milieu in which they are activated. Their ability to produce IFN-γ and TNF-α following microbial and mitogenic stimulation has been documented in numerous studies [9, 22, 25]. Peripheral blood and hepatic human MAIT cells produce little to no IL-17A in response to bacterial Ags or anti-CD3, but large quantities of this cytokine following stimulation with PMA and ionomycin [9, 10], an activation mode that bypasses TCR ligation. Of note, IL-7 can reportedly restore IL-17A production by anti-CD3/CD28-stimulated hepatic MAIT cells [10]. MAIT cells are found abundantly within the adipose tissue of both obese and non-obese adults [26, 27]. However, omental adipose tissue MAIT cells from obese subjects produce more IL-17 and less IFN-γ or TNF-α upon stimulation with PMA and ionomycin. Last but not the least, MAIT cells show a remarkable degree of heterogeneity at both single cell and population levels [25]. Consequently, their response patterns can be tailored to different challenges, microbial and perhaps also non-microbial in nature.
Although much of what we have learned about MAIT cells is owed to research in the area of infectious diseases, emerging evidence implicates these cells as both important players and attractive targets in various malignancies.
Mechanistic insights into possible MAIT cell roles in cancer—I: pro-tumorigenic effects?
As indicated above, some tissue-resident MAIT cells can launch TH17-skewed responses. On the other hand, IL-17A exerts pro-tumorigenic activities that can influence the clinical prognosis of a malignancy [28–32]. In the case of colorectal carcinoma (CRC) as an example, heavy expression of the TH17-related genes IL17A and RAR related orphan receptor C (RORC) and the accumulation of IL-17+ cells in the neoplastic lesions correlate with reduced disease-free survival [28]. Therefore, it is plausible to envisage a scenario in which IL-17-producing MAIT cells infiltrate tumor masses to the host’s detriment. A TH17 bias on the part of these lymphocytes could simply reflect the location where they encounter primary or metastatic tumors. Alternatively or additionally, such bias may be acquired or perpetuated within the tumor microenvironment (TME).
IL-17A may promote cancer via several mechanisms. Xie et al. found higher levels of IL-17A and IL-17 receptor A (IL-17RA) in human colon adenocarcinomas than in normal colon tissue, colon polyps diagnosed as benign adenomas, and tissue biopsies from patients with ulcerative colitis [33]. These changes were accompanied by increased levels of phosphorylated extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK) as well as augmented induction of matrix metalloproteinase (MMP)9, MMP2, B-cell lymphoma 2 (Bcl-2) and cyclin D1, downstream targets of IL-17A. Therefore, the IL-17A receptor signaling appears to be operational in the adenocarcinoma TME. Importantly, vascular endothelial growth factor (VEGF) and VEGF receptor expression levels were elevated and associated with enhanced angiogenesis, which was judged by immunohistochemical staining of microvessels for CD34 [33]. IL-17 upregulates the expression of VEGF [34] and also enhances its proangiogenic activity [35]. It may also potentiate the production of other angiogenic factors. Using human lung adenocarcinoma cell lines and tumor-bearing nude mice, Huang et al. demonstrated a link between IL-17A stimulation and the STAT1-dependent production of IL-6 and IL-8 in addition to VEGF [36]. These findings are particularly important since many, if not most, tumors rely on neoangiogenesis for their sustained growth and invasive behavior. Mouse models have also revealed that IL-17A can compromise the integrity of the lung endothelial barrier, thus allowing tumor cells to escape into the vasculature to establish pulmonary metastases [37]. Finally, IL-17A may facilitate the accumulation and/or expansion of suppressor cell populations within tumors. For instance, epithelial barrier disruption by a tumor in the colon may result in microbial invasion, thus eliciting inflammatory dendritic cells [38]. This can in turn give rise to IL-17A-polarized γδ T cells. Activated γδT17 cells secrete several cytokines other than IL-17A, including IL-8, GM-CSF and TNF-α, which may attract MDSCs into the tumor and promote their survival, expansion and/or retention [38]. The findings of a subsequent investigation suggest that CRC tumor-derived TGF-β1 induces CD39+FoxP3+ γδ T cells capable of producing high levels of IL-17A among other immunomodulatory, typically immunosuppressive, cytokines, which directly inhibit effector T cell functions and may also recruit MDSCs into the TME [39]. Several in vitro studies have proposed that IL-17A may indirectly promote macrophage differentiation towards an M2 phenotype by upregulating COX-2 and prostaglandin E2 (PGE2) in various tumor cell types [40, 41]. Unlike M1 macrophages, M2 macrophages assist in tumor growth and metastasis.
The above studies indicate that IL-17A, regardless of its source, can be a key player in creating a vicious circle of immunosuppression in TMEs (Fig. 1). Since MAIT cells are present among TILs [42–47] and can produce IL-17A in certain locations [10, 26, 27], they may contribute to early tumor progression. By the same token, one may theorize that TH17-polarized MAIT cells play an influential role in inflammation-associated cancers. Inflammatory bowel disease (IBD) is widely viewed as a major risk factor for colon carcinogenesis. Recent reports have found increased frequencies of MAIT cells within the inflammatory lesions of ulcerative colitis and Crohn’s disease in comparison with healthy, uninflamed intestinal tissue [48, 49]. Interestingly, MAIT cells sorted from the peripheral blood of IBD patients were found to secrete more IL-17 upon ex vivo stimulation with PMA and ionomycin when they were compared with MAIT cells isolated from healthy donors [48]. Furthermore, MAIT cell frequencies in the inflamed mucosa of ulcerative colitis patients could be correlated with clinical and endoscopic evidence of disease activity [49]. Although direct causation has yet to be established, we favor the hypothesis that MAIT cells play a pathogenic role in IBD and may drive the development of IBD-associated CRC.
