Abstract
CD8+ T cells can express NK-associated receptors (NKRs) that may regulate their cytolytic function. We have characterized the expression of several NKRs on peripheral blood CD8+ T cells from melanoma patients and compared them to age-matched healthy donors. The analysis performed includes HLA class I specific receptors (KIRs, LILRB1 and CD94/NKG2) and other NK receptors like CD57, CD56 and CD16. Melanoma patients showed a higher variability in the expression of NKRs on circulating CD8+ T cells than age-matched healthy donors. NKR expression on CD8+ T cells from melanoma patients showed a significant increase of KIR2DL2/L3/S2 (mAb gl183), CD244, CD57, CD56 and CD16. We have also found an increase of CD8+ CD28− CD27− T cells in melanoma patients. This subset represents terminally differentiated effector cells expressing CD244 and high levels of perforin. The expression of NKRs was also mainly restricted to this T cell subset. Altogether, circulating CD8+ T cells from melanoma patients display a distinct phenotype characterized by downregulation of costimulatory molecules and higher expression of NKRs. We suggest that the increased expression of NKRs on T cells may contribute to the final outcome of the immune response against melanoma both stimulating or inhibiting activation and differentiation to effector cells. Blocking inhibitory receptor function and enhancing activating receptors may represent new strategies with therapeutic potential against melanoma.
Keywords: NKR, KIR, CD94/NKG2, CD28, Tumor immunity, CTL, Co-stimulation
Introduction
Inhibitory and activating MHC-class I specific receptors were initially described as receptors present on NK cells that regulate lysis of target cells expressing the appropriate ligand [7, 27, 31, 38]. Several studies have demonstrated that the expression of these receptors is not exclusive to NK cells and it has been made clear that different subsets of T lymphocytes can express NK-associated receptors (NKRs) [35, 55]. NKRs expressed on T cells are functionally active and may act as regulators of T cell-mediated cytotoxicity [35, 64]. In humans, three families of HLA class-I specific NKRs have been identified including activating and inhibitory isoforms: Killer Immunoglobulin-like Receptors (KIRs), leukocyte immunoglobulin-like receptors (LILR; also termed Immunoglobulin Like Transcripts, ILT, or CD85) and C-type lectin receptors [27, 31, 37]. Together with these HLA class-I specific receptors, studies have described the expression on CD8+ T lymphocytes of non-HLA class I specific NKRs such as CD56, CD57 or CD244 that have been correlated with cell activation and may have functional implications in the immune response against tumour antigens [42, 45, 57, 59].
Tumour-specific CTLs can express HLA class-I specific NKRs and signalling via inhibitory receptors interferes with CTL effector function upon TCR engagement by MHC-peptide complexes [26, 54]. Several studies have investigated whether signalling via KIR can affect melanoma recognition by CTLs. Thus, infection of gp100-specific CTLs with a vaccine virus encoding human KIR3DL1 receptor [4] or the natural expression of CD94/NKG2A on Melan-A specific CTLs [54] resulted in inhibition of lysis of gp100 and Melan-A positive melanomas respectively. Consequently, the expression of the appropriate HLA class-I ligand by melanoma cells may regulate T cell function by crosslinking MHC-specific inhibitory receptors. In addition, melanoma cells that have HLA class-I loss of selective alleles can elude the inhibitory effect of HLA-specific NKRs. Thus, KIRD2L2 expression on an HLA-A24-restricted CTL clone from a melanoma patient inhibited lysis of autologous melanoma cells but did not affect lysis of a melanoma variant that had lost the expression of all HLA alleles except for HLA-A24, the restriction element [26]. Total or partial loss of HLA class I antigens is a frequent event in different tumours [1, 12, 32, 46, 47, 50]. In an extensive study of 61 melanoma cell lines analyzed by flow cytometry, it has been reported that 51.7% of melanoma cell lines had altered HLA class I phenotypes, including 3% of HLA class I total loss, 3% of HLA-ABC downregulation, 19.7% loss of heterozygosity and 26% of HLA-B downregulation [48]. Although inhibitory HLA class-I specific NKRs on CTLs can regulate melanoma cell lysis in vitro [54], their role in the control of the immune response in vivo has not yet been demonstrated. On the other hand, activating NKRs are also present in a subset of CD8+ T cells and can amplify signals mediated through the TCR acting as costimulatory molecules [52]. Thus, changes in the HLA class-I profile in melanoma cells can have profound effects on both CTL and NK cell responses [1, 12, 32, 46, 47, 50].
It has been recently demonstrated that, the analysis of KIR gene distribution and the frequency of inhibitory and activating KIR genes in melanoma patients and health control did not show significant differences in the Bulgarian population. However, an association of the inhibitory KIR2DL2/2DL3 genes with their HLA class I ligands was found more frequently in melanoma patients suggesting that the prevalence of inhibitory signals could be relevant for the outcome and progression of melanoma [40].
NKR+ T cells are almost totally absent in cord blood but have been found in increased quantity with age [17, 58]. NKR expression on CTLs has been found altered in several clinical conditions that involve chronic activation of the immune system such as HIV infection, tumors, rheumatoid arthritis or aging [2, 4, 6, 23, 59]. Thus, the expression of CD56 on CD8+ T cells has been correlated with the cytolytic effector function [45] and has been found in decreased quantities in HIV infected individuals [59]. In addition, the expression of CD57 on CD8+ T cells can define replicative senescence and antigen-induced apoptotic death [11].
