Abstract
Local interleukin 2 (IL-2) therapy is more effective against systemic tumours than systemic IL-2 therapy, but it remains unclear whether IL-2 should be injected intratumourally or peritumourally. To investigate this question, we treated DBA/2 mice bearing a large subcutaneous syngeneic SL2 lymphoma with either intra or peritumoural IL-2 therapy. Both applications enhanced survival, but intratumourally injected IL-2 was more effective than peritumourally injected IL-2. Tumours started to regress 4 days after IL-2 injection. Tumour cells died at the IL-2 injection site, although IL-2 is not directly cytotoxic for SL2 cells in vitro. Tumour cell death correlated well with oedema and extravascular erythrocytes, but less with leukocyte infiltrates. In mice bearing two s.c. tumours, intratumoural application therapy of IL-2 in one tumour caused decrease in size of both tumours in 4–9 days after therapy. However, the IL-2 treated tumours regressed more strongly than the untreated tumours. We conclude that vascular leakage and/or tissue destruction inside the tumour may contribute to the enhanced effect of intratumoural IL-2 therapy compared to peritumoural IL-2 therapy. Hence, we recommend applying of intratumoural rather than peritumoural IL-2 therapy.
Keywords: Interleukin 2, Intratumoural, Vascular leakage, Anticancer therapy, Mechanism
Introduction
Local interleukin-2 (IL-2) application is an effective therapy in mouse tumour models [6, 28], leading to T-cell mediated immunity [27, 28] and rejection of distant untreated tumours [8, 28, 31]. Local IL-2 is effective in bovine [11, 12, 15, 36], equine [37], canine, and feline [38] tumour patients. Also, human patients with papillary bladder carcinoma [10], mesothelioma [24], hepatocellular carcinoma [23], and nasopharyngeal carcinoma [39] can be cured by local IL-2 therapy. In spite of these remarkable clinical results of local IL-2 therapy, it is not clear where exactly IL-2 should be injected [4], intra or peritumourally. On the one hand, peritumoural IL-2 might have an advantage as IL-2 activates leukocytes, and most tumour infiltrating leukocytes are present in the tumour surroundings [30]. On the other hand, intratumoural IL-2 could lead to tumour necrosis due to vascular leakage in the tumour [3]. This paper compares: (a) the histological effects of IL-2 at the site of injection, (b) the therapeutical efficiency of intra and peritumoural injection of IL-2, and (c) the difference between IL-2 induced tumour rejection of treated tumours and of an untreated tumour in the same animal (systemic immunity).
Materials and methods
In vivo experiments
Female DBA/2 JIco mice 6–8 weeks old were obtained from Iffa Credo (France), and kept for 1 week prior to the experiments. Mice were kept in filter-top cages. DBA/2 derived Sutton lymphoma (SL2) was maintained by weekly intraperitoneal (i.p.) transplantation and lavage, as described in [28]. Mice were injected with 100 μl containing 2×105 SL2 cells subcutaneously (s.c.). After 10 days, the cells resulted in a tumour of 100–1,000 mm3 . Before therapy, all mice were randomised, with similar tumour sizes in each group. At day 0 (10 days after tumour inoculation), tumours were treated with a single injection of 1×106 IU human IL-2 (obtained from Chiron, Amsterdam, The Netherlands) in 100 μl PBS with 0.1% bovine serum albumin. PBS injections serve as controls for tissue damage caused by the large injection volume.
The s.c. tumours were measured with callipers in 3-D—length (l), width (w), and height (h). The tumour volume was estimated by π/6×l×w×h. Animals showing difficulty in moving or bearing tumours over 2,500 mm3 were euthanized. The day of euthanasia was recorded as the day of death. The research protocol was approved by the ethical committee for experimental animal research of the Faculty of Veterinary Medicine.
Injection marker
Charcoal (peat charcoal, activated Norit CAL) was co-injected subcutaneously with IL-2 as an injection marker [5]. Peat charcoal suspension (5%) in PBS was filtered through a mesh of 100 μm, autoclaved, and supplemented with 0.1% BSA. Injected charcoal caused no inflammation or tissue damage and remained local for at least 7 days; charcoal did not interact with the therapeutic efficiency of IL-2 or PBS treatment (data not shown).
