Abstract
5T4 is a tumor associated antigen that is expressed on the surface of a wide spectrum of human adenocarcinomas. The highly attenuated virus, modified vaccinia Ankara, has been engineered to express human 5T4 (h5T4). In a pre-clinical murine model, the recombinant virus (TroVax) induces protection against challenge with CT26–h5T4 (a syngeneic tumor line expressing h5T4). Anti-tumor activity is long lived, with protection still evident 6 months after the final vaccination. In a therapeutic setting, injection of mice with TroVax results in a reduction in tumor burden of >90%. Depletion of CD8+ T cells has no effect upon therapy in the active treatment model, whereas depletion of CD4+ T cells completely abrogates anti-tumor activity. In a prophylactic setting, depletion of CD4+ and CD8+ T cells after the induction of a h5T4 immune response has no deleterious effect on protection following challenge with CT26–h5T4. In light of these studies, the role of antibodies in protection against tumor challenge was investigated. 5T4 specific polyclonal serum decreased tumor burden by approximately 70%. Thus, we conclude that CD4+ T cells are essential for the induction of a protective immune response and that antibodies are the likely effector moiety in this xenogeneic murine tumor model.
Keywords: Rodent, T lymphocytes, Antibodies, Tumor immunity
Introduction
Cancer immunotherapy is dependent on the induction of an immune response which is capable of killing tumor cells but inducing no, or limited, deleterious autoimmune reactions. The identification of a tumor associated antigen (TAA) which is expressed on the tumor target (both primary and metastases) but absent from most normal tissues, is important for safe and effective immunotherapy. The human oncofetal antigen h5T4 is a 72 kDa surface glycoprotein containing leucine-rich repeats that is expressed at high levels on the placenta and also on a wide range of human carcinomas including colorectal, gastric and ovarian, but rarely on normal tissues [17, 20]. Human 5T4 is frequently expressed on metastases and such expression shown to be associated with poor prognosis [24]. Its restricted expression on normal tissues and high prevalence on many common human carcinomas make h5T4 an attractive target for cancer immunotherapy. In addition, its surface expression means that it could potentially be a target for both cytotoxic T cells (CTL) and antibody-mediated immune responses.
Identification of the protective mechanisms operating against tumors in vivo is important to enable optimization of immunotherapeutic approaches for the treatment of cancers. Several studies using murine tumor models have shown that either CD4+ [11, 22, 25] or CD8+ [1, 15, 28] T cells play crucial roles in the induction of efficacious anti-tumor immune responses. In order to achieve potent cellular and humoral responses, the target antigen(s) must be delivered in an optimal configuration. In this respect, recombinant poxviruses have been shown to be effective vectors for cancer immunotherapy in pre-clinical models [for review see 6] and those based on the highly attenuated vaccinia virus strain modified vaccinia Ankara (MVA), have shown efficacy in both infectious disease and cancer vaccine models [4, 18, 26]. To this end, we have used MVA as a vaccine vector to deliver h5T4. Previously, a recombinant MVA encoding h5T4 and a LacZ marker gene (MVA–h5T4), both inserted into deletion region II, was used in pre-clinical models [19]. Vaccination of mice with MVA–h5T4 induced a high degree of protection against challenge with a murine tumor cell line expressing h5T4 [19]. Whilst antibodies specific for h5T4 have been shown to mediate killing of tumor cells in vitro [21], no study has yet dissected the protective immune responses occurring in h5T4 tumor models in vivo. Our aim was to dissect the immune response occurring in this pre-clinical model using a vector which produced greater quantities of recombinant protein at an early stage in the infection cycle (thought to be important for the induction of more potent immune responses [3, 10]) and which was suitable for use in human clinical trials. Here we report the production of a recombinant MVA which expresses h5T4 at a higher level than the previously used MVA–h5T4 vector and which is suitable for clinical use (TroVax). Furthermore, we have dissected the protective immune response occurring in the pre-clinical murine tumor model and discuss the findings in the context of tumor immunotherapy.
Materials and methods
Animals and tumor cell lines
All animals used in this study were female Balb/c mice aged 8–10 weeks obtained from Harlan (Bicester, Oxon, UK) and maintained according to UK Home Office guidelines. Unless stated otherwise, all experiments used seven to ten mice per group. CT26 is a chemically induced colon carcinoma of Balb/c origin [2]. A stable CT26 cell line expressing human 5T4 (CT26–h5T4) was constructed with h5T4 cDNA, using the eukaryotic expression vector pIRES [19]. The CT26–CL25 cell line [30] stably expresses the E. coli LacZ gene.
Production of the recombinant viral vector (TroVax)
For insertion into deletion region III, a transfer vector, pTrV1b was constructed with the h5T4 coding sequence under the control of the modified H5 (mH5) promoter [31] and flanked by sequences homologous to deletion region III, into the plasmid pNEB 193 (New England Biolabs, Hitchin, Herts, UK). Cloning was carried out in the following sequence—Flank 1 was amplified by PCR using the following primer pairs:
5′ ggccaagcttGATAAAGCGTGTGCGTGTATAGAG 3′ +
5′ ccggctgcagctcgagTACCAGCCACCGAAAGAG 3′
(the HindIII and PstI sites used for cloning are underlined).
