Abstract
Plasmid DNA vaccine is an appealing cancer immunotherapy. However, it is a weak immunogen and immunization with plasmid DNA encoding self-antigens, such as melanoma-associated antigens, could not induce antitumor immunity because of tolerance. In this study, we investigated the feasibility of using a plasmid DNA encoding Xenopus laevis transforming growth factor-beta 5 (aTGF-β5) as an immunogen to induce neutralizing antibodies against murine TGF-β1 (mTGF-β1) and thus enhance the efficacy of plasmid DNA vaccine encoding murine tyrosinase-related protein 2 (mTRP-2) through neutralization of TGF-β. The results showed that immunization with aTGF-β5 resulted in the generation of mTGF-β1-neutralizing antibodies, and immunization with a combination of aTGF-β5 and mTRP-2 induced specific cytotoxic T lymphocytes (CTLs). On the contrary, immunization with mTRP-2 alone could not elicit the CTL response. Moreover, immunization of C57BL/6 wild-type mice with a combination of aTGF-β5 and mTRP-2 induced the protective and therapeutic antitumor immunity to B16F10 melanoma, whereas the antitumor activity was abrogated in both CD4-deficient mice and CD8-deficient mice on the C57BL/6 background. Our results indicate that immunization with aTGF-β5 is capable of breaking immune tolerance and induces mTGF-β1-neutralizing antibodies. Neutralization of TGF-β can enhance the efficacy of DNA vaccine encoding mTRP-2 and the induction of antitumor immunity by this immunization strategy is associated with CD4+ and CD8+ T cells.
Keywords: Melanoma, Tolerance, Transforming growth factor-beta, Cytotoxic T lymphocyte, DNA vaccine, Cancer immunotherapy
Introduction
Identification of tumor-associated antigens has boosted the development of therapeutic cancer vaccines [1, 2]. Studies of melanoma have shown that the immune system commonly recognizes the melanoma-associated antigens specifically expressed by melanocytes and melanomas, including gp100, MART-1, tyrosinase, tyrosinase-related protein 1 (TRP-1) and TRP-2. These melanoma-associated antigens are derived from normal nonmutated melanocyte lineage differentiation antigens and belong to self-antigens. They can be recognized by antibodies and by CD8+ and CD4+ T cells [3–5]. Recently, a number of therapeutic cancer vaccines based on these melanoma/melanocyte differentiation antigens such as MART-1, tyrosinase, TRP-2 and gp100 have been developed and are being evaluated in clinical trials. Plasmid DNA vaccine is an appealing cancer immunotherapy because it presents several advantages, including offering a cheap and stable source of antigen, inducing CD8+ CTL, CD4+ Th cells, as well as antibody responses to model antigens [6, 7]. However, it is generally considered as a weak immunogen; in addition, immunization with plasmid DNA encoding “self” melanoma-associated antigens, such as TRP-1 and TRP-2, could not induce antitumor immunity because of immune tolerance [8, 9].
One of the factors associated with immune tolerance is transforming growth factor-beta (TGF-β). TGF-β, especially TGF-β1, is a potent immunosuppressive cytokine. Immunosuppressive properties of TGF-β1 include inhibition of several dendritic cell (DC) functions [10, 11], inhibition of T-cell differentiation into cytotoxic T lymphocytes (CTLs) and Th cells, and inhibition of CD4+ T-cell differentiation into Th1 cells and Th2 cells [12, 13]. It has been found that there is a marked increase in the expression of TGF-β mRNA and protein in human cancers (in vivo). Moreover, high expression of TGF-β correlates with more advanced stages of malignancy and decreased survival [14]. In untreated breast cancer patients, plasma TGF-β1 levels were elevated and normalized after surgery [15]. In addition, TGF-β is directly produced by a great variety of tumor cells, such as B16F10 melanoma [16]. These findings suggest that TGF-β production by tumors could play an important role in reducing the effectiveness of plasmid DNA vaccines encoding “self” melanoma-associated antigens.