Several data indicate that NKRs are preferentially expressed on differentiated CD8+ CD28− T cells [39, 55, 59]. An increased proportion of CD28− T cells has been reported in aging and other situations of chronic immune stimulation, suggesting that NKR expression on differentiated CD8+ CD28− T cells constitutes a regulatory mechanism of effector function [20, 55, 59]. In addition, it has recently been demonstrated that the differential expression of HLA class-I specific NKRs on CTLs is correlated with transition from effector to memory T cells suggesting a role for NKRs in the survival of CD8+ memory T cells [46, 63, 69].
This work characterizes peripheral blood CD8+ T cells from melanoma patients in relation to the expression of several NKRs and costimulatory molecules. The analysis performed included the study of HLA class-I specific receptors (KIRs, LILRB1 and CD94/NKG2) and non-HLA class-I specific receptors (CD56, CD57, CD161 and CD16) related to the immune response against melanoma. As the expression of NKRs on CD8+ T cells is preferential on CD8+ CD28− T cells, we have also analyzed the expression of these NKRs on CD8+ T cells according to the CD28 phenotype.
Materials and methods
Patients
Seventeen melanoma patients were enrolled in this study (mean age ± SD was 43 ± 11). Patients were diagnosed by the Department of Plastic Surgery at the University Hospital Reina Sofía, Córdoba, Spain, and tumours were classified according to Clark staging (Table 1). Five patients had metastases at the time of this study. Nine healthy volunteers age matched were included as checks (mean age ± SD was 36±10). All subjects gave informed consent under the auspices of the appropriate Research and Ethics Committees.
Table 1.
Clinical characteristics of melanoma patients
| Patient no. | Age (years) | Sex | Breslow | Clark | Metastases |
|---|---|---|---|---|---|
| MEL 6 | 41 | M | NT | V | Yes |
| MEL 8 | 38 | M | 2.5 | III | No |
| MEL 9 | 49 | M | 2.6 | III | No |
| MEL 11 | 50 | M | 4.5 | III | No |
| MEL 13 | 40 | F | 6.2 | III | Yes |
| MEL 16 | 41 | M | 4 | IV | No |
| MEL 19 | 45 | M | 2.5 | III | No |
| MEL 20 | 38 | M | 0.75 | III | Yes |
| MEL 21 | 43 | M | 1.6 | III | Yes |
| MEL 22 | 28 | F | 0.76 | III | No |
| MEL 23 | 54 | F | NT | NT | No |
| MEL 25 | 49 | M | 2.6 | IV | No |
| MEL 27 | 57 | F | 1.8 | IV | Yes |
| MEL 28 | 22 | F | 2.3 | III | No |
| MEL 30 | 52 | M | 2 | III | No |
| MEL 31 | 60 | M | 0.8 | III | No |
| MEL 32 | 47 | M | 1.6 | III | No |
Peripheral blood was collected from patients and healthy donors and peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation over Histopaque-1077 (Sigma, St. Louis, MO, USA).
Flow cytometry
Peripheral blood mononuclear cells from melanoma patients and healthy volunteers were stained with the appropriate combination of the following monoclonal antibodies: peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (SK7) and anti-CD8 (SK1); phycoerythrin (PE)-conjugated, anti-CD8 (SK1), anti-CD16 (B73.1), anti-CD27 (L128), anti-CD28 (L293), CD45RA (L48), anti-CD56 (NCAM16.2), anti-KIR3DL1 (NKB1) and anti-CD161 (DX12) from BD Biosciences (San Jose, CA, USA), anti-CD94 (HP-3B1) from BD Pharmingen (San Diego, CA, USA), anti-CD244 (C.1.7), anti-KIR2DL2-2DL3/KIR2DS2 (gl183) and anti-KIR2DL1/KIR2DS1 (EB6) from Immunotech (Marseille, France), anti-NKG2D (149810), anti-NKG2A (131411) and anti-NKG2C (134591) from R&D Systems Europe (Abingdon, UK); fluorescein isothiocyanate (FITC)-conjugated anti-CD94 (HP-3D9) from Immunotech (Marseille, France), anti-CD28 (CD28.2), anti-CD85j (GHI/75), anti-perforin (δG9) from BD Pharmingen and anti-CD57 (HNK-1) from BD Biosciences. Purified anti-NKG2A (Z199) from Immunotech was used in indirect immunofluorescence. After incubation with the anti-NKG2A mAb (30 min. at 4°C), goat F(ab’)2 anti-mouse IgG (GAM-Ig) conjugated with TriColor (Caltag Laboratories, Burlingame, CA) was added during 30 min. and, after washing, the relevant FITC- and PE-conjugated mAbs were added. For intracellular perforin, after surface-marker labelling, cells were fixed with paraformaldehyde at a final concentration of 4% and permeabilized with 0.1% saponin before intracellular staining with anti-perforin-FITC.
Isotype matched negative control antibodies were used in all the experiments. Analysis was performed using a FACScalibur cytometer and Cell Quest software (Becton Dickinson). Acquisition and analysis was focused on the lymphocyte gate defined by forward and side scatter parameters carefully excluding the monocyte gate. The analysis of PBMCs was also focused on CD8bright T cell subset excluding CD8dim cells that are NK cells defined by the CD3− CD56+ phenotype (data not shown).
Statistical analysis
The differences in the means of measurements as between healthy persons in control and melanoma patients were studied by the Student’s t test for independent values using SPSS (version 9.0) software. The parameters studied had a normal distribution tested by the Kolmogorov-Smirnov test. A p value (two tailed) <0.05 was considered significant.