Histology
For routine histology, the s.c. tumours were dissected, fixed in 4% paraformaldehyde, and embedded in paraffin, or dissected tumours were frozen in liquid nitrogen and stored at −80°C. Frozen sections of 5 μm were fixated in cold acetone. Cryostat or paraffin sections of 5 μm were stained with haematoxylin and eosin (HE), May-Grünwald Giemsa (MGG) or methyl-green pyronine (MGP) [19, 21]. HE staining shows standard histology, whereas MGG is especially suitable for the demonstration of infiltrating leukocytes. The MGP method stains DNA blue and RNA pink. Dead cells can be easily discriminated from viable cells because of the absence of RNA [19, 21, 34]. RNA is both unstable and essential for living cells. Incubation of cells with RNAse A for 20 min removes all pink staining (J.J.L. Jacobs, unpublished data). MGP staining is the most appropriate method to determine cell death, independently of its mechanism [19–21]. The difference between the cytoplasm of dead (white) and viable cells (pink) can be seen easily. Therefore, it is possible to visually estimate white (dead cell) areas versus pink (viable cell) areas. The percentage of tumour cell death proximal and distal to the injection site was estimated by visual perception at 100-fold magnification of MGP-stained slides. Proximal was defined as an area within 150 μm of the injection site; distal was defined as areas located at least 300 μm from the injection site.
Statistical analyses
Differences in tumour sizes were subjected to Students’ t-test to reveal the statistical significance of differences. Survival curves were plotted with the Kaplan–Meier method, subjected to the Mantel–Haenszel log-rank tests, and, if appropriate, analysed with the log-rank test for trend.
Results
IL-2 treated tumours decreased significantly in size at day 4 compared to day 3 (Fig. 1). To study the mechanism of this decrease in tumour size, mice were killed for histology. In these histological experiments, we used five mice for peritumoural PBS injection, five mice for intratumoural PBS, and nine mice for peritumoural IL-2 injections (in one mouse no injection site could be recovered), and ten mice for intratumoural IL-2 injections. Proximal to the intratumoural injection, IL-2 causes a significant increase in cell death, leukocyte numbers, extravasated erythrocytes, and oedema (Fig. 2e). No large areas of cell death were found in the tumour after any PBS injection (Fig. 2a–c); peritumoural IL-2 injection (Fig. 2d) or distal from intratumoural IL-2 injections (Fig. 2f).
Fig. 1.
Relative tumour growth compared to day earlier. At day 0, mice were injected peritumourally with PBS (white bars), peritumoural IL-2 (dark grey bars), intratumoural PBS (light grey bars), or intratumoural IL-2 (black bars). For each day, the relative increase of the tumour size compared to the day before is given. Error bars indicate ±SEM
Fig. 2.
Methyl-green pyronine staining of treated tumours. Methyl-green pyronine stained tumours at day 4 after therapy. Tumour after peritumoural PBS injection (a). Tumour area proximal to intratumoural PBS injection site (b). Distant part of tumour after intratumoural PBS injection (c).Tumour after peritumoural IL-2 injection (d). Tumour area proximal to intratumoural IL-2 injection (e). Distant tumour area after intratumoural IL-2 injection (f). Methyl green stains DNA blue; pyronine stains RNA pink [19, 21]. The site of injection is marked black by charcoal (1). Areas without cells or only erythrocytes are white (2), areas with dead cells show blue nuclei with white cytoplasm (3); viable tumour areas are pink with blue nuclei (4).Dead cell areas are extensive near the injected IL-2 (e). Original magnification ×100
The area of cell death was significantly enlarged at the site of intratumoural IL-2 compared to distal to this site (Table 1), or any site after peritumoural IL-2 (Table 1). Intratumoural injections with PBS caused a small, albeit insignificant increase in cell death, possibly due to the injection volume. Infiltrating leukocytes were significantly increased proximal to the injection site after intratumoural IL-2. No significant differences were found between either proximal or distal to the intratumoural site with peritumoural injection (Table 1). Extravascular erythrocytes were highest proximally to the intratumoural IL-2 injection site, followed by after peritumoural IL-2, distally from the IL-2 injection site, and by PBS injection. Significant differences were found for proximal to the intratumoural IL-2 site and after peritumoural IL-2 compared to PBS. Proximal and distal locations from the intratumoural IL-2 injection site also differed significantly (Table 1). Oedema was significantly increased proximally to the intratumoural IL-2 injection site, compared to distally, after peritumoural IL-2, or after PBS injection (Table 1).
Table 1.