To demonstrate the effect of TGF-β on plasmid-based antitumor vaccines, we constructed the plasmid DNA encoding Xenopus laevis TGF-β5 (aTGF-β5) [17], of which the mature chain is approximately 76% identical to that of murine TGF-β1 (mTGF-β1) at the amino acid level [18], and investigated whether genetic immunization of mice with it would be effective in inducing TGF-β-neutralizing antibodies. TRP-2 belongs to the tyrosinase family of melanosomal enzymes involved in melanin synthesis and is expressed in melanocytes and most melanomas. The peptide SVYDFFVWL corresponding to amino acids 180-188 of murine TRP-2 (mTRP-2) was identified by Kb-restricted CTL specific for B16 melanoma cells [19]. Adoptive transfer of mTRP-2-specific CTL inhibits the growth of experimental melanoma lung metastases induced by an intravenous challenge with B16 melanoma cells [20], suggesting that mTRP-2 may represent a CTL-defined tumor rejection antigen in mice. Furthermore, we chose mTRP-2 as a model antigen to investigate whether immunization with plasmid DNA encoding aTGF-β5 would effectively enhance the effectiveness of plasmid DNA vaccines encoding self-antigens through neutralization of TGF-β1 by induced TGF-β-specific antibodies. The results demonstrated that immunization with aTGF-β5 broke tolerance to mTGF-β1 and induced neutralizing antibodies against mTGF-β1. Combined immunization with aTGF-β5 and mTRP-2 elicited mTRP-2-specific CTL response. Most importantly, combined immunization induced protective and therapeutic immunity to B16F10 melanoma.
Methods
Animals and cell lines
Six- to 12-week-old female C57BL/6 (H-2Kb) wild-type (WT) mice, CD4-deficient mice and CD8-deficient mice on the C57BL/6 background (Jackson Laboratory, Bar Harbor, ME, USA) were kept under specific pathogen-free conditions at Third Military Medical University in a conventional facility. Animal experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of Third Military Medical University. The murine melanoma B16F10 (H-2Kb) and the murine thymoma EL-4 (H-2Kb) were cultured in complete RPMI 1640 supplemented with 10% heat-inactivated FCS, 0.1 mM nonessential amino acids and 1 mM sodium pyruvate, 5×10−5 M 2-mercaptoethanol, 0.3% glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The transformed human embryonic kidney line 293 and the Chinese hamster ovary (CHO) cells were cultured in DMEM supplemented with 10% heat-inactivated FCS, 0.3% glutamine, 0.01 M Hepes, 100 U/ml penicillin and 100 μg/ml streptomycin. The mink lung epithelial cell line Mv 1 Lu was purchased from the American Type Culture Collection and cultured in Eagle’s minimal essential medium with Earle’s balanced salt solution supplemented with 10% heat-inactivated FCS, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin and 100 μg/ml streptomycin.