The following primer pairs were used for Flank 2:
5′ gcgcggtaccgctagcggcgcgccTTTGGAAAGTTTTATAGGTAGTTG 3′ +
5′ ccggaattcAACTAGTTTCCGGTGAATGTG 3′ (KpnI and EcoRI sites used for cloning are underlined). The mH5 promoter was produced by annealing the following two oligonucleotides:
tcgacTATTTATGATTATTTCTCGCTTTCAATTTAACACAACCCTCAAGAACCTTTGTATTTATTTTCAATTTTTaat
and
tAAAAATTGAAAATAAATACAAAGGTTCTTGAGGGTTGTGTTAAATTGAAAGCGAGAAATAATCATAAATAg
leaving PacI and SalI restriction enzyme site over hangs for cloning into the plasmid. The 5T4 coding region was amplified from the cloned cDNA using the following primers:
ttaagcggccgcAACCGCGAGCCGCGATG +
ccggctcgagTCAGACATCCGAGTTAGAAC,
(NotI and XhoI sites are underlined). The digested product was cloned into an intermediate cloning vector, excised with PmeI (upstream of the NotI site) and XhoI and ligated into the transfer vector.
The recombinant MVA virus expressing h5T4 (TroVax) was produced in chick embryo fibroblasts (CEFs) by concomitant infection with wild-type MVA (p581, a gift from NIH, Bethesda, MD) and transfection with pTrV1b.
Monolayers of CEFs (obtained from SPAFAS eggs by Charles River SPAFAS, USA and supplied by the Veterinary Laboratories Agency, Weybridge, Surrey, UK) were infected with wild-type MVA at a multiplicity of infection of 0.2 and after 1 h at 37°C, the virus was removed. The cells were transfected with pTrv1b (2 μg/well) using Fugene 6 (Boehringer Mannheim, Welwyn Garden City, Herts, UK) according to the manufacturers protocol.
Cells were harvested at 48 h post-transfection, and processed as described by Earl et al. [9]. Human 5T4 positive recombinant viral foci were detected by live immunostaining with H8 antibody and picked with sterile toothpicks. Overall the TroVax virus was subjected to a total of six rounds of plaque purification and two rounds of centrifugation through a sucrose cushion [9].
Comparison of protein expression by two vaccinia virus recombinants
Prior to experimental use, a comparison of h5T4 protein expression by MVA–h5T4 and the newly engineered TroVax vector was undertaken. CEFs were infected at an MOI of 1 with either MVA–h5T4 or TroVax, harvested 3 days later and resuspended in 1 ml PBS and stored frozen. Subsequently, samples were thawed and passed several times through a fine gauge needle, diluted in Laemmli buffer and heated to 95°C for 5 min. Samples were subjected to SDS-PAGE under non-reducing conditions on 4–20% Tris–glycine gels (Novagen, Nottingham, Notts, UK) along with known quantities of purified recombinant h5T4. Proteins were electro-blotted to Hybond P membrane (Amersham, Little Chalfont, Bucks, UK) and detected using the anti-h5T4 mAb (H8), followed by anti-mouse Ig FITC conjugate and subsequently sheep anti-FITC alkaline phosphatase conjugate. Blots were developed using ECF reagent (Amersham) scanned in a phosphorimager (Molecular Devices, Winnersh, Wokingham, UK) and the relative amounts of protein compared using Image Quant software (Molecular Dynamics, Washington, DC).
Recombinant protein production
Full-length h5T4 cDNA, lacking a termination codon, was cloned into pBluescript (Stratagene, La Jolla, CA) in-frame with a C-terminal enterokinase site, Myc epitope and His tag to enable subsequent detection and purification. The entire tagged h5T4 cassette was then sub-cloned into the pIRES-Neo vector (Clontech, Oxford, Oxfordshire, UK) and used to transfect CHO cells. Subsequently, a stable cell line expressing high levels of h5T4 (CHO–h5T4) was expanded in roller bottles in DMEM F12 Ham containing 1× MEM non-essential amino acids, glutamine (2 mM), FBS (10%) (all Sigma, Poole, Dorset, UK) and G418 (1 mg/ml) until confluent. 5T4 protein was purified by loading CHO–h5T4 cell lysates, solubilized in 8 M Urea, on to a pre-equilibrated Nickel HisTrap column (Pharmacia, Little Chalfont, Bucks, UK) and washing with phosphate buffer containing increasing concentrations of imidazole. Eluates were analyzed for protein content and those containing h5T4 were pooled and dialyzed extensively against PBS before a final analysis of protein concentration (Bradford assay), purity (silver stain SDS-PAGE) and integrity (western blot analysis with an anti-h5T4 monoclonal antibody) was undertaken.
Antisera and antibodies
Vaccinated mouse serum
Sera were recovered from groups of mice which had received two intraperitoneal (i.p.) vaccinations at 2 weekly intervals with 107 pfu MVA–LacZ (recombinant MVA expressing β-galactosidase) or TroVax diluted in formulation buffer (10 mM Tris, 140 mM NaCl, pH 7.7). A number of mice from within the same groups were challenged with the tumor cell line CT26–h5T4 to ensure that the group which had received TroVax were highly protected against tumor challenge (data not shown). Serum was stored at −20°C until required for use in passive transfer experiments.