Results
NKR expression on CD8+ T cells from melanoma patients
We have characterized peripheral blood CD8+ lymphocytes from melanoma patients in relation to the expression of NKRs. Since the expression of NKRs was found increased in elderly individuals, we have included in this study only melanoma patients under 60 years of age (range 22–60). Results were compared with those obtained from age-matched healthy donors. The analysis by multiparametric fluorescence of lymphocyte subpopulations in peripheral blood showed that the percentages of CD3+ and CD3+ CD8+ cells were not statistically different between healthy donors and melanoma patients (Table 2).
Table 2.
Distribution of lymphocyte subsets in peripheral blood
| Control | Melanoma | ||
|---|---|---|---|
| Percentage ± SD | Percentage ± SD | P | |
| CD3+ | 69.24 ± 10.72 | 57.74 ± 17.23 | NS |
| CD3+ aCD8+ | 21.92 ± 8.35 | 19.25 ± 8.18 | NS |
| CD8+ bCD28+ | 78.44 ± 9.33 | 66.25 ± 20.33 | 0.04 |
| CD8+ bCD28- | 21.55 ± 9.33 | 33.73 ± 20.31 | 0.04 |
a Values referred to double positive cells b Values referred to CD8+ T cells
We have analyzed CD8+ T cells for the expression of CD94, NKG2A, CD85j (LILRB1 or ILT2), KIR2DL1/2DS1 (EB6), KIR2DL2/2DL3/2DS2 (gl183), and KIR3DL1 (NKB1), CD16, CD56, CD57, CD161and CD244. The frequency of expression of these receptors was in the order NKG2D> CD244> CD57> CD94> CD56> CD161> CD85j> CD16> KIRs, both in healthy donors and melanoma patients. Thus, as shown in Fig. 1, NKG2D was expressed in the majority of CD8+ T cells, CD244, CD57 and CD94 at an intermediate percentage, whereas CD56, CD161, CD85j, CD16 and KIRs were expressed at low percentages. It is interesting to note that melanoma patients exhibited a greater variability in the expression of NKRs on CD8+ T cells than healthy controls (Fig. 1). A significant increase of the receptor identified by the monoclonal antibody gl183, was observed on CD8+ T cells from melanoma patients. Although CD85j was expressed only in a small population of CD8+ T cells from most individuals, two melanoma patients showed a high percentage (63%, 73%) of CD8+ CD85j+ T cells (Fig. 1). As CD94 can bind either to NKG2A or NKG2C to constitute the inhibitory or the activating receptor for HLA-E, we studied the expression of these markers on T lymphocytes from melanoma patients and healthy persons. Results in Fig. 2 indicate that no significant differences in the expression of NKG2A or NKG2C within the CD94+ T cells were found between healthy persons and melanoma patients. We have found that in most individuals the expression of NKG2A (63±20% of CD94+ T cells in healthy persons and 53±25% in melanoma patients) is higher than the expression of NKG2C in the same subset (20±13% and 21±15% of CD94+ T cells, respectively). Our results also showed a significant increase of CD244, CD57, CD56 and CD16 on CD8+ T cells from melanoma patients when compared to healthy donors (Fig. 1).
Fig. 1.
Percentages of NKR+ CD8+ T cells in healthy donors and melanoma patients. Expression of HLA-class I specific (left panel) and non-HLA-class I specific NKRs (right panel) on CD8+ T cells. Values represent individual and mean percentages of NKR+ within the CD8+ T cells subset in nine healthy donors (black dots) and 17 melanoma patients (white dots). *P<0.05
Fig. 2.
Coexpression of CD94 and NKG2A or NKG2C in T cells in a representative healthy persons (left panel) and melanoma patient (right panel). Values represent the percentage of cells expressing NKG2A or NKG2C within the CD94+ T cells
NKRs are preferentially expressed on CD8+ CD28− T cells
The expression of NKRs on CD8+ T cells was further analyzed in relation to the expression of the costimulatory molecule CD28. We observed that HLA class-I specific and non-HLA class-I specific NKRs were preferentially expressed on the CD8+ CD28− T cell subset both in healthy donors and melanoma patients (Fig. 3). In contrast, the expression of NKRs was very low on the CD8+ CD28+ T cell subset (Fig. 3). The comparative analysis of NKRs on CD8+ T cell subsets according to the CD28 phenotype among healthy donors and melanoma patients did not show significant differences. Since we have found a significant decrease of CD8+ CD28+ cells and an expansion of CD8+ CD28− T cell subset in melanoma patients (Table 2), these results suggest that the increased expression of NKRs on CD8+ T cells is the consequence of the expansion of the CD8+ CD28− T cell subset in melanoma patients.
Fig. 3.
Expression of NKRs on CD8+ T cells in healthy donors and melanoma patients according to the CD28 phenotype. Percentage of expression was analyzed gating on CD8+ CD28+ (upper panel) and CD8+ CD28− (lower panel) subsets. *P<0.05
CD8+ T cells from melanoma patients have high levels of perforin
Simultaneous detection of cell surface membrane antigens and intracellular content of perforin was made by flow-cytometry. Results showed a significant increase of the intracellular content of perforin on CD8+ T cells from melanoma patients when compared with healthy donors (Fig. 4a). Perforin was preferentially expressed in the CD8+ CD28– T cell subset. Although there were no significant differences in perforin expression when analyzed according to the CD28 phenotype (Fig. 4a), a significant increase in the perforin mean fluorescence channel was found in the CD8+ CD28– T cell subset from melanoma patients (Fig. 4b).