Tumour histology 4 days after therapy
| Treatment | Cell death (%) | Leukocytes (a.u.) | Erythrocytes (a.u.) | Oedema (a.u.) |
|---|---|---|---|---|
| Tumour histology after peritumoural IL-2 | ||||
| PBS | 28±13 | 0.0±0.0 | 0.0±0.0a | 0.4±0.4 |
| IL-2 | 59±6b | 0.4±0.3 | 1.2±0.5a | 0.4±0.3c |
| Proximal to intratumoural injection site | ||||
| PBS | 62±9d | 0.0±0.0e | 0.0±0.0f | 0.8±0.5g |
| IL-2 | 87±4b,d | 1.0±0.3e | 1.9±0.5f | 3.6±0.3c,g |
| Distal to intratumoural injection site | ||||
| PBS | 44±7 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| IL-2 | 59±5 | 0.0±0.0 | 0.7±0.3 | 0.0±0.0 |
| Difference between proximal and distal to the intratumoural injection site | ||||
| PBS | 18±9 | 0.0±0.0h | 0.0±0.0i | 0.8±0.5j |
| IL-2 | 28±6 | 1.0±0.3h | 1.2±0.3i | 3.6±0.3j |
All values ±SEM
a.u. arbitrary units (scale 0–5)
Significant differences are noted in pair-wise comparison of PBS and IL-2 treatment (a, d–j) or after peritumoural and intratumoural treatment (b, c): ap=0.05, bp=0.001, cp<0.0001, dp=0.05, ep=0.02, fp=0.005, gp=0.002, hp=0.02, ip=0.005, jp=0.002
Figure 3 shows the histology near the intratumoural injection site. At the intratumoural injection site, there are extravascular erythrocytes and a few leukocytes. Somewhat further away also tissue oedema can be found.
Fig. 3.

Tumour histology at day 4 after intratumoural IL-2 therapy. (a) Haematoxylin and Eosin staining of a tumour injected with intratumoural IL-2 at day 4. Areas are indicated (1) charcoal as IL-2 injection marker; (2) extravascular erythrocytes; (3) tissue oedema; (4) infiltrating macrophages. a Original magnification ×100. b Magnification of part of (a), ×400
Correlation of histological parameter values with tumour cell death may be an indicator of the mechanisms leading to the tumour destruction. Proximal to the injection site, we found significant correlations of tumour cell death with both oedema and erythrocyte extravasation, but only a very weak correlation with leukocyte infiltration (Fig. 4a). Obviously, these correlations could be either a concomitant effect of IL-2 injection (i.e. IL-2 induces vascular damages and tumour rejection, independently) or a genuine result in which vascular damage is directly related to tumour rejection. To distinguish between these two possibilities, we analysed these data within the group treated with intratumoural IL-2. Within group analysis of the intratumourally IL-2 treated mice showed similar slopes and also significant correlations of tumour cell death with both oedema and erythrocyte extravasation, but no correlation with leukocyte infiltration (data not shown). This shows that the correlations of tumour cell death with oedema and erythrocyte extravasation are not only due to the fact that all these factors are increased by the IL-2 injection, but also another, putative direct correlation may exist. No correlations were found for tumour cell death with oedema, erythrocyte extravasation, or leukocyte infiltration at distal sites (Fig. 4b).
Fig. 4.
Comparing cell death to other histological parameters of treated tumours. a Proximal to charcoal injection site; b distal to charcoal injection site. The percentage cell death is plotted at the x-axis against leukocytes (light grey diamonds), extravasated erythrocytes (dark grey triangles), and oedema values (black squares). Correlations with cell death are drawn for leukocyte (solid line), erythrocyte (broken line), and oedema values (dotted line). The slopes corrected for maximum values are for the proximal histology 1.4, 1.1, and 0.41 for oedema, erythrocytes, and leukocytes, respectively, with correlations (R2) of 0.44, 0.35, and 0.11, respectively. For the distal histology, the corrected slopes are 0.1, −0.01, and 0.1, respectively, with correlations (R2) of 0.03, 0.000, and 0.02 for oedema, erythrocytes, and leukocytes, respectively
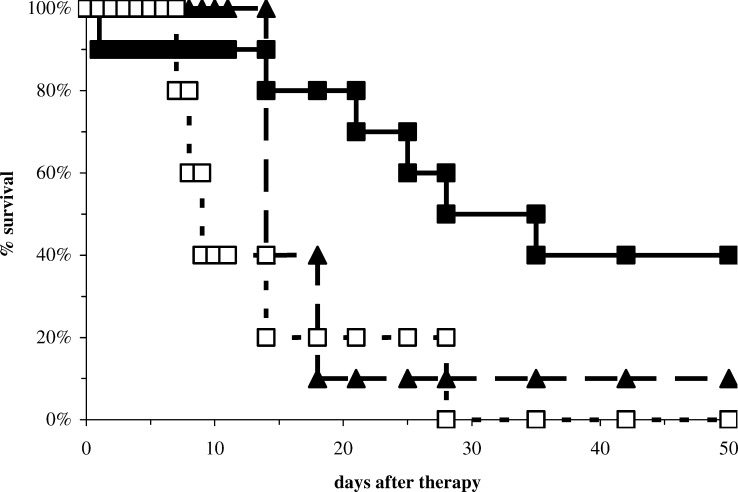
Next, we studied therapeutic efficiency of the different therapies. Tumour-bearing mice were injected with intratumoural IL-2, peritumoural IL-2, or PBS and followed for survival and analysed using the Kaplan–Meier survival analysis (Fig. 5). Intratumoural IL-2 increased survival time compared to the peritumoural IL-2 (hazard ratio=0.41; p=0.04). Intratumoural IL-2 also showed significant better survival than PBS treatment (hazard ratio=0.30; p=0.007). Peritumoural IL-2 appeared to be better than PBS treatment, albeit not significant (hazard ratio=056; p=0.10).