Peptide synthesis and construction of expression plasmids
The peptide SVYDFFVWL (mTRP-2aa180-188) was synthesized by Fmoc chemistry and purified by HPLC to >95% purity. The peptide was dissolved at 10 mg/ml in DMSO and stored at −20°C. To clone mTRP-2 and mTGF-β1 cDNAs from B16F10 cells, and aTGF-β5 cDNA from Xenopus laevis lung tissue, total RNA was isolated using TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN, USA) and the first-strand cDNA was synthesized using oligo(dT)15 primer. The PCR was performed using optimally matched primer pairs that generated cDNA clones encoding the entire proteins of mTRP-2, mTGF-β1 and aTGF-β5, respectively. The mTRP-2 primer sequences have been described [19]. The mTGF-β1 primer sequences were: forward primer, 5′-GCTTTCTCCCTCAACCTCA-3′, and reverse primer, 5′-GCTTCAGCTGCACTTGCA-3′. The aTGF-β5 primer sequences were: forward primer, 5′-CTGGTGCTGAGACTGTTGA-3′, and reverse primer, 5′-CTCCTTCTTCGACACTGGT-3′. The amplified products were inserted into pMD18-T plasmid (TaKaRa, Dalian, China) and then subcloned into pVAX1 plasmid (Invitrogen, Carlsbad, CA, USA) or pCI-neo plasmid (Promega, Beijing, China), which contains a cytomegalovirus promoter. The mTRP-2 inserted into pVAX1 was named as pVAX1-mTRP2; and mTGF-β1 and aTGF-β5 inserted into pCI-neo were named as pCI-mTGFβ1 and pCI-aTGFβ5, respectively. The full-length sequences of mTRP-2, mTGF-β1 and aTGF-β5 were confirmed by sequencing to be identical to those reported [17–19]. Plasmids for DNA vaccination were purified using Qiagen Endofree Plasmid Maxi Kit (Qiagen, Hilden, Germany). The expression of pVAX1-mTRP2 was confirmed in the transfected 293 cells by RT-PCR. The expression of pCI-mTGFβ1 and pCI-aTGFβ5 was confirmed in the transfected CHO cells by RT-PCR and with the use of anti-TGF-β antibodies (R&D Systems, Minneapolis, MN, USA) in Western blot assays.
Genetic immunization of mice
Purified DNA was resuspended in 0.01 M PBS at the final concentration of 1 mg/ml. Mice were immunized with 100 μg plasmid DNA by i.m. injection into the quadriceps muscle. For immunization with pCI-mTGFβ1 or pCI-aTGFβ5, mice were injected once a week for 4 weeks. For immunization with pVAX1-mTRP2, mice were injected once a week for 3 weeks. For immunization with a combination of pCI-aTGFβ5 and pVAX1-mTRP2, mice were injected with pVAX1-mTRP2 as described above, 1 week after the first injection of pCI-aTGFβ5. Additional control animals were injected with mock vectors or PBS. Groups of ten mice were inoculated s.c. in the flank with 2.5×103 B16F10 melanoma cells, and tumor growth was measured twice weekly using vernier calipers [21].
Detection of antibodies to TGF-β and analysis of plasma mTGF-β1 levels
Antibodies were detected in Western blot assays as described [22]. Briefly, the plasmids pCI-mTGFβ1 and pCI-aTGFβ5 were transfected into CHO cells by using the cationic liposomal transfection reagent DOTAP (Roche Molecular Biochemicals) as recommended by the manufacturer. After a 24-h incubation in DMEM medium supplemented with 10% FCS, the medium was replaced with serum-free DMEM medium. Transfected cells were incubated for an additional 48 h prior to supernatant collection. Serum-free supernatants from transfected cells were dialyzed against 0.2 M acetic acid, lyophilized, resuspended in sample buffer, subjected to 10% SDS-PAGE under nonreducing conditions and electroblotted onto PVDF membranes. Membranes were blocked for 1 h and reacted with pooled sera from groups of immunized mice at a 1:100 dilution for 1 h. Bound antibodies were detected with POD-labeled secondary antibody (Roche Molecular Biochemicals) and visualized using chemiluminescence on X-ray film.
The effect of the antibodies on the bioactivity of mTGF-β1 was investigated in the Mv 1 Lu bioassay system. Immunoglobulin (Ig) was purified from the pooled sera derived from the WT mice or control mice at day 7 after the last immunization using the Econo-Pac protein A kit (Bio-Rad, Hercules, CA, USA). The serum-free supernatant from pCI-mTGFβ1-transfected cells was acidified and then pre-incubated with the purified Ig for 1 h. The percentage of neutralization of bioactivity due to the Ig treatment was finally calculated [23].
The plasma mTGF-β1 levels from the WT mice at day 7 after the last immunization or from controls were analyzed by TGF-β1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA).
CTL assay
Animals were killed 1–2 weeks after the last immunization, and their spleens were removed and processed individually. A single cell suspension was obtained by smashing and filtering the organ through a sterile cell strainer and cleared of red blood cells using hypotonic shock. Forty million splenocytes were resuspended in 10 ml complete RPMI 1640 and cultured in a T25 flask with 10 units/ml recombinant murine interleukin 2 and 1 μg/ml mTRP-2 synthetic peptide. After 5 days at 37°C, effector cells were counted and tested for cytotoxic activity in a 6-h 51Cr release assay [24].