Fig. 4.
Analysis of perforin content on CD8+ T cells a Intracellular perforin content on CD8+ T cells, CD8+ CD28+ and CD8+ CD28− T cell subsets in healthy donors and melanoma patients. b Mean fluorescence channel of perforin on CD8+ CD28− subset. *P<0.05
CD28− CD27− CD8+ T cell subset is expanded in melanoma patients
The simultaneous staining of the costimulatory molecules CD27 and CD28 allows the identification of various stages of CD8+ T cell differentiation. Our results showed a significant decrease in the percentage of CD8+ T cells co-expressing of CD27 and CD28 and an increase of the CD28− CD27− CD8+ subset in melanoma patients when compared with healthy persons (Fig. 5), suggesting a shift from naïve to effector cytotoxic T lymphocytes in melanoma patients. Single CD27+ or CD28+ subsets were not significantly affected.
Fig. 5.
Co-expression of CD28 and CD27 molecules on CD8+ T cells from healthy donors (n=9) and melanoma patients (n=17). *P<0.05
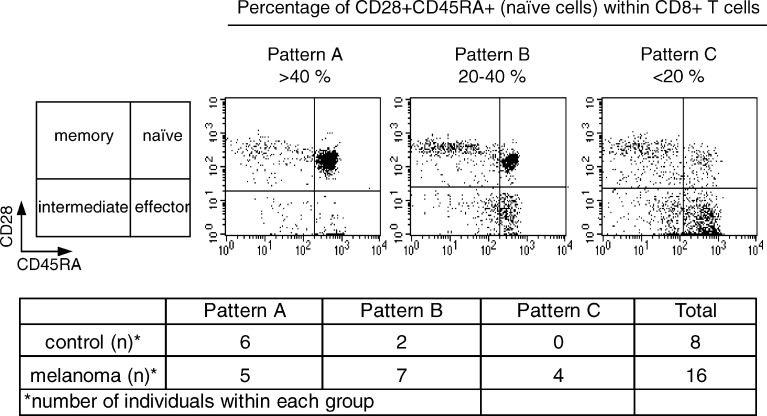
These cells are additionally characterized of these cells was done by studying the co-expression of CD28 and CD45RA on CD8+ T cells that distinguish several stages of differentiation [25]: naïve CD8+ T cells (CD28+ CD45RA+), memory cells (CD28+ CD45RA–) and effector cells (CD28– CD45RA+). The CD28– CD45RA– CD8+ T cells represent an intermediate differentiation stage to effector cells. This analysis demonstrated that three patterns can be distinguished in melanoma patients (Fig. 6) according to the percentage of naïve cells. Pattern A characterized by a high percentage of naïve cells (>40%), Pattern B with a lower percentage of naïve cells (20–40%) and Pattern C with the lowest percentage of naïve cells (<20%). The number of patients included in each group was: pattern A, 5 out of 16; pattern B, 7 out of 16 and pattern C, 4 out of 16. On the contrary, most healthy persons (6 out of 8) showed the pattern A, 2 out of 8 the pattern B and none showed the pattern C.
Fig. 6.
Representative dot plots showing the distribution of naïve, memory and effector CD8+ T cell subsets in the peripheral blood of healthy donors and melanoma patients according to the simultaneous staining with anti-CD28 and anti-CD45RA mAbs. Three distinct patterns can be distinguished according to the percentage of CD45RA+ CD28+ naïve T cells (upper panel). Pattern A characterized by a high percentage of naïve cells (>40%), pattern B with an intermediate percentage of naïve cells (20–40%) and pattern C with a low percentage of naïve cells (<20%). The numbers of healthy donors and melanoma patients with the corresponding phenotype are shown (lower panel)
Discussion
Previous studies have shown that NKRs can be expressed on several subsets of human peripheral blood CD8+ T cells [34, 35, 37, 55, 58, 64]. Further characterization of these subsets showed that NKR+ T cells express intracellular and surface markers characteristic of antigen-experienced T cells. The expression of inhibitory HLA class-I specific NKRs may govern TCR-mediated CD8+ T cell activation [35, 58, 67]. In contrast, activating NKR expressed on T cells can act as co-stimulatory molecules that augment TCR-mediated activation [52]. The expression of NKRs on CD8+ T cells has been found altered in several pathologies including infections and tumours and several changes in the expression of NKRs have been correlated with disease progression [13, 14, 19, 23, 34, 59].
We have analyzed the expression of HLA-specific NKRs on CD8+ T cells from melanoma patients. The analysis of the CD94 C-type lectin receptor shows that this molecule is expressed in a subset of CD8+ T cells and that CD94+ T cells express the inhibitory NKG2A molecule at higher levels than the activating NKG2C molecule both in melanoma and healthy donors. Thus, both forms of the receptor, inhibitory CD94-NKG2A/B and costimulator CD94-NKG2C/E receptors [52] are present in circulating T cells [30]. Differential expression of CD94-NKG2 isoforms has been observed on MART-1 specific T cells in different areas of melanoma. Whereas the inhibitory receptors were found within the vitiligo areas, both activating and inhibitory isoforms were found within the tumor [44]. Vetter et al., by RT-PCR, showed a predominant expression of CD94 associated with NKG2C/E molecules in tumour infiltrating lymphocytes (TIL). The reversal in the ratio of inhibitory to activating receptors in TIL versus peripheral blood lymphocytes could suggest either the presence of specific factors in the tumor microenvironment or the preferential homing of T cells with distinct stages of activation [66]. Furthermore, it has previously been demonstrated that melanoma cell lysis mediated by CTLs can be inhibited by CD94/NKG2A heterodimer [54] but the possible role of CD94-NKG2C/E as regulators of immune response against melanoma should be further analyzed. The ligand of CD94/NKG2 receptors is the nonclassical class I molecule HLA-E [8, 10, 28]. The expression of HLA-E on the cell surface of tumour cell lines (including melanoma cell lines) has been correlated inversely with the expression of other HLA class I molecules suggesting that HLA-E expression on tumour cells could regulate specific CTL-mediated cytotoxicity by interaction with inhibitory or costimulatory CD94/NKG2 receptors [32].