Fig. 5.
Survival after intra and peritumoural IL-2 therapy. Survival of mice after intratumoural IL-2 therapy (n=10; solid line with filled squares), peritumoural IL-2 (n=10; broken line with filled triangles), or PBS treatment (n=9; dotted line with open squares). Day 0 is the day of treatment
Figure 6 shows that either IL-2 therapy reduces the relative tumour size after day 5 (p<0.05) compared to PBS injections. At day 5, this reduction is to 58 and 49% of the PBS-treated tumour for the tumours treated with peritumoural and intratumoural IL-2, respectively. At day 11, these relative sizes are reduced even further to 27 and 9% of the PBS-treated tumours.
Fig. 6.
Tumour size changes after intra and peritumoural IL-2 therapy. Relative changes in tumour size of living mice after intratumoural IL-2 therapy (n=10; solid line with filled squares), peritumoural IL-2 (n=10; broken line with filled triangles), or PBS treatment (n=9; dotted line with open squares). Day 0 is the day of treatment
From day 9 onwards the relative tumour size is smaller (p<0.05) after intratumoural IL-2 than after peritumoural IL-2. Intratumourally treated tumours are 40% at day 9 and decrease to 25% at day 18 of peritumoural-treated tumours.
At days 14, 21, and 18, too few many mice had survived to show significant differences in tumour size, for the comparisons of PBS with peritumoural IL-2, peritumoural with intratumoural IL-2, and PBS with intratumoural IL-2, respectively (see Fig. 5).
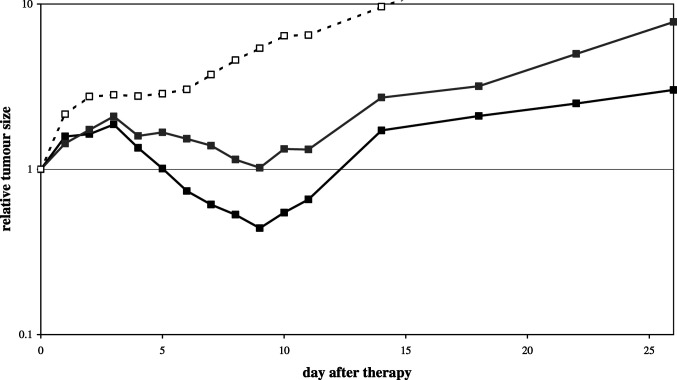
So far, our data have shown that the therapeutic effect of IL-2 injection into the tumour is superior to IL-2 injection near the tumour. Previously, we have shown that IL-2 also induces the rejection of another tumour in the same animal, possibly by inducing systemic immunity [8, 31]. If IL-2 does indeed exert a significant part of its action at the site of injection, as our data suggests, then the rejection of the second tumour would be less strong and/or later. This hypothesis was studied using mice with two subcutaneous tumours, one on the left and the other on the right flank. After randomisation, only one of these tumours was treated with an intratumoural IL-2 injection. The tumour volumes were normalised to 100% at day 0, and the kinetics of tumour rejection was followed. Tumour regression occurred between days 3 and 9 in both tumours in mice treated with IL-2 in one tumour (Fig. 7). Relative to the tumour size of day 3, sizes at day 9 decreased to 24 and 49% for directly and indirectly treated tumours, respectively, whereas untreated tumours are almost twice as big at day 9 compared to day 3. Thus, IL-2 is effective both in the treated tumour of a mouse, as well as in the same tumour at a distant site, but the IL-2 treated tumour regresses more strongly.