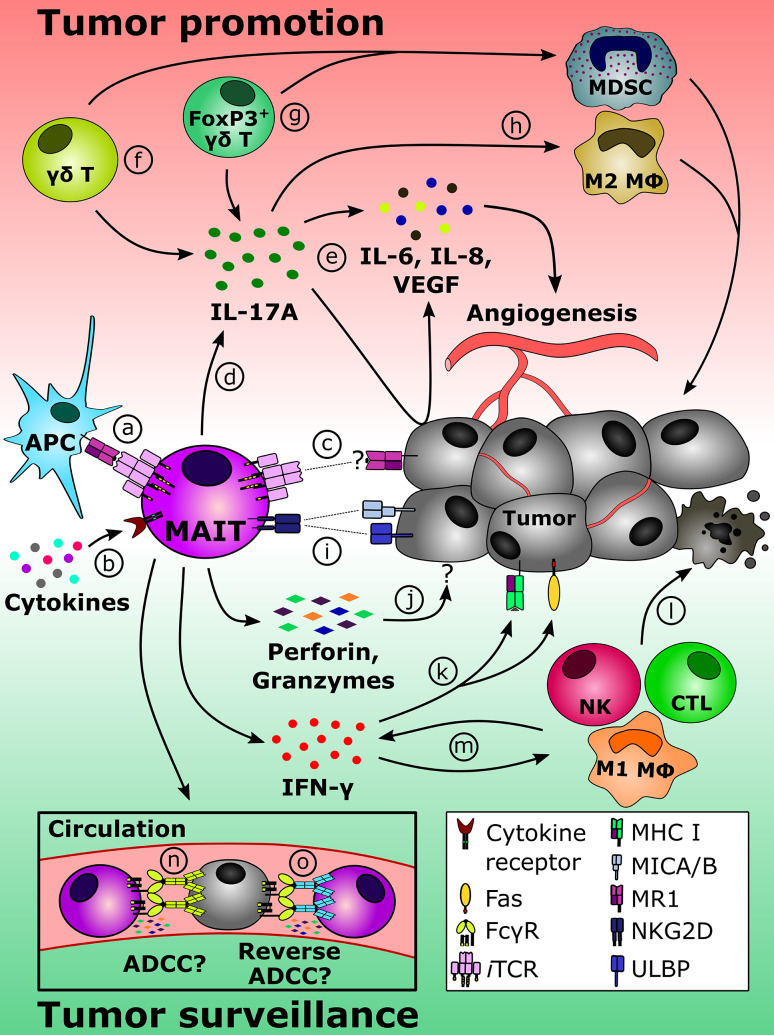
Fig. 1.
Potential MAIT cell roles in tumor promotion/progression and in antitumor immune surveillance. MAIT cells residing within and outside TMEs may recognize MR1-restricted Ags displayed by APCs (a) and/or respond to select cytokines including IL-7, IL-12 and IL-18 (b). There also exists a possibility that tumor cells themselves present MR1 ligands to directly activate MAIT cells (c). Once primed, MAIT cells in certain locations may release IL-17A (d) whose pro-tumorigenic effects include the induction of angiogenic factors such as IL-6, IL-8 and/or VEGF in tumor cells and other cell types (e). Therefore, MAIT cells can join forces with other sources of IL-17A within the TME, including γδT17 cells (f) and IL-17A-producing FoxP3+ γδ T cells (g) that facilitate the intratumoral accumulation of MDSCs. Regardless of its cellular origin, IL-17A can favor tumor progression also by promoting the differentiation of M2 macrophages (h). Conversely, a different scenario can be envisaged in which MAIT cells play a protective role against cancer. The expression of NKG2D by MAIT cells should enable them to sense the presence of MIC A/B and/or ULBPs on malignant cells (i). MAIT cells harbor a lethal arsenal and may release cytotoxic effector molecules (e.g., perforin and granzymes) upon target cell recognition (j). In addition, activated MAIT cells are among the most powerful sources of IFN-γ, a cytokine known to upregulate MHC class I and Fas (CD95) on tumor cells (k), thus making them prone to the cytolytic action of various killer cell types (l). IFN-γ also transactivates NK cells, CD8+ CTLs and M1 macrophages among other secondary effector cell types (m), thereby accelerating tumor cell demise (l). MAIT cells reportedly express CD16 (FcγRIII) in some individuals, which should allow for Ab-coated tumor cell destruction via ADCC (n). We propose that MAIT cells’ cytolytic potentials may be exploited in therapeutic settings where MAIT cell-specific Abs mediate the elimination of FcR+ tumor cells through reverse ADCC (o)
Obesity is considered a risk factor for several malignancies of the gastrointestinal tract. Low-grade, chronic inflammation that is associated with obesity is thought to be mediated, at least in part, by IL-17 [50], and adipose tissue MAIT cells produce elevated levels of IL-17 [26, 27]. Therefore, the possibility that adipose tissue TH17-skewed MAIT cells contribute to chronic inflammation culminating in cancer is not far-fetched.
Mechanistic insights into possible MAIT cell roles in cancer—II: participation in antitumor immune surveillance?
MAIT cells can rapidly produce copious amounts of IFN-γ, a potent cytokine with cytostatic, proapoptotic and immunostimulatory activities, all of which contribute to anticancer host responses. Genetic variation in IFN-γ signaling has been linked to susceptibility to oncogenesis. For instance, certain SNPs in IFN-γ or IFN-γR1 are associated with CRC risk or even post-diagnosis survival [51, 52].
In adenomatous polyposis coli (Apc)Min/+ mice, which provide a powerful model of inflammation-associated benign adenomatosis, heterozygous deletion of endogenous IFN-γ was shown to increase the number of adenomas and to potentiate progression to adenocarcinoma [53]. In the same study, RNA interference to knock down IFN-γR1 in the human CRC cell line HT-29 abrogated the anti-proliferative effect of exogenous IFN-γ. The direct growth inhibitory and/or apoptogenic effects of IFN-γ were also evident against multiple other human CRC cell lines, namely HCT116, LS174T, SW480 and SW620, and were attributed to the ability of this cytokine to induce microRNA-29b [54].
Downstream immunostimulatory effects of IFN-γ are well characterized. IFN-γ alters the Ag processing and presentation machinery of many cell types, including neoplastic cells that often express low levels of MHC class I molecules to avoid CD8+ CTL detection [55]. Exposure to IFN-γ can upregulate the expression of MHC I in tumor cells to aid in presentation of cognate peptides to CD8+ T cells. Under the circumstances where tumor cells express ample MHC I but still fail to activate naïve CD8+ T cells, due, for instance, to a lack of costimulatory molecules [56], IFN-γ may boost the cross-priming pathway of CTL generation [57]. IFN-γ is intimately involved in TH1 differentiation and TH1-mediated responses and in activation of macrophages. It can also turn tumor-associated macrophages into immunostimulatory M1 cells [58, 59]. Certain tumor cells produce TGF-β and IL-10 among other immunosuppressive factors as a ‘fight-back’ mechanism, which can be countered by IFN-γ [60]. On the other hand, the expression of monokine induced by gamma interferon [MIG; aka. C-X-C chemokine (ligand)9 (CXCL9)] and IFN-γ-inducible protein of 10 kDa (IP-10; aka. CXCL10) in certain tumor cells mediates tumor infiltration by T cells and inhibits angiogenesis by preventing VEGF production and endothelial cell proliferation [61]. It is noteworthy that IFN-γ-induced IP-10/MIG expression may occur secondarily to the action of IL-12 [62–64], a key immunomodulatory cytokine that has been used in experimental immunotherapy of cancer and that is known to vigorously activate MAIT cells.