Monoclonal antibody
The murine IgG1 monoclonal antibody H8 [17] recognizes a conformational epitope in h5T4. Prior to use in passive transfer experiments, the antibody was purified from hybridoma culture supernatant on a protein G column, dialyzed extensively and diluted to 150 μg/ml in sterile PBS.
Depletion monoclonal antibodies
Monoclonal antibodies specific for CD4 (YTS 191.1) and CD8 (YTS 169.4) were precipitated from hybridoma supernatants using a saturated solution of ammonium sulfate (70%). The precipitates were re-dissolved in PBS and dialyzed extensively against fresh PBS (both antibodies were a kind gift from Dr. Gould and Dr. Jones, Centre for Ecology and Hydrology, Oxford University, Oxford, UK).
Vaccination regimes
Antigen dose–response analysis
Groups of mice were injected at 0 and 4 weeks, via the i.p. route with either 105, 106 or 107 pfu MVA–LacZ or TroVax. Blood samples were taken 2 weeks after the final injection. The mice were then kept for 6 months and a further blood sample taken at 6 months to analyze the longevity of the antibody response. Subsequently, mice were challenged with a murine tumor line to determine their long-term protection against tumor growth, as detailed below.
Prophylactic treatment
Groups of mice were injected via the intra-peritoneal route with 107 pfu MVA–LacZ or TroVax on day 0 and again on day 14. Test bleeds were taken from tail veins 7–10 days after each vaccination and antibody titers determined by ELISA. Mice were challenged intravenously (i.v.) on day 28 with 5×105 cells of the syngeneic tumor line CT26–h5T4 suspended in 300 μl PBS. Twelve days post-challenge mice were euthanized, their lungs inflated with Indian ink and the number of tumor nodules counted.
Active treatment
Groups of mice were challenged i.v. on day 0 with 5×105 CT26–h5T4 cells. On days 3 and 10 post-challenge, mice were injected i.p. with 107 pfu MVA–LacZ or TroVax. Two days later (day 12) mice were euthanized, their lungs inflated with Indian ink and the number of nodules counted.
CD4+/CD8+ T cell depletion
Active treatment
Groups of mice received 6 mg (in 300 μl) αCD4 or αCD8 depletion antibodies via tail vein injection on day -3. On day 0, mice were challenged with 5×105 CT26–h5T4 cells by i.v. tail injection. Mice received 107 pfu MVA–LacZ or TroVax by i.p. injection on days 3 and 10. On day 12, mice were euthanized, the lungs inflated with Indian ink and the number of lung nodules counted. In addition, spleens were sampled to assess the efficiency of CD4+ and CD8+ T cell depletion by FACS analysis (data not shown).
Prophylactic treatment
To determine whether a pre-existing immune response was affected by depletion of CD4+ and CD8+ T cells, groups of mice received two injections of MVA–LacZ or TroVax on days 0 and 20. Mice were then challenged with CT26–h5T4 cells and 1 day later received 6 mg depletion antibodies. Animals were euthanized 12 days post-challenge and the number of lung nodules determined. The efficiency of CD4+ and CD8+ T cell depletion was again monitored by FACS.
Passive transfer of monoclonal and polyclonal antibodies
Groups of mice were challenged i.v. on day 0 with 5×105 CT26–h5T4 cells. On days 3 and 10, mice were injected i.v. with 50 μg H8 monoclonal antibody or with 300 μl neat serum recovered from animals vaccinated twice with MVA–LacZ or TroVax. On day 12, mice were euthanized and the number of lung nodules determined following staining with Indian ink.
Measurement of antibody titers
A standard ELISA was used for measurement of both h5T4 and MVA specific antibody titers. Briefly, 96-well plates (Immulon-4; Dynex, Worthing, West Sussex, UK) were coated overnight at 4°C in a humid environment with purified recombinant h5T4 (1 μg/ml) or MVA (5×106 pfu/ml) diluted in carbonate coating buffer pH 9.6. Following blocking with PBS + FBS (10%), primary mouse sera was diluted serially across the plate in PBS–Tween and incubated for 2 h at room temperature. Wells were then washed five times in PBS–Tween and incubated with a goat anti-mouse Ig HRP secondary antibody (DAKO) for 2 h at room temperature. Wells were washed five times with PBS–Tween and incubated with an OPD substrate (OPD-Fast; Sigma). The colorimetric change was monitored using an automated plate reader (Dynex).
Measurement of T cell responses
The cellular response induced following vaccination of mice with TroVax was monitored by IFNγ ELISPOT. Mice were vaccinated twice at 2 week intervals with 107 pfu MVA–LacZ or TroVax and splenocytes harvested 6 days after the second injection. Whole splenocytes were incubated in pre-coated IFNγ ELISPOT plates (Mabtech ELISPOT kit; Nacka Strand, Sweden; Millipore plates, Watford, Herts, UK). The antigens used to re-stimulate the splenocytes in vitro included CT26 cells stably expressing β-galactosidase (CT26–CL25) or h5T4 (CT26–h5T4) which had been pre-treated with Mitomycin C (Sigma; 75 μg/ml for 1 h at 37°C). In addition, a known H-2d restricted β-galactosidase peptide (TPHPARIGL; 12) was used. Splenocytes and antigens were co-cultured overnight at 37°C, 5% CO2 after which, plates were handled according to the manufacturer’s instructions. All plates were then read using an automated ELISPOT plate reader (AID, Strassberg, Germany).