Statistical analysis
Statistical analyses were performed using the variance test and Student’s t-test. A difference was considered significant at the conventional level of P<0.05.
Results
Immunization with aTGF-β5 induces mTGF-β1-neutralizing antibodies
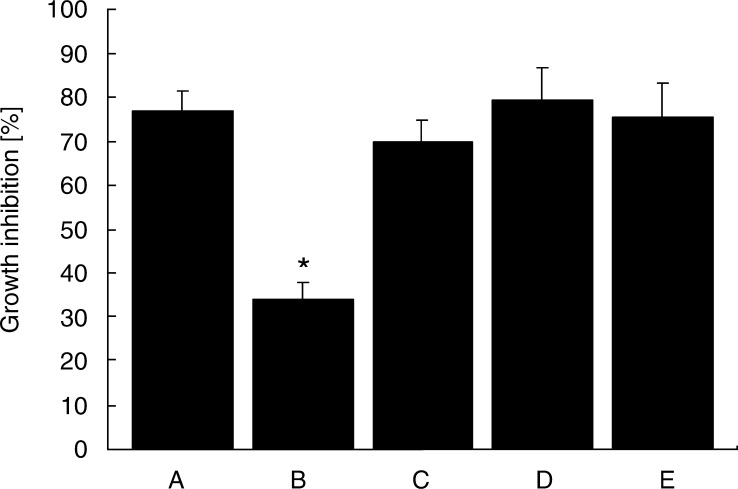
Wild-type mice were injected i.m. once in a week continuously for 4 weeks with pCI-aTGFβ5, pCI-mTGFβ1, pCI-neo or PBS. Induction of the antibodies specific for TGF-β was assessed by immunoblotting supernatants from CHO cells transfected with pCI-mTGFβ1 or pCI-aTGFβ5, which were separated by SDS-PAGE under nonreducing conditions and blotted onto PVDF membranes. Pooled sera from groups of mice immunized with aTGF-β5 reacted with two bands that represent the mature and precursor forms of aTGF-β5 with an apparent molecular weight of approximately 25 and 95 kDa and cross-reacted with mTGF-β1 (Fig. 1). Sera from mice injected with pCI-mTGFβ1, pCI-neo or PBS could not detect TGF-β (data not shown). The effect of the antibodies on the growth inhibition of Mv 1 Lu cells mediated by mTGF-β1 was assessed. The Ig from mice immunized with aTGF-β5 yielded significant suppression of the growth inhibition of Mv 1 Lu cells mediated by mTGF-β1. In contrast, Ig from mice injected with pCI-mTGFβ1, pCI-neo or PBS could not inhibit the bioactivity of mTGF-β1 (Fig. 2). Analysis of mTGF-β1 levels in plasma indicated normal levels in mice injected with pCI-mTGFβ1, pCI-neo or PBS, and the concentrations were significantly decreased in mice in which the antibodies were induced by immunization with aTGF-β5 (Fig. 3).
Fig. 1.

Induction of TGF-β-specific antibodies. Mice were immunized by i.m. injection of pCI-aTGFβ5. Sera samples were obtained at day 7 after the last immunization and tested for the presence of TGF-β-specific antibodies by immunoblotting supernatants from CHO cells transfected with pCI-mTGFβ1 (lane 1) or pCI-aTGFβ5 (lane 2), which were separated by SDS-PAGE under nonreducing conditions and blotted onto PVDF membranes.
Fig. 2.