MAIT cells abundantly express natural-killer group 2, member D (NKG2D) [9], a C-type lectin-like receptor that is also expressed by human NK, NKT, γδ T and activated CD8+ T cells. NKG2D is known to play a part in anticancer immune surveillance [65]. It binds to proteins encoded by the MHC class I polypeptide-related sequence (MIC) A and B loci and members of the UL16-binding protein/retinoic acid early transcript 1 (ULBP/RAET1) family [66, 67]. The engagement of NKG2D sets in motion an intracellular signaling cascade that culminates in degranulation of vesicles containing cytotoxic effector molecules. The highly restricted expression pattern of NKG2D ligands in healthy cells is therefore not surprising. By contrast, such ligands are rapidly inducible by stress or hyper-proliferation, thus allowing the immune system to sense the presence of stressed, abnormal and neoplastic cells [67].
McGilvray et al. demonstrated that the tumoral expression of MIC and RAET1G positively correlates with improved survival in CRC patients [66]. The authors also found that high expression levels of NKG2D ligands were frequently observed in TNM stage-I tumors but progressively lost in stage-II, -III and -IV tumors. Of interest, MIC expression could be correlated with the presence of CD16+ cells within the tumors. This was logically interpreted as evidence of tumor infiltration by NK cells and monocytes, both of which express CD16 along with NKG2D. Since human MAIT cells may too express CD16 at least in some individuals [68], it will be important to explore a possible link between MIC expression and tumor-infiltrating MAIT cell frequencies in CRC.
In their resting state, human peripheral blood MAIT cells constitutively express high levels of granzyme (GZM) A and GZM K, but only little to no GZM B and perforin [24]. However, after they encounter cells infected with certain bacterial pathogens, they swiftly upregulate perforin and GZM B, release the content of their granules as measured by CD107a expression, and kill infected cells in an MR1-dependent manner [23, 24].
Although MAIT cell-mediated cytotoxicity has been addressed mainly in the context of infection, direct and indirect lytic functions of these cells should be explored and potentially exploited in cancer. MAIT cells may indirectly participate in oncolysis through secreting IFN-γ, which can induce or increase Fas (CD95) expression on target cells [69], thus potentiating their Fas ligand-mediated lysis by various killer cell types. Finally, MAIT cells have been reported to express CD16 (FcγRIII) in some individuals [68]. Although this is not a consistent feature [25, 68], one can assume that CD16+ MAIT cells should be able to destroy tumor cells via antibody-dependent cell-mediated cytotoxicity (ADCC). It is also tempting to speculate that MAIT cells’ cytotoxic potentials may be harnessed through administration of Abs that could serve as a bridge between MAIT cells and FcR+ tumor cells, thus mediating reverse or redirected ADCC (Fig. 1). ADCC may be particularly important in preventing metastasis given the relatively high frequency of MAIT cells in the circulation [9, 10].
In summary, the ability of some MAIT cells to produce IFN-γ, to utilize the activating NKG2D receptor to engage malignant cells, and to potentially eliminate tumor cells, either directly or indirectly, lends support to the notion that MAIT cells may fulfill protective functions against cancer (Fig. 1).
MAIT cells in primary tumors
Several studies have reported the presence of human MAIT cells in solid tumors [42–46]. In an early report linking MAIT cells to cancer, Peterfalvi et al. [42] detected Vα7.2-Jα33 iTCRα transcripts in 8 out of 11 clear cell renal cell carcinoma samples as well as in 6 out of 8 brain tumor (glioblastoma and malignant meningioma) samples. In addition, all the eight kidney tumors expressing the MAIT clonotype also contained mRNA encoding Vβ2 and Vβ13, two TCR Vβ families preferentially utilized by human MAIT cells [70]. Moreover, MR1 mRNA and MAIT iTCRα transcripts were co-present in most tumor samples. This may be viewed as the earliest ‘circumstantial’ evidence that tumor-infiltrating MAIT cells can potentially be activated in situ by MR1-restricted ligands. Such ligands could be MR1-bound tumor antigens, if they exist, and synthetic mimetics of microbe-derived vitamin B metabolites as possible therapeutics.
While more recent studies have documented the infiltration of primary CRC tumors by MAIT cells [43–46], there is no clear consensus on the functionality and clinical significance of MAIT cells within and outside the CRC TME. Zabijak et al. used confocal microscopy to retrospectively trace MAIT cells in paired CRC tumor and neighboring healthy tissue samples [44]. They concluded that the more pronounced the presence of MAIT cells in CRC tumors, the poorer the patient outcome.
Won et al. found the absolute number of circulating MAIT cells to inversely correlate with the N (lymph node-based) staging of the tumors and with carcinoembryonic antigen (CEA) blood levels in a cohort of patients with mucosa-associated cancers including CRC [46]. In addition, there was a trend for inverse correlation between peripheral blood MAIT cell numbers and the tumor size. As such, the observed drop in circulating MAIT cell numbers was suggested to reflect the degree of cancer progression. Also interestingly, these investigators found in a colon cancer sub-cohort that peripheral blood MAIT cells expressed high levels of C-C chemokine receptor (CCR)6 and C-X-C chemokine receptor (CXCR)6. C-C chemokine (CCL)20 and CXCL16, the ligands for the aforementioned chemokine receptors, respectively, were also abundant at the mRNA level in paired tumor samples but not in the unaffected tissue. Furthermore, in transwell migration assays, MAIT cells were mobilized by CCL20, CXCL16 or both. Accordingly, the authors suggested that a numerical decline in the circulating MAIT cell compartment may be a consequence of MAIT cells’ tropism for the tumor tissue [46]. It is important to note, however, that while a separate report [45] and our own work (unpublished data) have found low percentages of MAIT cells in the peripheral blood of CRC patients, this is not always a reproducible observation [43].
Sundstrӧm and coworkers found elevated percentages of MAIT cells in CRC tumors in comparison with unaffected colon lamina propria, and this was not concomitant with a decrease in circulating MAIT cell frequencies [43]. They also evaluated several functional attributes of MAIT cells. In response to PMA and ionomycin, most colonic MAIT cells residing in unaffected tissues produced IFN-γ; some made TNF-α, IL-2 and GZM B; and only very few became IL-17A+. Importantly, compared with unaffected colonic tissue MAIT cells, tumor-derived MAIT cells exhibited a partially diminished capacity for IFN-γ production, whereas their IL-17A, IL-2, and GZM B levels remained unaltered. The authors also exposed peripheral blood MAIT cells isolated from healthy volunteers to a combination of IL-12 and IL-18 in the absence or presence of healthy colon tissue- or colon tumor-conditioned medium. Significantly fewer MAIT cells that were stimulated in the presence of tumor-conditioned medium were able to produce IFN-γ. Based on this observation, Sundstrӧm et al. proposed that the suppressed phenotype of tumor-infiltrating MAIT cells may be due to soluble factors released in the CRC TME [43]. Indeed, a previous study has reported that human CRC cell lines secrete TGF-β and PGE2, which can in turn prime lamina propria-derived mononuclear cells for enhanced production of the anti-inflammatory cytokine IL-10 [71].