Statistical analysis
Welch’s t-test was used to compare the effect of different treatment regimens on therapeutic outcome in both prophylactic and active treatment models in Balb/c mice. The Welch modification of Student’s two sample t-test permits the variance in the two groups to be different. Statistical analysis was undertaken using S-PLUS (R) version 6 software for Windows (Insightful Corporation, Seattle, WA).
Results
Generation of recombinant MVA expressing h5T4 (TroVax)
The transfer plasmid pTrV1b (Fig. 1) was used successfully to produce the TroVax recombinant. Integration of a single copy of both the H5 promoter and h5T4 coding sequence into the desired site of deletion region III of the virus was confirmed by both Southern blotting and sequencing of viral DNA (data not shown).
Fig. 1.
Site of transgene integration in TroVax. A schematic representation of the MVA genome showing HindIII restriction sites. The h5T4 cDNA, mH5 promoter and flanking regions of the transfer vector pTrV1b are integrated into the 164R gene of deletion region III. The site of the native H5 gene is also shown
Analysis and quantification of h5T4 expression by TroVax
In order to compare the h5T4 expression levels by the two vaccinia recombinants MVA–h5T4 and TroVax, CEFs were infected with each viral vector at an MOI of 1. Lysates of the infected cells were then analyzed by SDS-PAGE followed by western blotting using ECF and phosphorimage detection (Fig. 2). The relative intensities (volume) of the bands were measured and compared to a standard curve derived from known amounts of recombinant human 5T4 protein. The average expression level of h5T4 from TroVax was approximately eight times higher than that obtained for the MVA–h5T4 virus. Similar results were also obtained from infection of 293T human embryonic kidney cells (data not shown). In addition, the integrity of the expressed protein was confirmed by comparison to both molecular weight markers and to the purified recombinant protein.
Fig. 2.
Comparison of h5T4 expression in vitro by MVA–h5T4 and TroVax. Both recombinant viruses were expressed in CEF cells and infected cell lysates separated subsequently by SDS-PAGE. Western blotting, ECF detection and phosphorimage analysis were carried out. The immunoblot illustrates the 5T4 expression by CEF cells infected with either TroVax (lane 1), MVA–h5T4 (lane 2) or wild-type MVA (lane 4) as well as uninfected CEFs (lane 5). Known amounts of recombinant h5T4 (37.5, 75, 125 and 250 ng in lanes 6, 7, 8 and 9, respectively) were used to facilitate quantification. Molecular weight markers are illustrated
TroVax induces a potent h5T4-specific antibody, but weak IFNγ ELISPOT response
To analyze the ex vivo T cell response induced following two injections with MVA–LacZ or TroVax, an IFNγ ELISPOT assay was used. Results illustrated in Fig. 3 demonstrate the induction of a potent β-galactosidase specific response in mice vaccinated with MVA–LacZ but a much weaker response to h5T4 in TroVax immunized mice.
Fig. 3.
IFNγ ELISPOT demonstrating that TroVax induces a weak h5T4 specific cellular response, whereas MVA–LacZ induces a potent β-galactosidase specific response. Whole splenocytes from mice vaccinated with MVA–LacZ (solid black bars) or TroVax (hatched bars) were stimulated in vitro with CT26 cells expressing either β-galactosidase (CT26–CL25) or h5T4 (CT26–h5T4) or a β-galactosidase peptide. Responses are illustrated as the number of spot forming units (SFU) per well (specific numbers are given above each histogram bar)
Protection against tumor challenge and the h5T4-specific antibody response are long-lived following injection with TroVax
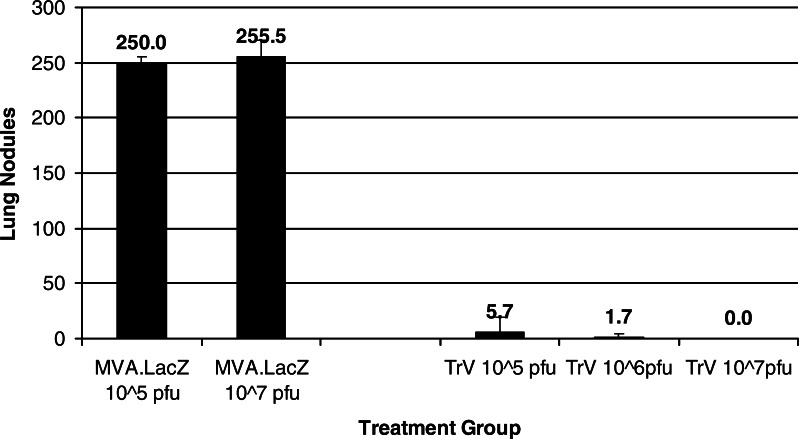
To determine the effect that the viral inoculum has on the observed immune response and subsequent protection against tumor challenge, mice were injected twice with either 105, 106 or 107 pfu MVA–LacZ or TroVax. To assess the longevity of the immune response, blood samples were taken 0.5 and 6 months after the last injection and the 5T4 specific antibody titer determined by ELISA (Fig. 4). As expected, the antibody titer was higher 2 weeks after the final injection compared to 6 months and was greatest in mice receiving the highest viral inoculum. The same groups of mice were challenged with CT26–h5T4 cells 6 months after receiving the final injection. All groups of mice immunized with TroVax were highly protected compared to those animals receiving MVA–LacZ (P<0.01% for all test groups; Fig. 5). In particular, mice receiving the highest inoculum (and having the highest antibody titer) showed a complete absence of tumors.