The percentage of KIR+ CD8+ T cells was very low both in healthy donors and melanoma patients, with values below 5% in most individuals. The possible functional relevance of these results are difficult to evaluate since the mAbs used in our study recognize both the inhibitory and activating forms of the receptors and also because the KIR expression depends on the individual genotype. In this respect it is interesting that, the recent report by Naumova et al. [40] showing no significant differences among KIR genotypes between melanoma patients and healthy persons. The expression of the LILRB1 receptor, CD85j, was low in the majority of the individuals and no statistically significant differences were found between melanoma patients and healthy donors. However, two melanoma patients showed a high percentage of CD85j+ CD8+ T cells (63%, 73%). The expression of the inhibitory receptor CD85j on CD8+ T cells has been shown increased in some patients with lymphoproliferative disorders [14]. This receptor has been found to interact through its amino-terminal Ig domain with several classical HLA class I molecules and the nonclassical HLA-G and HLA-F molecules [16, 29, 41]. UL18, a human CMV protein homologous to HLA class I, has also been identified as a ligand for CD85j [15, 18].
We have also observed that circulating CD8+ T cells from melanoma patients have a significant increase of CD56 and CD57 expression. CD56 surface expression has been correlated with cytolytic effector function of CD8+ T cells [45]. Furthermore, it has been described that in vitro stimulation with anti-CD3 leads to an expansion of CD3+ CD56+ T cells that display cytotoxic ability against several tumours including melanoma [24]. It is interesting to note that the expression of CD57 on CD8+ T cells has been associated with chronic antigen stimulation and considered as a marker of proliferative inability [11]. Thus an expansion of CD57+ CD28− CD8+ T cells was also found in myeloma patients probably as the consequence of persistent antigen stimulation [56]. Altogether, our results suggest that the expansion and accumulation of circulating CD56+ and CD57+ T cells in melanoma patients is also caused by antigen-driven activation of specific T cells.
The expression of NKRs on CD8+ T cells from melanoma patients is highly variable suggesting that lymphocyte activity may differ considerably between melanoma patients, as previously described by Speiser et al. on CD3+ T cells [55]. Moreover, the expression of NKRs on CD8+ T cells is preferential in the CD28− subset [5, 13, 55, 59]. Our results showing that circulating CD8+ T cells from melanoma patients have a decreased expression of the costimulatory molecule CD28 when compared with age-matched healthy donors confirm and extend previous work by Martinez-Escribano et al. [33]. It could be speculated that the increase in the percentage of CD8+ CD28− cells is related with the tumour load and that patients with metastatic melanoma should have a greater expansion of the CD8+ CD28− T cell subset. However, our preliminary results show that only one out of the five patients with metastatic melanoma has a significant decrease in the expression of CD28 (data not shown) suggesting that metastatic melanoma patients have a deficient CD8 mediated immune response against the tumour. Further studies are necessary to test this possibility. When the analysis of NKRs was performed according to the CD28 phenotype, no statistically significant differences between melanoma patients and healthy donors were found, suggesting that the increase of NKR+ CD8+ T cells observed in melanoma patients is the consequence of CD8+ CD28− T cell expansions and that the high variability observed in the expression of NKRs might be related to the differences in the proportion of CD8+ CD28− T cells among melanoma patients.
It has been proposed that the CD8+ CD28− T cell subset observed in vivo arose as a consequence of repeated rounds of antigen-driven proliferation [20–22]. In vitro experiments clearly showed that CD28− cells are derived from CD28+ precursors and can be considered as terminally differentiated antigen experienced effector cells that emerge and expand in response to chronic antigenic stimulation [43, 62]. In head and neck cancer patients an expanded proportion of CD8+ T cells lacking CD28 has been observed. In these patients CD8+ CD28− T cells showed an increased apoptosis rate that might suggest that chronic antigenic stimulation drives the recruitment, differentiation and finally death of these cells [62]. After surgical resection of the tumour the proportions of CD28− and CD28+ CD8+ T cells were found normalized [62]. In addition, the downregulation of CD28 on CD8+ T cells has been previously described in many others clinical conditions that involve chronic activation of the immune system as HIV infection or aging [9, 13, 20, 59, 68].
Recent studies show that the simultaneous staining of CD28 and CD27 molecules defines several stages of T cell differentiation [3, 49, 60]. We have found that melanoma patients had a decrease of the naïve CD8+ CD28+ CD27+ T cell subset while the CD28− CD27− CD8+ T cell subset was expanded. Recently, this CD28− CD27− CD8+ T cell subset has been characterized by the expression of perforin, granzyme B, and CD95L [65] and by an increased cytotoxicity and a decreased proliferative potential [25]. Downregulation of CD28 and CD27 has been correlated to the differentiation status of antigen-specific T cells [60, 61, 65].