MAIT cells in metastatic CRC
Our group recently evaluated the presence and functional competence of hepatic MAIT cells in patients with colorectal liver metastasis (CRLM) [47]. We found MAIT cells to be only marginally less abundant within the metastatic tumors than in healthy liver samples. In fact, the mean frequency of MAIT cells in the CRLM TME was more similar to that in the surrounding healthy tissue (~ 30%) than to their reported frequencies in the peripheral blood [9, 10], unaffected gut lamina propria [9, 43], or colon adenocarcinomas [43]. Therefore, at least a large fraction of intratumoral MAIT cells appear to be of local tissue origin. They may actively penetrate the metastatic masses or become ensnared within the tumors gradually forming in the liver. These possibilities are not mutually exclusive. Metastatic tumor cells may also recruit additional MAIT cells from the peripheral blood and distal sites as previously proposed in the case of primary CRC [46].
We employed a panel of TCR-independent and -dependent stimuli to address the functionality of hepatic MAIT cells in CRLM [47]. When exposed, ex vivo, to recombinant human IL-12 and IL-18, tumor-infiltrating MAIT cells were severely impaired in their IFN-γ production capacity. This was despite their adequate CD212 and CD218a expression. We also attempted to activate tumor-infiltrating MAIT cells with SEB or Klebsiella pneumoniae lysate, albeit to little avail. By comparison, MAIT cells isolated from the healthy liver compartment produced substantial levels of IFN-γ in response to the combination of IL-12 and IL-18, SEB or Klebsiella lysate, and those residing in the tumor margin were only partially active. Signaling through CD212/CD218a is TCR-independent. SEB activates MAIT cells in a TCR/IL-12/IL-18-dependent but MR1-independent fashion [21], and Klebsiella lysate is a crude source of MR1-restricted TCR ligands of MAIT cells [22, 72]. The above findings led us to conclude that: (i) the physical proximity of MAIT cells to the CRLM TME can dictate their functional competence or incompetence; and (ii) MAIT cells’ dysfunctions within the CRLM lesions are seemingly broad-ranging and involve both TCR and cytokine receptor signaling pathways. Consequently, therapeutic targeting of each pathway alone may not be sufficient to overcome such impairments.
It should be noted that when we exposed MAIT cells to a combination of OKT3 (an anti-human CD3 mAb) and IL-12, they became undetectable, due perhaps to TCR internalization or apoptotic death, which can both result from cellular activation. Therefore, intrametastatic MAIT cells may have not completely lost their ability to respond to potent agonists and environmental cues.
In our study, the expression of GZM B and IFN-γ by MAIT cells followed the same pattern. Intratumoral MAIT cells produced moderately less GZM B when they were compared with those extracted from healthy hepatic tissue samples [47]. Finally, our analyses in a small sub-cohort of patients revealed only negligible IL-17A expression by MAIT cells regardless of their location within the liver. If reproducible in larger CRLM cohorts, this finding should indicate that the failure of MAIT cells to produce IFN-γ in the metastatic tumor bed represents broad functional impairments as opposed to a shift from an IFN-γ+ TH1-type to IL-17A+ TH17-type phenotype. We propose that the CRLM TME actively suppresses MAIT cells. We did not find any marked enhancement in the expression levels of several classic anergy/exhaustion markers [PD-1, T cell immunoglobulin and mucin-3 (TIM-3) and lymphocyte-activation gene 3 (LAG-3)] (our unpublished data). Whether soluble factors such as IL-10, TGF-β and PGE2 contribute directly or indirectly to defective MAIT cell responses within the CRLM TME warrants further investigation.
MAIT cells as therapeutic targets
MAIT cells constitute an attractive target in cancer immunotherapy for multiple reasons. First, they occupy strategic locations in mucosal layers, the primary sites of malignant transformation for many mucosa-associated cancers. Second, they are detectable in primary and metastatic cancers [42–47]. Third, human MAIT cells comprise a significant population of innate-like T cells in the peripheral blood and in the liver [9, 10], a common destination for circulating tumor cells. Therefore, exploiting MAIT cells’ anticancer potentials may keep cancer cells in check to prevent their spread to vital organs or even help eradicate established metastases. Fourth, many MAIT cells have effector memory-like characteristics [9] and can be quickly activated through TCR and cytokine receptor triggering [14]. Fifth, the ample expression of IFN-γ and cytotoxic effector molecules by many MAIT cells should enable them to participate in anticancer responses. Sixth, unlike conventional T cells that recognize peptide Ags displayed by the highly polymorphic MHC molecules, MAIT cells are restricted by the monomorphic molecule MR1 [4]. MR1-restricted ligands should thus work beyond the HLA restriction barrier in genetically diverse human populations. Seventh, MAIT cells heavily express multi-drug resistance protein 1 (MDR1), which renders them refractory to certain chemotherapeutic agents [9]. Therefore, they are likely to retain at least some of their effector functions post-chemotherapy.
Dusseaux et al. reported that MAIT cells were almost the only MDR1 expressors among effector memory CD4− T cell populations in the peripheral blood, which was consistent with their ability to effectively efflux the fluorescent dye rhodamine 123 [9]. Moreover, in a cohort of breast cancer patients, the absolute number of peripheral blood MAIT cells remained stable during and after treatment with six cycles of a chemotherapeutic regimen containing epirubicin, an anthracycline that is expelled by MDR1. This was unlike naïve or memory conventional T cell subsets whose blood counts dropped to varying degrees.
We recently demonstrated that preoperative chemotherapy with FOLFOX [Leucovorin Calcium (Folinic Acid)/5-Fluorouracil/Oxaliplatin] or with a combination of FOLFOX and bevacizumab does not lessen either the frequency or the IFN-γ production capacity of MAIT cells in the peripheral blood, healthy liver tissue or metastatic tumors of patients with CRLM [47].
MAIT cells are not equally resistant to all chemotherapeutic agents. In a longitudinal study, Novak et al. enumerated peripheral blood MAIT cells in patients with hematological malignancies who received a course of myeloablative conditioning (e.g., with a combination of carmustin, etoposide, cytarabine and melphalan) before they underwent autologous CD34+ stem cell transplantation [73]. The full numerical recovery of MAIT cells was achieved only in 33% and 44% of patients on days 60 and 100 post-transplantation, respectively. Although interesting, the findings of this study needs to be interpreted cautiously since a direct comparison between stem cell recipients with and without myeloablative conditioning was not possible. Nevertheless, combining MAIT cell agonists/stimuli with chemotherapies that do not deplete or incapacitate MAIT cells may be a tempting therapeutic option for some cancers.