Fig. 4.
h5T4 specific antibody titers remain high 6 months after the last injection with TroVax. The 5T4 antibody responses of mice immunized twice with either 105, 106 or 107 pfu TroVax was compared by ELISA 0.5 months and 6 months after receiving the final injection. Results are expressed as the mean OD ± SD. Serum dilutions are detailed in the figure legend
Fig. 5.
Vaccination with TroVax induces long-lived protection against tumor challenge. Mice were challenged with 5×105 CT26–h5T4 cells 6 months following the last vaccination. Twelve days post-challenge animals were euthanized and the number of lung nodules determined per mouse. Results show the mean number of lung nodules ± SD per experimental group
TroVax has a therapeutic effect on pre-existing tumors
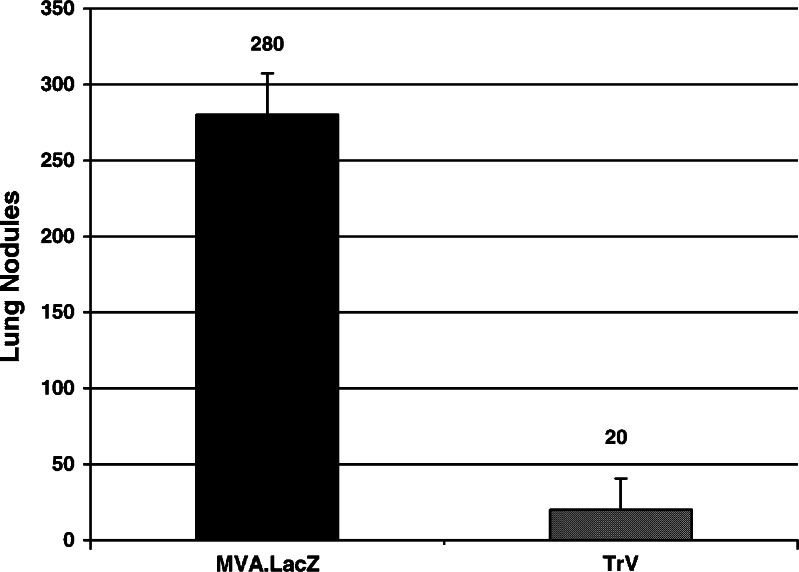
Having established that TroVax can induce a potent and long-lived anti-tumor response in this murine model, we subsequently studied the more clinically relevant scenario of therapeutic intervention. Groups of mice already harboring a tumor load received two injections of 107 pfu MVA–LacZ or TroVax on days 3 and 10 post-challenge. Lung nodules were enumerated on day 12. A reduction in tumor load of >90% was observed in TroVax vaccinated mice (P<0.01%) compared to those receiving MVA–LacZ (Fig. 6).
Fig. 6.
Immunization with TroVax can actively treat a pre-existing tumor load. Mice were injected with 1×107 pfu MVA–LacZ or TroVax on days 3 and 10 post-challenge. On day 12 animals were euthanized and the number of lung nodules determined per mouse. Results show the mean number of lung nodules ± SD per experimental group
CD4+ but not CD8+ T cells are required for h5T4 directed immunotherapy
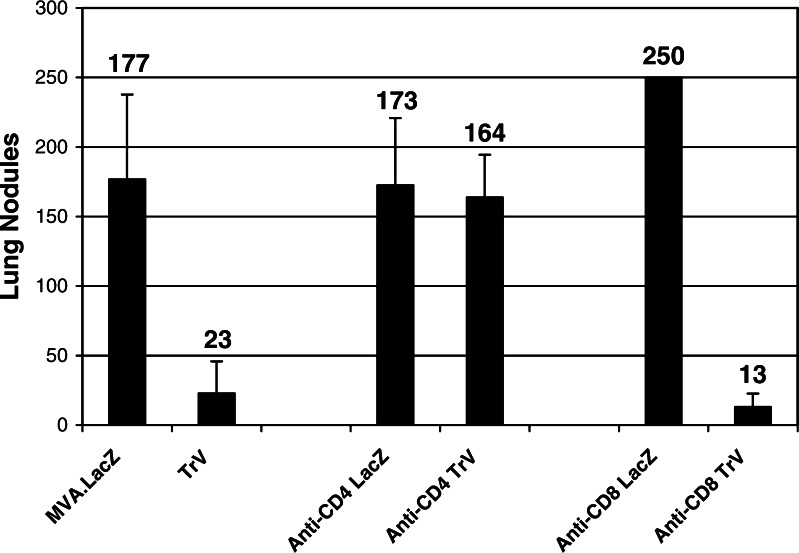
The role of CD4+ and CD8+ T cells in the induction of an efficacious anti-tumor immune response was investigated in the active treatment model using depletion monoclonal antibodies. Mice were depleted of CD4+ or CD8+ T cells prior to injection with MVA–LacZ or TroVax on days 3 and 10. The efficiency of depletion was monitored at the end of the experiment on day 12. Both CD4+ and CD8+ T cells were reduced to <1% of the total splenocyte population (data not shown). Active treatment following vaccination with TroVax was totally abrogated in mice depleted of CD4+ T cells (Fig. 7; not significant compared to MVA–LacZ control group). In contrast, mice depleted of CD8+ T cells showed >90% reduction in tumor load (P<0.1%) which was comparable to non-depleted, TroVax vaccinated mice (P<5%).