Within CD8+ CD28− T cells several subpopulations can be distinguished based on the expression of surface and intracellular markers. The increased mean fluorescent channel of perforin within the CD8+ CD28− T cell subset in melanoma patients compared to healthy persons may indicate an active immune response against the tumour. It has been previously described that CD244 expression on CD8+ T cells is correlated with perforin content [53] indicating that CD28− CD27− CD8+ T cells expanded in melanoma patients are terminally differentiated effector cells that are CD244+ and express high levels of perforin.
Based on the coexpression of CD28 and CD45RA, different CD8+ T cells subsets can be defined: naïve T cells (CD28+ CD45RA+), memory T cells (CD28+ CD45RA−) and effector T cells (CD28− CD45RA+) [25]. Whereas the percentage of naïve cells was higher than 40% in 75% of healthy persons, only 31% of melanoma patients showed this phenotype. Interestingly, 25% of melanoma patients had less than 20% of naïve CD8+ T cells further suggesting that a significant expansion of terminally differentiated CD8 effector cells can be found in a high proportion of melanoma patients.
Once generated, tumour-specific effector T cells must undergo clonal expansion in order to maintain control over the tumour. Chronic exposure to tumour antigens either during tumour development or during immunotherapy may hamper the ability of the immune system to prevent cancer occurance or progression as, after multiple rounds of antigenic stimulation, T cells can undergo replicative senescence, comprising irreversible cell cycle arrest and alterations in effector function. In addition to irreversible growth arrest, senescent cells acquire resistance to apoptotic stimuli and show increased levels of bcl2 expression [21]. Together with CD28 as hallmark of immunosenescence, CD57 has also been associated with senescence and is expressed by the majority of CD28− T cells [11, 36, 51, 56].
In conclusion, we have found that circulating CD8+ T cells from melanoma patients display a distinct effector phenotype characterized by downregulation of costimulatory molecules and high expression of NKRs and perforin. The increased percentage of these cells is probably related to the acquisition of an effector phenotype as the consequence of tumour antigen stimulation. We suggest that the increased expression of NKRs on T cells may contribute to the final outcome of the immune response against melanoma, either stimulating or inhibiting the function of CD8 effector cells. Blocking inhibitory receptor function and enhancing activating receptors may represent new strategies with therapeutic potential against melanoma.
Acknowledgements
This work was supported by grants QLRT-2001-00668 (Outcome and Impact of Specific Treatment in European Research on Melanoma, OISTER) and QLK6-CT2002-02283 (T cells in Ageing, T-CIA) from the 5th Framework Program of the European Union, FIS01/0478, FIS03/1383 (to R.S.), FIS00/0853 (to R.T.) from the Ministry of Health, SAF2003-05184 (to R.T.) from the Ministry of Science and Technology and 03/2 (to R.T.) from the “Consejería de Sanidad y Consumo” Junta de Extremadura, (Spain).
Abbreviations
- CTL
Cytotoxic T Lymphocyte
- Ig
Immunoglobulin
- ILT
Immunoglobulin like transcripts
- ITIM
Immunoreceptor tyrosine-based inhibition motif
- ITAM
Immunoreceptor tyrosine-based activation motif
- KIR
Killer cell Immunoglobulin-like receptors
- LILR
Leukocyte immunoglobulin-like receptors
- NK cell
Natural killer cell
- NKR
Natural killer cell-associated receptor
- TCR
T cell receptor
References
- 1.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andre P, Brunet C, Guia S, Gallais H, Sampol J, Vivier E, Dignat-George F. Differential regulation of killer cell Ig-like receptors and CD94 lectin-like dimers on NK and T lymphocytes from HIV-1-infected individuals. Eur J Immunol. 1999;29:1076. doi: 10.1002/(SICI)1521-4141(199904)29:04<1076::AID-IMMU1076>3.3.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 4.Bakker AB, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J Immunol. 1998;160:5239. [PubMed] [Google Scholar]
- 5.Becker JC, Vetter CS, Schrama D, Brocker EB, thor Straten P. Differential expression of CD28 and CD94/NKG2 on T cells with identical TCR beta variable regions in primary melanoma and sentinel lymph node. Eur J Immunol. 2000;30:3699. doi: 10.1002/1521-4141(200012)30:12<3699::AID-IMMU3699>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253. doi: 10.1016/S0531-5565(98)00076-X. [DOI] [PubMed] [Google Scholar]
- 7.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637. doi: 10.1016/S0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 8.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borthwick NJ, Bofill M, Gombert WM, Akbar AN, Medina E, Sagawa K, Lipman MC, Johnson MA, Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS. 1994;8:431. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 11.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera T, Lopez-Nevot MA, Gaforio JJ, Ruiz-Cabello F, Garrido F. Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother. 2003;52:1. doi: 10.1007/s00262-002-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casado JG, DelaRosa O, Peralbo E, Tarazona R, Solana R. CD28 downregulation and expression of NK associated receptors on T cells in aging and situations of chronic activation of the immune system. In: Pawelec G, editor. Basic biology and clinical impact of immunosenescence. Amsterdam: Elsevier; 2003. p. 123. [Google Scholar]