Certain bacterial preparations and synthetic riboflavin precursor derivatives have been demonstrated to upregulate CD69, an early activation marker, on mouse MAIT cells in vivo [74]. MAIT cell Ags formed through non-enzymatic Schiff base condensation of 5-amino-6-d-ribitylaminouracil, a riboflavin synthesis intermediate, with α-dicarbonyl compounds arising from mammalian glycolysis or bacterial metabolism (e.g., glyoxal and methylglyoxal) are inherently unstable and prone to hydrolysis or spontaneous intramolecular cyclization when assembled in water [75]. However, preparation of such Ags in DMSO first was found to prevent their decomposition in water while retaining their in vitro and in vivo biological activities [75]. MAIT cell Ags with improved stability are logistically suitable for transportation, preparation and utilization in preclinical and clinical settings.
MAIT cell ligands are not limited to vitamin B metabolites of microbial origin. A recent study has revealed that a variety of readily available drugs and drug-like molecules, fragments and derivatives, including salicylates and diclofenac metabolites, can be loaded onto MR1 for presentation to MAIT cells [76]. Several such molecules were found to inhibit MAIT cell responses while a few others, most notably diclofenac metabolites, behaved agonistically towards these cells. Therefore, these molecules may serve to modulate MAIT cell responses in a variety of conditions including cancer.
Future investigations will need to address the efficacy of MAIT cell agonists, when delivered alone or in conjunction with chemotherapeutic agents and/or IL-12/IL-18, into TMEs. As a word of caution, systemic administration of MAIT cell ligands/stimuli may result in their hyperactivation followed by an anergic or exhausted state with compromised antimicrobial functions [21]. Therefore, supplementary treatment with antibiotics and/or checkpoint inhibitors may be warranted to ward off opportunistic infections.
Outstanding questions and concluding remarks
Despite intense recent investigations on MAIT cells, our understanding of their roles in malignancy is at a rudimentary level. MAIT cell are present in primary and metastatic tumors including CRC and their hepatic metastases. However, whether they play protective or pathogenic roles in various phases of cancer, from initial neoplastic transformation to formation of distant metastases, is far from clear. This may be dictated by the tissue localization of MAIT cells and the type of cytokines and other effector molecules they harbor. In this review, we have argued that preferential production of IFN-γ or IL-17A by MAIT cells likely promotes anticancer immunity or tumorigenesis, respectively (Fig. 1). However, one should never ignore the atypical or unexpected roles of these cytokines, especially IFN-γ, in malignancies [77, 78].
The presence of cytokine receptor-positive cells in distinct TMEs and their biological behavior in response to MAIT cell cytokines will need to be addressed. These could be tumor cells themselves, stromal and endothelial cells, and tumor-infiltrating effector, regulatory and suppressor cells of hematopoietic origin. Therefore, it is pertinent to explore the degree, nature and impact of cross-talk between MAIT cells and neighboring cells within TMEs. Such interactions may lead to bystander immune cell activation, collateral tissue damage and/or the spread of cancer.
MAIT cells typically express CD8 as their co-receptor, but CD4+ and CD4−CD8− minorities also exist. Furthermore, not all MR1-restricted T cells express TRAV1-2. Tetramer staining has revealed a subset of human MAIT cells that unusually use TRAV12-2 in their TCRα chain [79]. It will be important to compare various MAIT cell subsets for their anti- or pro-cancer potentials.
Dias et al. have recently demonstrated that MAIT cells exhibit biased TCR Vβ usage when dealing with different bacterial and fungal pathogens [25]. Interestingly, TCR Vβ usage by MAIT cells could also affect their MR1-restricted cytokine production when recognizing the same pathogen. Finally, the authors presented compelling evidence for functional heterogeneity of IL-12/IL-18-stimulated MAIT cells depending on their expression levels of NK cell-associated markers. To what extent this type of heterogeneity, flexibility or adaptation may be at play and biologically meaningful in cancer remains to be elucidated.
The chemoresistance of tissue-resident and tumor-penetrating MAIT cells, or lack thereof, should be comprehensively addressed as future treatments may benefit from combining MAIT cell ligands, relevant cytokines and chemotherapeutic agents. Finally, the role of MAIT cells at the interface between microbiome and cancer, especially in malignancies that originate from or metastasize to mucosal layers, remains an open question.
The availability of useful mouse models as experimental tools will enable mechanistic in vivo studies on MAIT cells and expedite progress in this area of research. Unlike humans, conventional mouse strains have only a tiny MAIT cell compartment [80]. This justified the generation of invariant Vα19 TCR transgenic (iVα19 tg) mice with an enlarged pool of MAIT cells [81]. These mice can be used as a source of MAIT cells for technical purposes and for in vitro studies. However, iVα19 tg mouse MAIT cells do not fully simulate those isolated from wild-type mice or humans [11]. Recently, CAST/EiJ mice were found to have an unusually high number of MAIT cells in their T cell repertoire [80], approximately 20 times more than in the commonly used C57BL/6J strain. The abundance of MAIT cells in mice is predetermined by their genetic background, not by the microbiota they harbor. Olivier Lantz’s group identified the region responsible for the increased frequency of MAIT cells in CAST/EiJ mice and created a congenic strain on the C57BL/6 background, namely B6-MAITCAST mice, in which MAIT cells are frequent [80]. These mice mimic humans in terms of MAIT cell frequencies and should, along with their MR1 knockout counterparts, provide valuable models in which to study MAIT cell roles in cancer.
Depending on whether MAIT cells are beneficial or detrimental to cancer patients/hosts, we propose that they can be activated or inhibited in therapeutic settings. MR1-restricted MAIT cell agonists and antagonists can be used in patients irrespective of the HLA allomorphs they express. MAIT cells’ resistance to certain chemotherapeutic drugs should permit the application of such agents in combination with MAIT cell agonists and stimulatory cytokines in systemic or tumor-targeted treatment regimens. The time is now ripe to study MAIT cell functions in malignancy and to potentially use the lessons to be learnt towards designing novel and efficient immunotherapies.