Effect of the antibodies on the bioactivity of mTGF-β1. Supernatant from pCI-mTGFβ1-transfected CHO cells was collected, acidified and lyophilized as mTGF-β1. The growth inhibition of Mv 1 Lu cells mediated by mTGF-β1 was assessed by treatment with mTGF-β1 alone (a) or together with purified Ig derived from mice immunized with pCI-aTGFβ5 (b), pCI-mTGFβ1 (c), pCI-neo (d) or PBS (e) in the Mv 1 Lu bioassay system. *P<0.05 compared with the group treated with mTGF-β1 alone.
Fig. 3.
Plasma levels of mTGF-β1 in untreated controls (a) and mice immunized with pCI-aTGFβ5 (b), pCI-mTGFβ1 (c), pCI-neo (d) or PBS (e). *P<0.0005 compared with controls.
Immunization with a combination of aTGF-β5 and mTRP-2 induces CTLs specific for a Kb-binding peptide derived from mTRP-2
The WT mice were immunized with mTRP-2 alone or together with aTGF-β5. Splenocytes were harvested from mice 1–2 weeks after the last immunization and restimulated in vitro with the mTRP-2aa180-188 synthetic peptide and tested in 6-h 51Cr release assays 5 days later. As shown in Fig. 4, immunization with a combination of aTGF-β5 and mTRP-2 induced a vigorous CTL activity against B16F10 cells and mTRP-2aa180-188-pulsed EL-4 cells, and not against unpulsed EL-4 cells. Immunization with mTRP-2 alone, in contrast, did not induce significant CTL activity (data not shown).
Fig. 4.
Induction of mTRP-2-specific CTLs. Mice were immunized with a combination of aTGF-β5 and mTRP-2. One or 2 weeks later, splenocytes were harvested and cultured for 5 days in the presence of 1 μg/ml mTRP-2aa180-188 and 10 units/ml recombinant murine interleukin 2, and tested in 6-h 51Cr release assays. Curves represent the cytotoxicity against mTRP-2aa180-188-pulsed EL-4 (filled circles), B16F10 (squares) and unpulsed EL-4 (open circles). Values are expressed as percentage (average of triplicates) of specific 51Cr release (percentage of specific lysis) at the indicated E/T ratios.
Induction of protective antitumor immunity by immunization with a combination of aTGF-β5 and mTRP-2
The WT mice were immunized with pCI-aTGFβ5, pCI-mTGFβ1 or pCI-neo. One week after the last immunization, mice were challenged with B16F10 cells. Results showed that similar growth of tumor was observed in all groups of mice including controls (data not shown). Furthermore, we tested the functional activity in vivo of the CTL immune response induced by immunization with a combination of aTGF-β5 and mTRP-2. Mice were immunized with mTRP-2 alone or together with aTGF-β5 and challenged with B16F10 cells. Significant protection against tumor growth was observed only in mice immunized with a combination of aTGF-β5 and mTRP-2 (P<0.0005), and 60% of tumor-bearing mice survived for over 50 days, whereas immunization with mTRP-2 alone resulted in survival time similar to that of controls (Fig. 5).
Fig. 5.
Induction of the protective antitumor immunity. Mice were immunized with a combination of aTGF-β5 and mTRP-2 (filled circles), mTRP-2 alone (squares) or pVAX1 (triangles), or were injected with PBS as controls (open circles). One week after the last immunization, mice were challenged with 2.5×103 B16F10 cells. Values are expressed as percentage of survival animals at the indicated time after tumor challenge.
Roles of CD4+ and CD8+ T cells in the antitumor immunity induced by immunization with a combination of aTGF-β5 and mTRP-2
Wild-type mice, CD4-deficient mice and CD8-deficient mice were immunized with a combination of aTGF-β5 and mTRP-2. Additional WT mice were injected with PBS as controls. One week later, mice were challenged with B16F10 cells. Figure 6 shows that the survival time of tumor-bearing WT mice, which were immunized with a combination of aTGF-β5 and mTRP-2, was significantly prolonged. On the other hand, the antitumor activity was significantly abrogated in both CD4-deficient mice and CD8-deficient mice with a survival time similar to that of controls.
Fig. 6.