Fig. 7.
Relative tumour size in a two-tumour model. Each mouse had two tumours, one treated with intratumoural IL-2 injection, and the other left untreated. Relative tumour size is normalised to day 0 in mice (n=10). The relative size changes of the IL-2 treated tumour (black solid line with black solid squares) and the untreated tumour (grey solid line with grey solid squares) are shown. The relative size changes of PBS treated tumours are shown for reference (open squares). Statistical significant differences (p<0.05) start at day 4 (IL-2 treated tumour versus PBS treated tumour), day 5 (the untreated tumours in IL-2 treated mice versus PBS treated tumour), and at day 6 (IL-2 treated versus untreated tumours in the same mice). Statistical significance of differences is lost after day 10 when the number of mice decreases due to death of mice (due to tumour load)
Discussion
Loco-regional or local IL-2 therapy is more effective against systemic tumours than systemic IL-2 therapy [4]. Leukocyte infiltration at inflammatory sites requires local activation of leukocytes and endothelial cells in a coordinated and defined temporal sequence. It has been hypothesised that in the case of systemic IL-2 therapy, infiltration of leukocytes at tumour sites is suboptimal due to the failure of coordination of these localising events [7]. Although local IL-2 can be applied with therapeutic efficiency both intratumourally and peritumourally, it has not been studied which application is more effective [4]. In this paper, we assess this question and conclude that in this DBA/2—SL2 lymphoma model intratumoural IL-2 therapy is more effective than peritumoural IL-2 (Figs. 5, 6).
IL-2 is not directly cytotoxic to SL2 tumour cells in vitro [28], but 4 days after IL-2 injection the tumour starts to decrease in size (Fig. 1). At the site of injection, IL-2 is associated with tumour cell death (Fig. 2). We found only a limited correlation of leukocyte infiltration with tumour cell death (Table 1). This is in line with earlier reports that show that only a few leukocytes are present near a large area of tumour cell death [29, 30]. In agreement with other earlier findings [3, 9], we found oedema and extravasated erythrocytes associated with tumour cell death (Table 1; Figs. 2, 3, 4). More specifically, we found this only in the proximity of injected IL-2, but not in other regions of the treated tumour.
Our data suggest that IL-2 causes vascular damage and/or leakage. Vascular leakage syndrome is a major toxic side effect associated with systemic IL-2 therapy [13, 14, 35]. Vascular leakage has also been described in local IL-2 therapy [3, 9, 22]. We now show that after intratumoural IL-2 injection vascular leakage occurs more strongly at the site of injection than (a) elsewhere in the tumour, and (b) after peritumoural IL-2 injection (Table 1). The leakage found was specific for tumour vasculature, as the dose of IL-2 we injected did not cause vascular leakage in normal skin tissue of tumour-bearing mice (data not shown).
Vascular leak syndrome has a complex aetiology involving damage to the vascular endothelial cells, extravasation of fluids and proteins, interstitial oedema, and tissue destruction. IL-2 can induce vascular leakage by activating leukocytes, by directly acting on the endothelial cells, or by both mechanisms. At the injection site, IL-2 probably acts directly at the endothelial cells, since vascular leakage is much more severe at the site of injection than elsewhere (Table 1). Endothelial cells are directly responsive to IL-2 as they express the IL-2Rαβγ complex [1] and IL-2 can damage endothelial cells directly [2]. The activated endothelium can trigger disseminated intravascular coagulation via activation of the coagulation cascade [26].
It is stated above that IL-2 causes vascualar damage at the site of injection. Our results also indicated that intratumourally injected IL-2 has a systemic vascular anti-tumour effect. First, the shrinking of treated tumour is more than could be caused by vascular damage at the injection site. Second, also peritumourally treated tumours shrink. And, most important, also regression was found in the distant untreated tumour (Fig. 7). Regressions after peritumoural IL-2, intratumoural IL-2, and the concomitant untreated tumours occur between days 4 and 9 after IL-2 injection (Figs. 6, 7), thus faster than expected from de novo generated immune cells. The systemic effect is probably mediated through cells, as the distance would dilute IL-2 too much to cause vascular leakage.
Cellular anti-tumour effects could be directed at endothelial cells or directly at tumour cells. The rejection of the tumour occurs very suddenly and very rapidly, which would indicate that vascular damage is responsible for this. Also, the finding that the tumour starts growing after necrosis of a major area [31] (Fig. 7) suggests that it is not caused by destruction of individual tumour cells. Local IL-2 eventually leads to systemic immunity [27, 28]. However, rejection of the tumour bulk may not be due to specific memory as tumours may grow out after rejection [31].