- 14.Casado LF, Granados E, Algara P, Navarro F, Martinez-Frejo M, Lopez-Botet M. High expression of the ILT2 (LIR-1) inhibitory receptor for major histocompatibility complex class I molecules on clonal expansions of T large granular lymphocytes in asymptomatic patients. Haematologica. 2001;86:457. [PubMed] [Google Scholar]
- 15.Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11:603. doi: 10.1016/S1074-7613(00)80135-1. [DOI] [PubMed] [Google Scholar]
- 16.Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cookson S, Reen D. IL-15 drives neonatal T cells to acquire CD56 and become activated effector cells. Blood. 2003;102:2195. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 18.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu ML. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273. doi: 10.1016/S1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 19.De Maria A, Moretta L. HLA-class I-specific inhibitory receptors in HIV-1 infection. Hum Immunol. 2000;61:74. doi: 10.1016/S0198-8859(99)00169-X. [DOI] [PubMed] [Google Scholar]
- 20.Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol. 1997;21:471. doi: 10.1016/S0145-305X(97)00027-X. [DOI] [PubMed] [Google Scholar]
- 21.Effros RB. Replicative senescence of CD8 T cells: potential effects on cancer immune surveillance and immunotherapy. Cancer Immunol Immunother. 2004;53:925. doi: 10.1007/s00262-004-0508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion. Immunol Today. 1997;18:450. doi: 10.1016/S0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 23.Galiani MD, Aguado E, Tarazona R, Romero P, Molina I, Santamaria M, Solana R, Pena J. Expression of killer inhibitory receptors on cytotoxic cells from HIV-1-infected individuals. Clin Exp Immunol. 1999;115:472. doi: 10.1046/j.1365-2249.1999.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gritzapis AD, Dimitroulopoulos D, Paraskevas E, Baxevanis CN, Papamichail M. Large-scale expansion of CD3(+)CD56(+) lymphocytes capable of lysing autologous tumor cells with cytokine-rich supernatants. Cancer Immunol Immunother. 2002;51:440. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamann D, Roos MT, van Lier RA. Faces and phases of human CD8 T-cell development. Immunol Today. 1999;20:177. doi: 10.1016/S0167-5699(99)01444-9. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, De Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199. doi: 10.1016/S1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 27.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 28.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepin EJ, Bastin JM, Allan DS, Roncador G, Braud VM, Mason DY, van der Merwe PA, McMichael AJ, Bell JI, Powis SH, O’Callaghan CA. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol. 2000;30:3552. doi: 10.1002/1521-4141(200012)30:12<3552::AID-IMMU3552>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Botet M, Bellon T. Natural killer cell activation and inhibition by receptors for MHC class I. Curr Opin Immunol. 1999;11:301. doi: 10.1016/S0952-7915(99)80048-X. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Botet M, Bellon T, Llano M, Navarro F, Garcia P, de Miguel M. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol. 2000;61:7. doi: 10.1016/S0198-8859(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 32.Marin R, Ruiz-Cabello F, Pedrinaci S, Mendez R, Jimenez P, Geraghty DE, Garrido F. Analysis of HLA-E expression in human tumors. Immunogenetics. 2003;54:767. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Escribano JA, Hernandez-Caselles T, Campillo JA, Campos M, Frias JF, Garcia-Alonso A, Alvarez-Lopez MR. Changes in the number of CD80(+), CD86(+), and CD28(+) peripheral blood lymphocytes have prognostic value in melanoma patients. Hum Immunol. 2003;64:796. doi: 10.1016/S0198-8859(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 34.Mingari MC, Ponte M, Cantoni C, Vitale C, Schiavetti F, Bertone S, Bellomo R, Cappai AT, Biassoni R. HLA-class I-specific inhibitory receptors in human cytolytic T lymphocytes: molecular characterization, distribution in lymphoid tissues and co-expression by individual T cells. Int Immunol. 1997;9:485. doi: 10.1093/intimm/9.4.485. [DOI] [PubMed] [Google Scholar]
- 35.Mingari MC, Ponte M, Vitale C, Bellomo R, Moretta L. Expression of HLA class I-specific inhibitory receptors in human cytolytic T lymphocytes: a regulated mechanism that controls T-cell activation and function. Hum Immunol. 2000;61:44. doi: 10.1016/S0198-8859(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587. [PubMed] [Google Scholar]
- 37.Moretta A, Moretta L. HLA class I specific inhibitory receptors. Curr Opin Immunol. 1997;9:694. doi: 10.1016/S0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 38.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420. doi: 10.1016/S0167-5699(00)01673-X. [DOI] [PubMed] [Google Scholar]
- 39.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F, Cascinelli N, Parmiani G, Anichini A. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535. [PubMed] [Google Scholar]
- 40.Naumova E, Mihaylova A, Stoitchkov K, Ivanova M, Quin L, Toneva M. Genetic polymorphism of NK receptors and their ligands in melanoma patients: prevalence of inhibitory over activating signals. Cancer Immunol Immunother. 2005;54:1172. doi: 10.1007/s00262-004-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro F, Llano M, Bellon T, Colonna M, Geraghty DE, Lopez-Botet M. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur J Immunol. 1999;29:277. doi: 10.1002/(SICI)1521-4141(199901)29:01<277::AID-IMMU277>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, Hiraide H, Sekine I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawelec G, Wagner W, Adibzadeh M, Engel A. T cell immunosenescence in vitro and in vivo. Exp Gerontol. 1999;34:419. doi: 10.1016/S0531-5565(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen LO, Vetter CS, Mingari MC, Andersen MH, thor Straten P, Brocker EB, Becker JC. Differential expression of inhibitory or activating CD94/NKG2 subtypes on MART-1-reactive T cells in vitiligo versus melanoma: a case report. J Invest Dermatol. 2002;118:595. doi: 10.1046/j.1523-1747.2002.01698.x. [DOI] [PubMed] [Google Scholar]