Roles of CD4+ and CD8+ T cells in the antitumor immunity. WT mice (filled circles), CD4-deficient mice (squares) and CD8-deficient mice ( triangles) were immunized with a combination of aTGF-β5 and mTRP-2, and additional WT mice were injected with PBS as controls (open circles). One week later, mice were challenged with 2.5×103 B16F10 cells. Values are expressed as percentage of survival animals at the indicated time after tumor challenge.
Induction of therapeutic antitumor immunity by immunization with a combination of aTGF-β5 and mTRP-2
The therapeutic efficacy of the combination of aTGF-β5 and mTRP-2 was next tested in the B16F10 tumor model. WT mice were inoculated with B16F10 cells first and then treated with a combination of aTGF-β5 and mTRP-2, mTRP-2 alone or PBS at day 1 after tumor inoculation. Treatment with a combination of aTGF-β5 and mTRP-2 resulted in significant antitumor activity, and 50% of mice survived for over 50 days (Fig. 7). The survival of the tumor-bearing mice treated with a combination of aTGF-β5 and mTRP-2 was significantly greater than that of controls (P<0.05).
Fig. 7.
Induction of the therapeutic antitumor immunity. Mice were inoculated with 2.5×103 B16F10 melanoma cells first and then treated with a combination of aTGF-β5 and mTRP-2 (filled circles), mTRP-2 alone (squares) or were injected with PBS as controls (open circles) at day 1 after tumor inoculation. Values are expressed as percentage of survival animals at the indicated time after tumor inoculation.
Discussion
In this study, we first observed that the active immunization of mice with plasmid DNA encoding aTGF-β5 induced the antibodies that cross-react with mTGF-β1 (Fig. 1) and inhibit the bioactivity of mTGF-β1 (Fig. 2). Moreover, plasma mTGF-β1 levels were significantly decreased in mice immunized with aTGF-β5 (Fig. 3). Our results clearly show that immunization with aTGF-β5 can break tolerance and induce mTGF-β1-neutralizing antibodies. In contrast, specific antibodies were not detected in CD4-deficient mice immunized with aTGF-β5 (data not shown). Similar observations have been reported that genetic immunization with xenogeneic antigens induces specific antibodies or CTLs against murine homologues [25, 26]. It is known that CD4+ T cells can steer and amplify immune responses through the secretion of cytokines and expression of surface molecules [27, 28]. Wei et al. [25] reported that the autoantibodies against vascular endothelial growth factor can be induced in mice by plasmid DNA immunization with Xenopus laevis vascular endothelial growth factor and induction of the autoantibodies has depended on CD4+ T cells. For the antibody-dependent immunity, CD4+ T cells can be required at the immunization phase as well as at the effector phase [29]. These findings suggest that induction of mTGF-β1-neutralizing antibodies may involve CD4+ T cells.
Because CTL plays a major role in the rejection of B16 melanoma [24], we investigated the effect of immunization with aTGF-β5 on the efficacy of plasmid DNA encoding mTRP-2 to elicit antitumor CTLs. We found that immunization with a combination of aTGF-β5 and mTRP-2 resulted in the induction of mTRP-2aa180-188-specific CTLs (Fig. 4). Of importance, induction of the specific CTLs was associated with protective and therapeutic immunity to B16F10 melanoma (Fig. 5 and Fig. 7). In contrast, immunization with mTRP-2 alone did not lead to detectable induction of mTRP-2aa180-188-specific CTLs. It has been reported that neutralization of TGF-β1 with TGF-β1-neutralizing antibody results in complete restoration of suppressed interleukin 2 responsiveness of T cells from EL-4-bearing mice [30]. Blockade of TGF-β signaling in T cells allows generation of tumor-specific CTLs and renders mice resistant to tumor growth [16]. From our findings reported in the present and previous studies, it is reasonable to deduce that the mTGF-β1-neutralizing antibodies induced by immunization with aTGF-β5 may enhance the efficacy of plasmid DNA encoding mTRP-2 and help break self-tolerance to mTRP-2 by neutralizing TGF-β.