Acknowledgements
We thank Joshua Choi and Courtney Meilleur from the Haeryfar Laboratory for participating in helpful discussions on the theme of this review.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- Apc
Adenomatous polyposis coli
- Bcl-2
B-cell lymphoma 2
- CCL
C-C chemokine [ligand]
- CCR
C-C chemokine receptor
- CEA
Carcinoembryonic antigen
- CRC
Colorectal carcinoma
- CRLM
Colorectal liver metastasis
- CXCL
C-X-C chemokine [ligand]
- CXCR
C-X-C chemokine receptor
- ERK
Extracellular signal-regulated kinase
- FOLFOX
Leucovorin Calcium (Folinic Acid)/5-Fluorouracil/Oxaliplatin
- GZM
Granzyme
- IBD
Inflammatory bowel disease
- IL-17RA
IL-17 receptor A
- iNKT
Invariant natural killer T [cell]
- IP-10
IFN-γ-inducible protein of 10 kDa
- iTCRα
Invariant TCR α [chain]
- iVα19 tg
Invariant Vα19 TCR transgenic [mice]
- JNK
c-Jun N-terminal kinase
- LAG-3
Lymphocyte-activation gene 3
- MAIT
Mucosa-associated invariant T [cell]
- MDR1
Multi-drug resistance protein 1
- MIC
MHC class I polypeptide-related sequence
- MIG
Monokine induced by gamma interferon
- MMP
Matrix metalloproteinase
- MR1
MHC-related protein 1
- NKG2D
Natural-killer group 2, member D
- PGE2
Prostaglandin E2
- RORC
RAR related orphan receptor C
- SEB
Staphylococcal enterotoxin B
- TH1
T helper 1
- TH17
T helper 17
- TIM-3
T cell immunoglobulin and mucin-3
- TME(s)
Tumor microenvironment(s)
- TRAJ
T cell receptor alpha joining
- TRAV
T cell receptor alpha variable
- ULBP/RAET
UL16-binding protein/retinoic acid early transcript
- VEGF
Vascular endothelial growth factor
Author contributions
CRS and PTR participated in collection of the relevant literature and in preparation of the manuscript. SMMH conceived the theme and organization of the manuscript, participated in collection of the relevant literature, and wrote the manuscript.
Funding
The authors’ cited research on the subject was partially funded by a Canadian Institutes of Health Research (CIHR) operating grant (MOP-130465) to S.M. Mansour Haeryfar. Christopher R. Shaler is a CIHR postdoctoral fellowship recipient.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4 + and CD4-8- T cells in mice and humans. J Exp Med. 1994;180(3):1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189(12):1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi H, Hirai M, Kurosawa Y, Hashimoto K. A highly conserved major histocompatibility complex class I-related gene in mammals. Biochem Biophys Res Commun. 1997;238(3):697–702. doi: 10.1006/bbrc.1997.7379. [DOI] [PubMed] [Google Scholar]
- 6.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 7.Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, Soudais C, Lantz O. Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol. 2013;191(12):6002–6009. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 8.Koay HF, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, Russ BE, Nold-Petry CA, Nold MF, Bedoui S, Chen Z, Corbett AJ, Eckle SB, Meehan B, d’Udekem Y, Konstantinov IE, Lappas M, Liu L, Goodnow CC, Fairlie DP, Rossjohn J, Chong MM, Kedzierska K, Berzins SP, Belz GT, McCluskey J, Uldrich AP, Godfrey DI, Pellicci DG. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17(11):1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 9.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 10.Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, Lee KH, Gehring AJ, De Libero G, Bertoletti A. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 11.Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SB, Meehan B, Chen Z, Whittle B, Liu L, Fairlie DP, Goodnow CC, McCluskey J, Rossjohn J, Uldrich AP, Pellicci DG, Godfrey DI. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212(7):1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, Uldrich AP, Birkinshaw RW, Patel O, Kostenko L, Meehan B, Kedzierska K, Liu L, Fairlie DP, Hansen TH, Godfrey DI, Rossjohn J, McCluskey J, Kjer-Nielsen L. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210(11):2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van Sinderen D, Mak JY, Fairlie DP, Kjer-Nielsen L, Rossjohn J, McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 14.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P, Willberg CB. CD161 + + CD8 + T cells, including the MAIT cell subset, are specifically activated by IL-12 + IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salou M, Franciszkiewicz K, Lantz O. MAIT cells in infectious diseases. Curr Opin Immunol. 2017;48:7–14. doi: 10.1016/j.coi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 16.van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, de Lara C, Cole S, Vasanawathana S, Limpitikul W, Malasit P, Young D, Denney L, consortium Moore S-H, Fabris MD, Giordani P, Oo MT, Laidlaw YH, Dustin SM, Ho LB, Thompson LP, Ramamurthy FM, Mongkolsapaya N, Willberg J, Screaton CB, Klenerman GR. MAIT cells are activated during human viral infections. Nat Commun. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J, Xu J, Doherty PC, McCluskey J, Kedzierska K. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci USA. 2016;113(36):10133–10138. doi: 10.1073/pnas.1610750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattler A, Dang-Heine C, Reinke P, Babel N. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur J Immunol. 2015;45(8):2286–2298. doi: 10.1002/eji.201445313. [DOI] [PubMed] [Google Scholar]
- 19.Leeansyah E, Svard J, Dias J, Buggert M, Nystrom J, Quigley MF, Moll M, Sonnerborg A, Nowak P, Sandberg JK. Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 infection. PLoS Pathog. 2015;11(8):e1005072. doi: 10.1371/journal.ppat.1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaan M, Hullegie SJ, Beudeker BJ, Kreefft K, van Oord GW, Groothuismink ZM, van Tilborg M, Rijnders B, de Knegt RJ, Claassen MA, Boonstra A. Frequencies of circulating MAIT Cells are diminished in chronic HCV, HIV and HCV/HIV co-infection and do not recover during therapy. PLoS ONE. 2016;11(7):e0159243. doi: 10.1371/journal.pone.0159243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaler CR, Choi J, Rudak PT, Memarnejadian A, Szabo PA, Tun-Abraham ME, Rossjohn J, Corbett AJ, McCluskey J, McCormick JK, Lantz O, Hernandez-Alejandro R, Haeryfar SMM. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol. 2017;15(6):e2001930. doi: 10.1371/journal.pbio.2001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, Core M, Sleurs D, Serriari NE, Treiner E, Hivroz C, Sansonetti P, Gougeon ML, Soudais C, Lantz O. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9(10):e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, Klenerman P. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8(2):429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci USA. 2017;114(27):E5434E5443. doi: 10.1073/pnas.1705759114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, Da Silva J, Allatif O, Rossjohn J, Kjer-Nielsen L, McCluskey J, Ledoux S, Genser L, Torcivia A, Soudais C, Lantz O, Boitard C, Aron-Wisnewsky J, Larger E, Clement K, Lehuen A. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest. 2015;125(4):1752–1762. doi: 10.1172/JCI78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, Geoghegan J, Cody D, O’Connell J, Winter DC, Doherty DG, Lynch L, O’Shea D, Hogan AE. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194(12):5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 28.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett. 2012;148(2):144–150. doi: 10.1016/j.imlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhong F, Cui D, Tao H, Du H, Xing C. IL-17A-producing T cells and associated cytokines are involved in the progression of gastric cancer. Oncol Rep. 2015;34(5):2365–2374. doi: 10.3892/or.2015.4246. [DOI] [PubMed] [Google Scholar]
- 32.Madkouri R, Kaderbhai CG, Bertaut A, Truntzer C, Vincent J, Aubriot-Lorton MH, Farah W, Limagne E, Ladoire S, Boidot R, Derangere V, Ghiringhelli F. Immune classifications with cytotoxic CD8(+) and Th17 infiltrates are predictors of clinical prognosis in glioblastoma. Oncoimmunology. 2017;6(6):e1321186. doi: 10.1080/2162402X.2017.1321186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Z, Qu Y, Leng Y, Sun W, Ma S, Wei J, Hu J, Zhang X. Human colon carcinogenesis is associated with increased interleukin-17-driven inflammatory responses. Drug Des Devel Ther. 2015;9:1679–1689. doi: 10.2147/DDDT.S79431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93(1):39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98(2):189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Huang Q, Duan L, Qian X, Fan J, Lv Z, Zhang X, Han J, Wu F, Guo M, Hu G, Du J, Chen C, Jin Y. IL-17 promotes angiogenic factors IL-6, IL-8, and Vegf production via Stat1 in lung adenocarcinoma. Sci Rep. 2016;6:36551. doi: 10.1038/srep36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulig P, Burkhard S, Mikita-Geoffroy J, Croxford AL, Hovelmeyer N, Gyulveszi G, Gorzelanny C, Waisman A, Borsig L, Becher B. IL17A-mediated endothelial breach promotes metastasis formation. Cancer Immunol Res. 2016;4(1):26–32. doi: 10.1158/2326-6066.CIR-15-0154. [DOI] [PubMed] [Google Scholar]
- 38.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, Chen Z, Wang K, Zhang T, Xu J, Han Y, Zhang T, Wu X, Wang J, Gong W, Zheng S, Qiu F, Yan J, Huang J. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu G, Wu P, Cheng P, Zhang Z, Wang Z, Yu X, Shao X, Wu D, Ye J, Zhang T, Wang X, Qiu F, Yan J, Huang J. Tumor-infiltrating CD39(+)gammadeltaTregs are novel immunosuppressive T cells in human colorectal cancer. Oncoimmunology. 2017;6(2):e1277305. doi: 10.1080/2162402X.2016.1277305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, Ren F, Liao H, Pu Q, Wang T, You Z. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7(7):1091–1100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Liu L, Zhang Q, Liu S, Ge D, You Z. Interleukin-17 indirectly promotes M2 macrophage differentiation through stimulation of COX-2/PGE2 pathway in the cancer cells. Cancer Res Treat. 2014;46(3):297–306. doi: 10.4143/crt.2014.46.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, Szekeres-Bartho J, Szereday L, Illes Z. Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. 2008;20(12):1517–1525. doi: 10.1093/intimm/dxn111. [DOI] [PubMed] [Google Scholar]
- 43.Sundstrom P, Ahlmanner F, Akeus P, Sundquist M, Alsen S, Yrlid U, Borjesson L, Sjoling A, Gustavsson B, Wong SB, Quiding-Jarbrink M. Human mucosa-associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN-gamma. J Immunol. 2015;195(7):3472–3481. doi: 10.4049/jimmunol.1500258. [DOI] [PubMed] [Google Scholar]
- 44.Zabijak L, Attencourt C, Guignant C, Chatelain D, Marcelo P, Marolleau JP, Treiner E. Increased tumor infiltration by mucosal-associated invariant T cells correlates with poor survival in colorectal cancer patients. Cancer Immunol Immunother. 2015;64(12):1601–1608. doi: 10.1007/s00262-015-1764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, Li M, Ni J, Li C, Wang L, Jiang Y. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. 2016;6:20358. doi: 10.1038/srep20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Won EJ, Ju JK, Cho YN, Jin HM, Park KJ, Kim TJ, Kwon YS, Kee HJ, Kim JC, Kee SJ, Park YW. Clinical relevance of circulating mucosal-associated invariant T cell levels and their anti-cancer activity in patients with mucosal-associated cancer. Oncotarget. 2016;7(46):76274–76290. doi: 10.18632/oncotarget.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaler CR, Tun-Abraham ME, Skaro AI, Khazaie K, Corbett AJ, Mele T, Hernandez-Alejandro R, Haeryfar SMM. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol Immunother. 2017;66(12):1563–1575. doi: 10.1007/s00262-017-2050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, Dupas JL, Treiner E. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176(2):266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haga K, Chiba A, Shibuya T, Osada T, Ishikawa D, Kodani T, Nomura O, Watanabe S, Miyake S. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol. 2016;31(5):965–972. doi: 10.1111/jgh.13242. [DOI] [PubMed] [Google Scholar]
- 50.Chehimi Marwa, Vidal Hubert, Eljaafari Assia. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. Journal of Clinical Medicine. 2017;6(7):68. doi: 10.3390/jcm6070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slattery ML, Lundgreen A, Bondurant KL, Wolff RK. Interferon-signaling pathway: associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis. 2011;32(11):1660–1667. doi: 10.1093/carcin/bgr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu S, Pardini B, Cheng B, Naccarati A, Huhn S, Vymetalkova V, Vodickova L, Buchler T, Hemminki K, Vodicka P, Forsti A. Single nucleotide polymorphisms within interferon signaling pathway genes are associated with colorectal cancer susceptibility and survival. PLoS ONE. 2014;9(10):e111061. doi: 10.1371/journal.pone.0111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Wang Y, Song Z, Chu J, Qu X. Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. J Interferon Cytokine Res. 2015;35(4):273–280. doi: 10.1089/jir.2014.0132. [DOI] [PubMed] [Google Scholar]