Fig. 7.
Therapeutic treatment of immunocompetent or CD4 or CD8 depleted (anti-CD4 and anti-CD8, respectively) mice following challenge with CT26–h5T4. Results show the mean number of lung nodules ± SD per experimental group
Removal of CD4+ or CD8+ T cells following the induction of a h5T4-specific immune response has no effect on protection against tumor challenge
Mice, which had already received two injections of MVA–LacZ or TroVax, were depleted of CD4+ or CD8+ T cells to assess the importance of each cellular sub-set in the context of a pre-existing h5T4-specific immune response. Blood samples were taken after depletion of T cells and the h5T4 specific antibody titer determined by ELISA. Removal of either cellular sub-set had no effect on the antibody titer compared to non-depleted mice (data not shown). In addition, removal of either CD4+ or CD8+ T cells had no effect on the observed protection against tumor challenge (Fig. 8; P<0.1% in both depleted groups).
Fig. 8.
Depletion of CD4+ or CD8+ T cells following the generation of a 5T4 specific immune response has no effect on the observed reduction in tumor burden. Mice were immunized twice with MVA–LacZ or TroVax, challenged with CT26–h5T4 cells and 1 day later depleted of CD4+ or CD8+ T cells (anti-CD4 and anti-CD8, respectively). Results show the mean number of lung nodules ± SD per experimental group
Passive transfer of antibodies specific for h5T4 can confer protection against tumor challenge
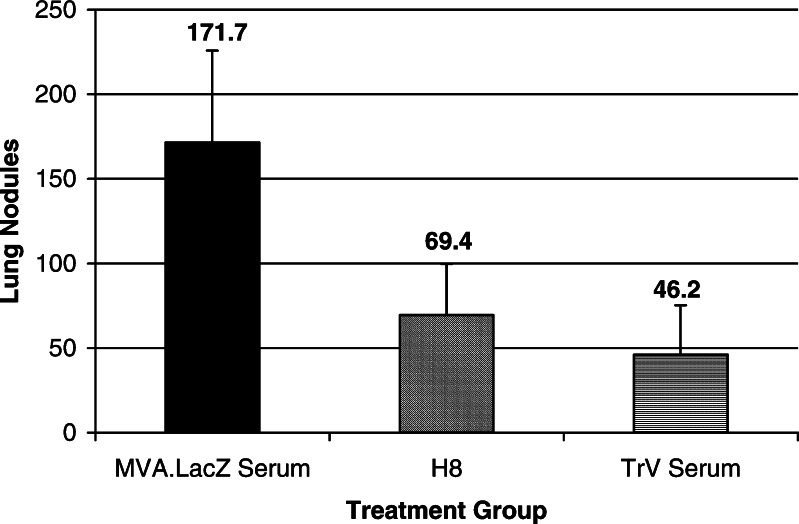
The role of 5T4 specific antibodies in tumor elimination was assessed by transferring either a murine IgG1 monoclonal antibody specific for h5T4 (H8) or polyclonal serum recovered from TroVax vaccinated mice (VMS) to animals harboring pre-existing tumors. Both monoclonal and polyclonal antibodies induced a reduction in tumor burden of 60 and 73%, respectively (Fig. 9; P<0.5% and P<0.2%, respectively).
Fig. 9.
Passive transfer of H8 monoclonal antibody or TroVax serum on days 3 and 10 post-challenge significantly reduces tumor burden measured on day 12. Results show the mean number of lung nodules ± SD per experimental group
Discussion
The pre-clinical results presented here provide further support for the use of the TAA 5T4 in cancer immunotherapy. To enable the use of a recombinant vaccinia virus encoding h5T4 in a phase I/II clinical trial, a new recombinant vector was engineered to optimize expression of the tumor antigen. We have been able to increase production of the recombinant protein in vitro compared to the prototype MVA–h5T4 construct [19] by placing h5T4 expression under control of the mH5 promoter rather than the synthetic promoter, Psyn and by the removal of 90 bases of 5′ UTR. Such enhanced expression of h5T4 observed in vitro, should result in the induction of a greater h5T4 specific antibody titer in vivo by TroVax compared to the prototype MVA–h5T4 construct. The immune response induced following vaccination with TroVax is long lasting and retains the capacity to protect mice against tumor growth even when challenge is delayed until 6 months after the last immunization.