Here, we observed that WT mice vaccinated with aTGF-β5 were not protected from challenge by B16F10 melanoma cells, though mTGF-β1-neutralizing antibodies were induced. Immunization with a combination of aTGF-β5 and mTRP-2 did not induce antitumor immunity in CD4-deficient mice. At the same time, the antitumor activity was also abrogated in CD8-deficient mice (Fig. 6). These findings suggest that the antitumor effect induced by this immunization strategy may require the participation of both CD4+ and CD8+ T cells, and CD8+ CTL is a major component responsible for antitumor activity. It is known that CD8+ CTLs are major effector cells involved in immunologically specific tumor destruction in vivo, and CD4+ T cells are essential for controlling this CD8+ T-cell-dependent tumor eradication [31, 32]. It has been reported that antitumor immunity can be induced by genetic immunization with mTRP-2 linked to foreign helper sequences and has depended on both CD4+ and CD8+ T cells in melanoma models [33]. Furthermore, CD4+ T cells and CD8+ T cells have been reported to be required for the induction of sufficient antitumor immunity in mice by DCs transduced with melanoma-associated antigen gp100 or vaccination with a recombinant adenovirus encoding mTRP-2 [34, 35]. However, induction of antitumor immunity, in some cases, is independent of CD4+ T cells [21, 36]. In the present study, we do not have enough data to conclude whether CD4+ T cells are necessary for the induction of mTRP-2-specific CTLs under the contribution of mTGF-β1-neutralizing antibodies.
In conclusion, our results show that genetic immunization with aTGF-β5 is able to break tolerance and induce mTGF-β1-neutralizing antibodies. The neutralization of mTGF-β1 may contribute to breaking of self-tolerance to mTRP-2 by immunization with plasmid DNA encoding mTRP-2 and enhance the efficacy of plasmid DNA encoding mTRP-2 to induce antitumor immunity against growth of B16F10 melanoma.
Acknowledgements
This work was supported by National Key Basic Research Program of China (2001CB510001) and Key Project of the National Science Foundation of China.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst. 1996;88:1635. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]
- 3.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Rivoltini L, Mancini M, Markus NR, Robbins PF, Kawakami Y, Rosenberg SA. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci U S A. 1994;91:9461. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 7.Piechocki MP, Pilon SA, Wei WZ. Complementary antitumor immunity induced by plasmid DNA encoding secreted and cytoplasmic human ErbB-2. J Immunol. 2001;167:3367. doi: 10.4049/jimmunol.167.6.3367. [DOI] [PubMed] [Google Scholar]
- 8.Steitz J, Bruck J, Steinbrink K, Enk A, Knop J, Tuting T. Genetic immunization of mice with human tyrosinase-related protein 2: implications for the immunotherapy of melanoma. Int J Cancer. 2000;86:89. doi: 10.1002/(SICI)1097-0215(20000401)86:1<89::AID-IJC14>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Weber LW, Bowne WB, Wolchok JD, Srinivasan R, Qin J, Moroi Y, Clynes R, Song P, Lewis JJ, Houghton AN. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102:1258. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860. [PubMed] [Google Scholar]
- 11.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512. doi: 10.1182/blood.V98.5.1512. [DOI] [PubMed] [Google Scholar]
- 12.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 14.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303. [PubMed] [Google Scholar]
- 15.Kong FM, Anscher MS, Murase T, Abbott BD, Iglehart JD, Jirtle RL. Elevated plasma transforming growth factor-beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg. 1995;222:155. doi: 10.1097/00000658-199508000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockage of transforming growth factor-β signaling in T cells. Nat Med. 2001;7:1118. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 17.Kondaiah P, Sands MJ, Smith JM, Fields A, Roberts AB, Sporn MB, Melton DA. Identification of a novel transforming growth factor-beta (TGF-beta 5) mRNA in Xenopus laevis. J Biol Chem. 1990;265:1089. [PubMed] [Google Scholar]