- 54.Yuan L, Zhou C, Lu Y, Hong M, Zhang Z, Zhang Z, Chang Y, Zhang C, Li X. IFN-gamma-mediated IRF1/miR-29b feedback loop suppresses colorectal cancer cell growth and metastasis by repressing IGF1. Cancer Lett. 2015;359(1):136–147. doi: 10.1016/j.canlet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Reeves E, James E. Antigen processing and immune regulation in the response to tumours. Immunology. 2017;150(1):16–24. doi: 10.1111/imm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen W, Masterman KA, Basta S, Haeryfar SM, Dimopoulos N, Knowles B, Bennink JR, Yewdell JW. Cross-priming of CD8 + T cells by viral and tumor antigens is a robust phenomenon. Eur J Immunol. 2004;34(1):194–199. doi: 10.1002/eji.200324257. [DOI] [PubMed] [Google Scholar]
- 57.Deauvieau F, Ollion V, Doffin AC, Achard C, Fonteneau JF, Verronese E, Durand I, Ghittoni R, Marvel J, Dezutter-Dambuyant C, Walzer T, Vie H, Perrot I, Goutagny N, Caux C, Valladeau-Guilemond J. Human natural killer cells promote cross-presentation of tumor cell-derived antigens by dendritic cells. Int J Cancer. 2015;136(5):1085–1094. doi: 10.1002/ijc.29087. [DOI] [PubMed] [Google Scholar]
- 58.Jeannin P, Duluc D, Delneste Y. IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: regulation by IFN-gamma. Immunotherapy. 2011;3(4 Suppl):23–26. doi: 10.2217/imt.11.30. [DOI] [PubMed] [Google Scholar]
- 59.Sun T, Yang Y, Luo X, Cheng Y, Zhang M, Wang K, Ge C. Inhibition of tumor angiogenesis by interferon-gamma by suppression of tumor-associated macrophage differentiation. Oncol Res. 2014;21(5):227–235. doi: 10.3727/096504014X13890370410285. [DOI] [PubMed] [Google Scholar]
- 60.Naganuma H, Sasaki A, Satoh E, Nagasaka M, Nakano S, Isoe S, Nukui H. Down-regulation of transforming growth factor-beta and interleukin-10 secretion from malignant glioma cells by cytokines and anticancer drugs. J Neurooncol. 1998;39(3):227–236. doi: 10.1023/A:1005902120612. [DOI] [PubMed] [Google Scholar]
- 61.Beatty GL, Paterson Y. Regulation of tumor growth by IFN-gamma in cancer immunotherapy. Immunol Res. 2001;24(2):201–210. doi: 10.1385/IR:24:2:201. [DOI] [PubMed] [Google Scholar]
- 62.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87(9):3877–3882. [PubMed] [Google Scholar]
- 63.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, Gri G, Wysocka M, Kim JE, Liu L, Liao F, Farber JM, Pestka S, Trinchieri G, Lee WM. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9(1):25–34. doi: 10.1016/S1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 64.Bukowski RM, Rayman P, Molto L, Tannenbaum CS, Olencki T, Peereboom D, Tubbs R, McLain D, Budd GT, Griffin T, Novick A, Hamilton TA, Finke J. Interferon-gamma and CXC chemokine induction by interleukin 12 in renal cell carcinoma. Clin Cancer Res. 1999;5(10):2780–2789. [PubMed] [Google Scholar]
- 65.Lopez-Soto A, Huergo-Zapico L, Acebes-Huerta A, Villa-Alvarez M, Gonzalez S. NKG2D signaling in cancer immunosurveillance. Int J Cancer. 2015;136(8):1741–1750. doi: 10.1002/ijc.28775. [DOI] [PubMed] [Google Scholar]
- 66.McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, Trowsdale J, Durrant LG. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res. 2009;15(22):6993–7002. doi: 10.1158/1078-0432.CCR-09-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanier LL. NKG2D Receptor and its ligands in host defense. Cancer Immunol Res. 2015;3(6):575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brozova J, Karlova I, Novak J. Analysis of the phenotype and function of the subpopulations of mucosal-associated invariant T cells. Scand J Immunol. 2016;84(4):245–251. doi: 10.1111/sji.12467. [DOI] [PubMed] [Google Scholar]
- 69.Mullbacher A, Lobigs M, Hla RT, Tran T, Stehle T, Simon MM. Antigen-dependent release of IFN-gamma by cytotoxic T cells up-regulates Fas on target cells and facilitates exocytosis-independent specific target cell lysis. J Immunol. 2002;169(1):145–150. doi: 10.4049/jimmunol.169.1.145. [DOI] [PubMed] [Google Scholar]
- 70.Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, Lee B, Poidinger M, Zolezzi F, Quagliata L, Sander P, Newell E, Bertoletti A, Terracciano L, De Libero G, Mori L. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 71.Kucharzik T, Lugering N, Winde G, Domschke W, Stoll R. Colon carcinoma cell lines stimulate monocytes and lamina propria mononuclear cells to produce IL-10. Clin Exp Immunol. 1997;110(2):296–302. doi: 10.1111/j.1365-2249.1997.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 73.Novak J, Dobrovolny J, Brozova J, Novakova L, Kozak T. Recovery of mucosal-associated invariant T cells after myeloablative chemotherapy and autologous peripheral blood stem cell transplantation. Clin Exp Med. 2016;16(4):529–537. doi: 10.1007/s10238-015-0384-z. [DOI] [PubMed] [Google Scholar]
- 74.Soudais C, Samassa F, Sarkis M, Le Bourhis L, Bessoles S, Blanot D, Herve M, Schmidt F, Mengin-Lecreulx D, Lantz O. In vitro and in vivo analysis of the gram-negative bacteria-derived riboflavin precursor derivatives activating mouse MAIT cells. J Immunol. 2015;194(10):4641–4649. doi: 10.4049/jimmunol.1403224. [DOI] [PubMed] [Google Scholar]
- 75.Mak JY, Xu W, Reid RC, Corbett AJ, Meehan BS, Wang H, Chen Z, Rossjohn J, McCluskey J, Liu L, Fairlie DP. Stabilizing short-lived Schiff base derivatives of 5-aminouracils that activate mucosal-associated invariant T cells. Nat Commun. 2017;8:14599. doi: 10.1038/ncomms14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keller AN, Eckle SB, Xu W, Liu L, Hughes VA, Mak JY, Meehan BS, Pediongco T, Birkinshaw RW, Chen Z, Wang H, D’Souza C, Kjer-Nielsen L, Gherardin NA, Godfrey DI, Kostenko L, Corbett AJ, Purcell AW, Fairlie DP, McCluskey J, Rossjohn J. Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat Immunol. 2017;18(4):402–411. doi: 10.1038/ni.3679. [DOI] [PubMed] [Google Scholar]
- 77.Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17(19):6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kursunel MA, Esendagli G. The untold story of IFN-gamma in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81. doi: 10.1016/j.cytogfr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Meermeier EW, Laugel BF, Sewell AK, Corbett AJ, Rossjohn J, McCluskey J, Harriff MJ, Franks T, Gold MC, Lewinsohn DM. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat Commun. 2016;7:12506. doi: 10.1038/ncomms12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui Y, Franciszkiewicz K, Mburu YK, Mondot S, Le Bourhis L, Premel V, Martin E, Kachaner A, Duban L, Ingersoll MA, Rabot S, Jaubert J, De Villartay JP, Soudais C, Lantz O. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J Clin Invest. 2015;125(11):4171–4185. doi: 10.1172/JCI82424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7(3):e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]