Several cells have been implicated as putative anti-tumour effectors. These cells include neutrophils [25], humorally induced cytotoxicity of macrophages [33], and CD8+ [18] and CD4− CD8− [32] T lymphocytes. Infiltration of some cells, especially macrophages, could also be the result of tumour cell death [3]. We chose to study tumour histology at the earliest time point of regression (Fig. 1). This minimises the risk of finding cells, like macrophages, that are attracted by dead tumour cells.
Looking early also reduces the risk of effector cells leaving the site of rejection. Thus, our data argue against a significant role of leukocytes in local tumour destruction at day 4. An alternative explanation is that leukocytes have exerted their anti-endothelial effects while remaining in circulation. Those cells would be responsible, but not be detected by histology at any time point. This effect can be studied by the depletion of each of the cell types mentioned. If deletion of all cells still causes vascular leakage at the site of injection, it would show the direct action of IL-2 on endothelial cells.
No hard data exist on intratumoural versus peritumoural IL-2. Often, a tumour is treated with only at one of these sites, without a rationale. Nevertheless, most often beneficial results were obtained when patients had received IL-2 intratumourally [23, 24, 39, 40]. In the case of bladder carcinoma, IL-2 is instilled in the bladder after partial tumour resection [10]. Since this is not intratumoural injection, this can be argued to be a peritumoural IL-2 application. The difference from peritumoural application is that IL-2 is applied to the centre of the incompletely resected bladder tumour. Thus, IL-2 could still induce vascular leakage directly inside the tumour. Similarly, administration of IL-2 to lung metastases of renal cell carcinoma by inhalation [16, 17] probably implies direct contact between IL-2 and metastases. In short, in all highly effective therapy protocols, IL-2 is in direct contact with the tumour or more specifically its vasculature.
Taken together, IL-2 appears to have two anti-vascular effects; one directly at the site of injection, the other is systemic against tumour vasculature. The anti-vascular effect at the site of IL-2 injection is optimal if IL-2 is injected inside the tumour.
In conclusion, we advise that local IL-2 in clinical practice should be applied inside the tumour, as this is more effective than near the tumour. The improved effect of intratumoural IL-2 is related to the induction of vascular leakage in association with cell death at the site of intratumoural IL-2 injection.
Acknowledgements
We thank Chiron (Amsterdam) for the kind gift of IL-2.
References
- 1.Angiolillo AL, Kanegane H, Sgadari C, Reaman GH, Tosato G. Interleukin-15 promotes angiogenesis in vivo. Biochem Biophys Res Commun. 1997;223:231. doi: 10.1006/bbrc.1997.6435. [DOI] [PubMed] [Google Scholar]
- 2.Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Nat Acad Sci USA. 1999;96:3957. doi: 10.1073/pnas.96.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baselmans AH, Koten JW, Battermann JJ, Van Dijk JE, Den Otter W. The mechanism of regression of solid SL2 lymphosarcoma after local IL-2 therapy. Cancer Immunol Immunother. 2002;51:492. doi: 10.1007/s00262-002-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernsen MR, Tang JW, Everse LA, Koten JW, Den Den Otter W. Interleukin 2 (IL-2) therapy: potential advantages of locoregional versus systemic administration. Cancer Treat Rev. 1999;25:73. doi: 10.1053/ctrv.1998.0115. [DOI] [PubMed] [Google Scholar]
- 5.Bonhomme-Faivre L, Depraetere P, Savelli MP, Amdidouche D, Bizi E, Seiller M, Orbach-Arbouys S. Charcoal suspension for tumour labeling modifies macrophage activity in mice. Life Sci. 2000;66:817. doi: 10.1016/S0024-3205(99)00654-2. [DOI] [PubMed] [Google Scholar]
- 6.Bubenik J, Indrova M, Toulcova A. Immunotherapy of murine sarcomas with interleukin 2. I. Local administration of human recombinant IL-2 preparations. Folia Biol (Praha) 1986;32:384. [PubMed] [Google Scholar]