Using a CT26–h5T4 murine tumor model, we have demonstrated that the depletion of CD8+ T cells in both prophylactic and active treatment settings has no effect on the observed protection following tumor challenge. This is consistent with the detection of a weak h5T4 specific T cell response by ELISPOT following vaccination of mice with TroVax. Heterologous prime-boost regimens have previously been shown to enhance antigen-specific CD8+ T cell responses [5, 16]. However, we have used heterologous prime-boost regimens using MVA and avian poxvirus vectors expressing h5T4, and were unable to induce strong h5T4 specific CD8+ T cell responses (data not shown). A similar observation has been made previously in a murine self antigen model [22], in which immunization of mice with a recombinant vaccinia virus expressing murine TRP-1 induced vitiligo and protection against tumor challenge. In these mice, a strong TRP-1 specific antibody response was observed but no CTL could be detected even after repeated rounds of antigenic restimulation in vitro. Furthermore, the use of both MHC class II knockout mice or wild-type animals depleted of CD4+ T cells suggested an important role for T helper cells in the induction of vitiligo and protection against tumor challenge. By depleting CD4+ T cells prior to, and following vaccination with TroVax, we have demonstrated that CD4+ T cells are essential for the induction of protection, but are not required directly for tumor destruction. This observation was further supported by the ability of antibody alone (either monoclonal or polyclonal) to confer significant protection against tumor challenge in passive transfer studies. In contrast to the results reported here, antibody was found to have little or no role in protection against tumor challenge in a CEA tumor model [1]. Like h5T4, CEA is a large, highly glycosylated, membrane associated tumor antigen and therefore also accessible to attack by antibody. However, in a murine model in which recombinant vaccinia virus was also used as the vehicle to deliver the tumor antigen (CEA), CD8+ T cells were found to be essential for protection against tumor challenge. CEA specific antibodies appeared to have no role in tumor destruction (although this was not demonstrated directly). Thus, in two similar murine models both using recombinant vaccinia virus expressing cell surface tumor antigens, different effector mechanisms appear to be operating. However, the identification of immune effector mechanisms operating in a xenogeneic animal model must be interpreted with caution when extrapolating to cancer immunotherapy in man. The 5T4-specific immune responses induced in this xenogeneic model are likely to be both qualitatively and quantitatively different from those induced by TroVax in man. However, such data provide guidance for the analysis of immune responses in man. The induction of a 5T4-specific antibody response in man may be advantageous in the context of tumor therapy against membrane antigens, since antibody mediated effector mechanisms would not be affected by MHC class I down-regulation in tumor cells.
The induction of a therapeutic response in this murine tumor model is particularly encouraging given the timing of treatment. Mice were challenged on day 0, vaccinated on days 3 and 10 and culled on day 12. If antibody is the sole effector arm in this murine model, then isotype switching, affinity maturation and antibody titer are unlikely to be optimal by the time the experiment is concluded. However, vaccination on days 3 and 10 consistently induced a greater therapeutic effect than the passive transfer of a 5T4 specific monoclonal antibody or polyclonal serum from mice vaccinated twice with TroVax. It is possible that the monoclonal antibody had a poorer therapeutic effect owing to its monospecificity or the isotype of the molecule. H8 is of the IgG1 isotype which is known to be a less efficient mediator of ADCC than IgG2a [7]. Further possibilities are simply that the antibody concentration used in the passive transfer experiments was non-optimal or that injection with a recombinant MVA enhances NK cell activity. In a TRP-1 self-antigen model, Hara et al. [14] used a monoclonal antibody to confer protection against tumor challenge in both prophylactic and active treatment settings. Approximately 40-fold more mAb was used in the TRP-1 model than used in this h5T4 murine tumor model. It is more difficult to explain why the polyclonal serum does not cause such an efficacious anti-tumor response as vaccination. The 5T4 specific antibody titer measured on day 12 following vaccination on days 3 and 10 is approximately eightfold lower than the serum used in passive transfer experiments (data not shown). Since 300 μl polyclonal serum is transferred on both occasions, this should result in a higher concentration of circulating 5T4 specific antibodies than that induced by the injections on days 3 and 10, thus one might expect a more efficacious anti-tumor response. However, the half-life of the transferred serum may be insufficient to maintain high levels of circulating therapeutic antibodies or facilitate accumulation at the tumor site, compared to the long-lived production of h5T4 specific antibodies induced by vaccination with TroVax. The mode of action of both mono- and polyclonal antibodies remains to be determined. It is possible that the 5T4 specific antibodies could act directly through ligation of the surface protein and inhibit growth signaling [23] or by the induction of apoptosis [13, 27, 29]. The more likely possibility is that antibodies act through antibody-dependent cell-mediated cytotoxicity (ADCC) or complement fixation as seen with the anti-her2/neu antibody, herceptin [8].
In conclusion, we have shown that vaccination of mice with TroVax induces a potent immune response that is sufficient to induce tumor protection in both prophylactic and active treatment settings. In this murine model, the effector arm is CD4 dependent and antibody-mediated. The ability of TroVax to induce a 5T4 specific immune response in humans is now being assessed in a phase I/II clinical trial in late-stage colorectal cancer patients.