- 45.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 46.Riteau B, Menier C, Khalil-Daher I, Martinozzi S, Pla M, Dausset J, Carosella ED, Rouas-Freiss N. HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol. 2001;13:193. doi: 10.1093/intimm/13.2.193. [DOI] [PubMed] [Google Scholar]
- 47.Rivoltini L, Barracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A, Restifo NP, Topalian SL, Simonis TB, Rosenberg SA. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149. [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez T, Mendez R, Roberts CH, Ruiz-Cabello F, Dodi TA, Nevot MA, Paco L, Maleno I, Marsh SG, Pawelec G, Garrido F. High frequency of homozygosity of the HLA region in melanoma cell lines reveals a pattern compatible with extensive loss of heterozygosity. Cancer Immunol Immunother. 2005;54:141. doi: 10.1007/s00262-004-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rufer N, Zippelius A, Batard P, Pittet MJ, Kurth I, Corthesy P, Cerottini JC, Leyvraz S, Roosnek E, Nabholz M, Romero P. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood. 2003;102:1779. doi: 10.1182/blood-2003-02-0420. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Cabello F, Klein E, Garrido F. MHC antigens on human tumors. Immunol Lett. 1991;29:181. doi: 10.1016/0165-2478(91)90168-A. [DOI] [PubMed] [Google Scholar]
- 51.Scheuring UJ, Sabzevari H, Theofilopoulos AN. Proliferative arrest and cell cycle regulation in CD8(+)CD28(-) versus CD8(+)CD28(+) T cells. Hum Immunol. 2002;63:1000. doi: 10.1016/S0198-8859(02)00683-3. [DOI] [PubMed] [Google Scholar]
- 52.Snyder MR, Weyand CM, Goronzy JJ. The double life of NK receptors: stimulation or co-stimulation. Trends Immunol. 2004;25:25. doi: 10.1016/j.it.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Speiser DE, Colonna M, Ayyoub M, Cella M, Pittet MJ, Batard P, Valmori D, Guillaume P, Lienard D, Cerottini JC, Romero P. The activatory receptor 2B4 is expressed in vivo by human CD8+ effector alpha beta T cells. J Immunol. 2001;167:6165. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- 54.Speiser DE, Pittet MJ, Valmori D, Dunbar R, Rimoldi D, Lienard D, MacDonald HR, Cerottini JC, Cerundolo V, Romero P. In vivo expression of natural killer cell inhibitory receptors by human melanoma-specific cytolytic T lymphocytes. J Exp Med. 1999;190:775. doi: 10.1084/jem.190.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speiser DE, Valmori D, Rimoldi D, Pittet MJ, Lienard D, Cerundolo V, MacDonald HR, Cerottini JC, Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 56.Sze DM, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, Baxter AG, Fazekas dSG, Basten A, Joshua DE. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(-) compartment. Blood. 2001;98:2817. doi: 10.1182/blood.V98.9.2817. [DOI] [PubMed] [Google Scholar]
- 57.Tarazona R, Casado JG, Soto R, DelaRosa O, Peralbo E, Rioja L, Pena J, Solana R. Expression of NK-associated receptors on cytotoxic T cells from melanoma patients: a two-edged sword. Cancer Immunol Immunother. 2004;53:911. doi: 10.1007/s00262-004-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77. doi: 10.1016/S0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 59.Tarazona R, DelaRosa O, Casado JG, Torre-Cisneros J, Villanueva JL, Galiani MD, Pena J, Solana R. NK-associated receptors on CD8 T cells from treatment-naive HIV-infected individuals: defective expression of CD56. AIDS. 2002;16:197. doi: 10.1097/00002030-200201250-00008. [DOI] [PubMed] [Google Scholar]
- 60.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 61.Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol. 2004;34:999. doi: 10.1002/eji.200324478. [DOI] [PubMed] [Google Scholar]
- 62.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ugolini S, Arpin C, Anfossi N, Walzer T, Cambiaggi A, Forster R, Lipp M, Toes RE, Melief CJ, Marvel J, Vivier E. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat Immunol. 2001;2:430. doi: 10.1038/85246. [DOI] [PubMed] [Google Scholar]
- 64.Ugolini S, Vivier E. Regulation of T cell function by NK cell receptors for classical MHC class I molecules. Curr Opin Immunol. 2000;12:295. doi: 10.1016/S0952-7915(00)00090-X. [DOI] [PubMed] [Google Scholar]
- 65.van Baarle D, Kostense S, van Oers MH, Hamann D, Miedema F. Failing immune control as a result of impaired CD8+ T-cell maturation: CD27 might provide a clue. Trends Immunol. 2002;23:586. doi: 10.1016/S1471-4906(02)02326-8. [DOI] [PubMed] [Google Scholar]
- 66.Vetter CS, thor Straten P, Terheyden P, Zeuthen J, Brocker EB, Becker JC. Expression of CD94/NKG2 subtypes on tumor-infiltrating lymphocytes in primary and metastatic melanoma. J Invest Dermatol. 2000;114:941. doi: 10.1046/j.1523-1747.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 67.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol. 2004;4:190. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 68.Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28- CD8+ T-cell population. Immunology. 1999;98:443. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]