- 7.Carlos TM. Leukocyte recruitment at sites of tumour: dissonant orchestration. J Leukoc Biol. 2001;70:171. [PubMed] [Google Scholar]
- 8.Characiejus D, Dullens HFJ, Den Otter W. Mechanisms of tumour rejection in the murine DBA/2-SL2 concomitant immunity system. Cancer Immunol Immunother. 1990;32:179. doi: 10.1007/BF01771454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Mik HJ, Koten JW, Maas RA, Dullens HF, Den Otter W. Tumour regression by IL-2 mediated stagnation of blood flow. In Vivo. 1991;5:679. [PubMed] [Google Scholar]
- 10.Den Otter W, Dobrowolski Z, Bugajski A, Papla B, Van Der Meijden APM, Koten JW, Boon TA, Siedlar M, Zembala M. Intravesical interleukin-2 in T1 papillary bladder carcinoma: regression of marker lesion in 8 of 10 patients. J Urol. 1998;159:1183. doi: 10.1097/00005392-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Den Otter W, Hill FWG, Klein WR, Koten JW, Steerenberg PA, De Mulder PHM, Rhode C, Stewart R, Faber JA, Ruitenberg EJ, Rutten VPMG. Therapy of bovine ocular squamous-cell carcinoma with local doses of interleukin-2: 67% complete regressions after 20 months of follow-up. Cancer Immunol Immunother. 1995;41:10. doi: 10.1007/s002620050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den Otter W, Hill FWG, Klein WR, Koten JW, Steerenberg PA, De Mulder PHM, Rutten VPMG, Ruitenberg EJ. Low doses of interleukin-2 can cure large bovine ocular squamous cell carcinoma. Anticancer Res. 1993;13:2453. [PubMed] [Google Scholar]
- 13.Edwards M, Schuschke D, Abney D, Miller F. Interleukin-2 acutely induces protein leakage from the microcirculation. J Surg Res. 1991;50:609. doi: 10.1016/0022-4804(91)90050-V. [DOI] [PubMed] [Google Scholar]
- 14.Harada Y, Yahara I. Pathogenesis of toxicity with human-derived interleukin-2 in experimental animals. Int Rev Exp Pathol. 1993;34:37. [PubMed] [Google Scholar]
- 15.Hill FWG, Klein WR, Hoyer MJ, Rutten VPMG, Kock N, Koten JW, Steerenberg PA, Ruitenberg EJ, Den Otter W. Antitumor effect of locally injected low doses of recombinant human interleukin-2 in bovine vulval papilloma and carcinoma. Vet Immunol Immunopathol. 1994;41:19. doi: 10.1016/0165-2427(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 16.Huland E, Heinzer H, Huland H, Yung R. Overview of interleukin-2 inhalation therapy. Cancer J Sci Am. 2000;6(Suppl 1):104. [PubMed] [Google Scholar]
- 17.Huland E, Huland H, Heinzer H. Interleukin-2 by inhalation: local therapy for metastatic renal cell carcinoma. J Urol. 1992;147:344. doi: 10.1016/s0022-5347(17)37233-6. [DOI] [PubMed] [Google Scholar]
- 18.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Fillion P, van Hagen D, Robinson BWS, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumour cells and tumour-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JJL, Lehé C, Cammans KDA, Das PK, Elliott GR. Methyl green-pyronine staining of porcine organotypic skin explant cultures: an alternative model for screening skin irritants. ATLA. 2000;28:279. doi: 10.1177/026119290002800206. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JJL, Lehé C, Cammans KDA, Das PK, Elliott GR (2000) Screening of skin irritant by RNA detection as a viability marker in porcine organotypic skin explants. In: Progress in the reduction refinement replacement of animal experimentation, vol 31A, pp 601
- 21.Jacobs JJL, Lehé C, Cammans KDA, Das PK, Elliott GR. An in vitro model for detecting skin irritants: methyl green-pyronine staining of human skin explant cultures. Toxicol Vitro. 2002;16:581. doi: 10.1016/S0887-2333(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 22.Koten JW, Van Luyn MJA, Cadée JA, Brouwer L, Hennink WE, Bijleveld C, Den Otter W. IL-2 loaded dextran microspheres with attractive histocompatibility properties for local IL-2 cancer therapy. Cytokine. 2003;24:57. doi: 10.1016/S1043-4666(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 23.Krastev Z, Koltchakov V, Popov D, Alexiev A, Koten J-W, Den Otter W. A case of hepatocellular carcinoma (HCC): treatment with local application of alcohol and interleukin 2 (IL-2) Cancer Immunol Immunother. 2003;50:1647. [PubMed] [Google Scholar]