Acknowledgments
The authors would like to thank Pauline Henbest for her technical expertise and assistance and Peter Treasure for statistical support.
References
- 1.Abrams SI, Hodge JW, McLaughlin JP, Steinberg SM, Kantor JA, Schlom J. Adoptive immunotherapy as an in vivo model to explore antitumor mechanisms by a recombinant anticancer vaccine. J Immunother. 1997;20:48. doi: 10.1097/00002371-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Brattain MG, Strobel-Stevens J, Fine D, Webb M, Sarrif AM. Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 1980;40:2142. [PubMed] [Google Scholar]
- 3.Bronte V, Carroll MW, Goletz TJ, Wang M, Rosenberg SA, Moss B, Restifo NP. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll MW, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;24:198. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 5.Carroll MW, Overwijk WW, Chamberlain RS, Rosenberg SA, Moss B, Restifo NP. Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model. Vaccine. 1997;15:387. doi: 10.1016/S0264-410X(96)00195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll MW, Restifo NP. Poxviruses as vectors for cancer immunotherapy. In: Stern PL, Beverly PCL, Carroll MW, editors. Cancer vaccines and immunotherapy. Cambridge, UK: Cambridge University Press; 2000. pp. 47–65. [Google Scholar]
- 7.Clark MR. IgG effector mechanisms. Chem Immunol. 1997;65:88. [PubMed] [Google Scholar]
- 8.Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27:1533. doi: 10.1016/S0301-472X(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 9.Earl P, Wyatt LS, Moss B, Carroll MW (1998) Generation of vaccinia virus recombinant viruses. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) Current protocols in molecular biology, supplement 43, vol 2. Wiley Interscience, New York, NY, pp16.17.1–16.17.19
- 10.Earl PL, Hugin AW, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eck SC, Turka LA. Adoptive transfer enables tumor rejection targeted against a self-antigen without the induction of autoimmunity. Cancer Res. 2001;61:3077. [PubMed] [Google Scholar]
- 12.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971. [PubMed] [Google Scholar]
- 13.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody for integrin alphabeta 3. Clin Cancer Res. 2000;6:3056. [PubMed] [Google Scholar]
- 14.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995;182:1609. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins WG, Gold JS, Dyall R, Wolchok JD, Bowne WB, Srinivasan R, Houghton AN, Lewis JJ. Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery. 2000;128:273. doi: 10.1067/msy.2000.107421. [DOI] [PubMed] [Google Scholar]
- 16.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759. doi: 10.1016/S0264-410X(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 17.Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239. doi: 10.1038/bjc.1988.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;15:11341. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulryan K, Ryan MG, Myers KA, Shaw D, Wang W, Kingsman SM, Stern PL, Carroll MW. Attenuated recombinant vaccinia virus expressing oncofetal antigen (tumor-associated antigen) 5T4 induces active therapy of established tumors. Mol Cancer Ther. 2002;1:1129. [PubMed] [Google Scholar]
- 20.Myers KA, Rahi-Saund V, Davison MD, Young JA, Cheater AJ, Stern PL. Isolation of a cDNA encoding 5T4 oncofetal trophoblast glycoprotein. J Biol Chem. 1994;269:9319. [PubMed] [Google Scholar]
- 21.Myers KA, Ryan MG, Stern PL, Shaw D, Embelton MJ, Kingsman SM, Carroll MW. Targeting immune effector molecules to human tumor cells through genetic delivery of 5T4-specific scFv fusion proteins. Caner Gene Ther. 2002;9:884. doi: 10.1038/sj.cgt.7700513. [DOI] [PubMed] [Google Scholar]
- 22.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26:60. [PubMed] [Google Scholar]
- 24.Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steitz J, Bruck J, Knop J, Tuting T. Adenovirus-transduced dendritic cells stimulate cellular immunity to melanoma via a CD4+ T cell-dependent mechanism. Gene Ther. 2001;8:1255. doi: 10.1038/sj.gt.3301521. [DOI] [PubMed] [Google Scholar]
- 26.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse E, Rabbitts TH. Intracellular antibody-caspase-mediated cell killing: an approach for application in cancer therapy. Proc Natl Acad Sci USA. 2000;97:12266. doi: 10.1073/pnas.97.22.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuting T, Gambotto A, DeLeo A, Lotze MT, Robbins PD, Storkus WJ. Induction of tumor antigen-specific immunity using plasmid DNA immunization in mice. Cancer Gene Ther. 1999;6:73. doi: 10.1038/sj.cgt.7700020. [DOI] [PubMed] [Google Scholar]
- 29.Vollmers HP, Zimmermann U, Krenn V, Timmermann W, Illert B, Hensel F, Hermann R, Theide A, Wilhelm M, Ruckle-Lanz H, Reindl L, Muller-Hermelink HK. Adjuvant therapy for gastric adenocarcinoma with the apoptosis-inducing human monoclonal antibody SC-1: first clinical and histopathological results. Oncol Rep. 1998;5:549. doi: 10.3892/or.5.3.549. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685. [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt LS, Shors ST, Murphy BR, Moss B. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine. 1996;14:1451. doi: 10.1016/S0264-410X(96)00072-2. [DOI] [PubMed] [Google Scholar]