- 18.Derynck R, Jarrett JA, Chen EY, Goeddel DV. The murine transforming growth factor-beta precursor. J Biol Chem. 1986;261:4377. [PubMed] [Google Scholar]
- 19.Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada M, Tamada K, Abe K, Li T, Onoe Y, Tada H, Tatsugami K, Ando T, Kimura G, Nomoto K. Characterization of B16 melanoma-specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 1998;47:198. doi: 10.1007/s002620050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miconnet I, Coste I, Beermann F, Haeuw JF, Cerottini JC, Bonnefoy JY, Romero P, Renno T. Cancer vaccine design: a novel bacterial adjuvant for peptide-specific CTL induction. J Immunol. 2001;166:4612. doi: 10.4049/jimmunol.166.7.4612. [DOI] [PubMed] [Google Scholar]
- 22.Brunner AM, Marquardt H, Malacko AR, Lioubin MN, Purchio AF. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989;264:13660. [PubMed] [Google Scholar]
- 23.Su HC, Ishikawa R, Biron CA. Transforming growth factor-beta expression and natural killer cell responses during virus infection of normal, nude, and SCID mice. J Immunol. 1993;151:4874. [PubMed] [Google Scholar]
- 24.Mendiratta SK, Thai G, Eslahi NK, Thull NM, Matar M, Bronte V, Pericle F. Therapeutic tumor immunity induced by polyimmunization with melanoma antigens gp100 and TRP-2. Cancer Res. 2001;61:859. [PubMed] [Google Scholar]
- 25.Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, Shu JM, Lu CJ, Niu T, Kang B, Mao YQ, Liu F, Wen YJ, Lei S, Luo F, Zhou LQ, Peng F, Jiang Y, Liu JY, Zhou H, Wang QR, He QM, Xiao F, Lou YY, Xie XJ, Li Q, Wu Y, Ding ZY, Hu B, Hu M, Zhang W. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci U S A. 2001;98:11545. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, Lewis JJ, Houghton AN. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz RH. T cell clonal anergy. Curr Opin Immunol. 1997;9:351. doi: 10.1016/S0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263. doi: 10.1016/S0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 29.Pardoll DM. Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci U S A. 1999;96:5340. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda H, Shiraishi A. TGF-β contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol. 1996;156:73. [PubMed] [Google Scholar]
- 31.Svane IM, Boesen M, Engel AM. The role of cytotoxic T-lymphocytes in the prevention and immune surveillance of tumors–lessons from normal and immunodeficient mice. Med Oncol. 1999;16:223. doi: 10.1007/BF02785868. [DOI] [PubMed] [Google Scholar]
- 32.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269. doi: 10.1016/S1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 33.Steitz J, Bruck J, Gambotto A, Knop J, Tuting T. Genetic immunization with a melanocytic self-antigen linked to foreign helper sequences breaks tolerance and induces autoimmunity and tumor immunity. Gene Ther. 2002;9:208. doi: 10.1038/sj.gt.3301634. [DOI] [PubMed] [Google Scholar]
- 34.Okada N, Masunaga Y, Okada Y, Mizuguchi H, Iiyama S, Mori N, Sasaki A, Nakagawa S, Mayumi T, Hayakawa T, Fujita T, Yamamoto A. Dendritic cells transduced with gp100 gene by RGD fiber-mutant adenovirus vectors are highly efficacious in generating anti-B16BL6 melanoma immunity in mice. Gene Ther. 2003;10:1891. doi: 10.1038/sj.gt.3302090. [DOI] [PubMed] [Google Scholar]
- 35.Perricone MA, Claussen KA, Smith KA, Kaplan JM, Piraino S, Shankara S, Roberts BL. Immunogene therapy for murine melanoma using recombinant adenoviral vectors expressing melanoma-associated antigens. Mol Ther. 2000;1:275. doi: 10.1006/mthe.2000.0029. [DOI] [PubMed] [Google Scholar]
- 36.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, Overwijk WW, Restifo NP, Melief CJ, Offringa R, Allison JP. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]