- 24.Krastev Z, Koltchakov V, Vladov N, Popov D, Milev A, Koten JW, Den Otter W. A mesothelioma that is sensitive to locally applied IL-2. Cancer Immunol Immunother. 2001;50:226. doi: 10.1007/s002620100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Gyorffy S, Lee S, Kwok CS. Effect of recombinant human interleukin 2 on neutrophil adherence to endothelial cells in vitro. Inflammation. 1996;20:361. doi: 10.1007/BF01486739. [DOI] [PubMed] [Google Scholar]
- 26.Locker GJ, Kapiotis S, Veitl M, Mader RM, Stoiser B, Kofler J, Sieder AE, Rainer H, Steger GG, Mannhalter C, Wagner OF. Activation of endothelium by immunotherapy with interleukin-2 in patients with malignant disorders. Br J Haematol. 1999;105:912. doi: 10.1046/j.1365-2141.1999.01453.x. [DOI] [PubMed] [Google Scholar]
- 27.Maas RA, Becker MJ, Weimar IS, De Nooy JC, Dullens HFJ, Den Otter W. Transfer of tumour immunity by both CD4+ and CD8+ tumour infiltrating T lymphocytes activated in vivo by IL-2 therapy of tumour bearing mice. Immunobiology. 1993;188:281. doi: 10.1016/s0171-2985(11)80236-6. [DOI] [PubMed] [Google Scholar]
- 28.Maas RA, Dullens HFJ, De Jong WH, Den Otter W. Immunotherapy of mice with a large burden of disseminated lymphoma with low-dose interleukin 2. Cancer Res. 1989;49:7037. [PubMed] [Google Scholar]
- 29.Maas RA, Dullens HFJ, Den Otter W. Mechanisms of tumour regression induced by low doses of interleukin-2. In Vivo. 1991;5:637. [PubMed] [Google Scholar]
- 30.Maas RA, Dullens HFJ, Henk D, Van Weering J, De Mik HJ, Koten JW, Belger RJ, Den Otter W. Histological analysis of IL-2 induced regression of murine solid SL2-tumors. Biotherapy. 1993;6:83. doi: 10.1007/BF01877421. [DOI] [PubMed] [Google Scholar]
- 31.Maas RA, Van Weering DH, Dullens HFJ, Den Otter W. Intratumoral low-dose interleukin-2 induces rejection of distant solid tumour. Cancer Immunol Immunother. 1991;33:389. doi: 10.1007/BF01741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masztalerz A, Everse LA, Den Otter W. Presence of cytotoxic B220+ CD3+ CD4− CD8− cells correlates with the therapeutic efficacy of lymphoma treatment with IL-2 and/or IL-12. J Immunother. 2004;27:107. doi: 10.1097/00002371-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Masztalerz A, Van Rooijen N, Den Otter W, Everse LA. Mechanisms of macrophage cytotoxicity in IL-2 and IL-12 mediated tumour regression. Cancer Immunol Immunother. 2003;52:235. doi: 10.1007/s00262-003-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffitt P. A methyl green-pyronin technique for demonstrating cell death in the murine tumour S180. Cell Biol International. 1994;18:677. doi: 10.1006/cbir.1994.1096. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735. [PubMed] [Google Scholar]
- 36.Rutten VPMG, Klein WR, De Jong WA, Misdorp W, Den Otter W, Steerenberg PA, De Jong WH, Ruitenberg EJ. Local interleukin-2 therapy in bovine ocular squamous cell carcinoma. A pilot study. Cancer Immunol Immunother. 1989;30:165. doi: 10.1007/BF01669425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spoormakers TJP, Klein WR, Jacobs JJL, Van Den Ingh TSGAM, Koten JW, Den Otter W. Comparison of the efficacy of local treatment of equine sarcoids with IL-2 or cisplatin/IL-2. Cancer Immunol Immunother. 2003;52:179. doi: 10.1007/s00262-002-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziekman PGPM. Local low-dose IL-2 therapy of tumours in companion animals. Anticancer Res. 1999;19:2016. [Google Scholar]
- 39.Jacobs JJL, Hordijk GJ, Jürgenliemk-Schulz IM, Terhaard CHJ, Koten JW, Battermann JJ, Den Otter W. Treatment of stage III-IV nasopharyngeal carcinomas by external beam irradiation and local low doses of IL-2. Cancer Immunol Immunother 54(in press) (DOI: 10.1007/s00262-004-0641-6) [DOI] [PMC free article] [PubMed]
- 40.Krastev Z, Koltchakov V, Tomova R, Deredjian S, Alexiev A, Popov D, Tomov B, Bijleveld C, Koten JW, Jacobs JJL, Den Otter W. Locoregional IL-2 therapy in terminal tumour cases presented in our clinic of gastroenterology: intratumoural IL-2 has beneficial effects in 6 out of 7 patients. (Submitted)