Abstract
Fragile X syndrome is a neurodevelopmental disorder that is caused by the silencing of a single gene on the X chromosome, the Fragile X Mental Retardation 1 (FMR1) gene. Affected individuals display a unique neurocognitive phenotype that includes significant impairment in inhibitory control, selective attention, working memory, and visual-spatial cognition. In contrast, little is known about the trajectory and specificity of any cognitive impairment associated with the fragile X premutation (i.e., “carrier-status”) or its relationship with the recently identified neurodegenerative disorder, fragile X-associated tremor/ataxia syndrome (FXTAS). In the present study, we evaluated a broad sample of forty PM males aged 18-69 years matched on age and IQ to 67 unaffected comparison males. Performance was compared across a range of cognitive domains known to be impaired in Fragile X syndrome (i.e., “full mutation”). Tremor was also assessed using a self-report neurological questionnaire. Premutation males displayed statistically significant deficits in their ability to inhibit prepotent responses, differentiating them from comparison males from age 30 onwards. With increasing age, the two groups follow different trajectories, with premutation males developing progressively more severe problems in inhibitory control. This deficit also has a strong co-occurrence in males displaying FXTAS-related symptomatology (p<0.001). Selective attention was also impaired in premutation males but did not show any disproportionate aging effect. No other cognitive deficits were observed. We conclude that an Inhibitory deficit and its impact across the lifespan are specifically associated with the fragile X premutation status, and may be a precursor for development of a more severe form of cognitive impairment or dementia, which has been reported in patients with the diagnosis of FXTAS.
Introduction
Fragile X syndrome is the most common hereditary cause of developmental delay in males affecting 1 in 4000 live births (de Vries, Halley et al., 1998; Kooy et al 2000; Turner et al 1996). The syndrome is caused by a defect in the Fragile X Mental Retardation 1 (FMR1) gene, located near the end of the long arm of the X chromosome. The FMR1 gene carries a CGG trinucleotide repeat region that in unaffected individuals is usually in the range of 7 – 60 repeats. However, in Fragile X syndrome there is an expansion of these repeats to over 200, known as the full-mutation. At this so-called threshold level, the FMR1 gene is no longer transcribed, resulting in a lack of the encoded protein, the Fragile X mental retardation protein – FMRP. It is the absence of FMRP during early brain development that results in the characteristic cognitive phenotype associated with this syndrome.
Until recently, individuals who carry FMR1 allele expansions with between 55 – ~200 repeats, classified as premutation for Fragile X syndrome (i.e., carrier status), were generally thought to be free from phenotypic effects. However, apparently premutations can be unstable across successive generations eventually giving rise to the Fragile X full-mutation with further expansion past threshold (Kooy, 2003; O’Donnell & Warren, 2002). Both males and females can be carriers of Fragile X syndrome. The absence of observable phenotypic differences in carriers of Fragile X stands in marked contrast the effects of the full mutation (>200 repeats), which results in the moderate to severe mental retardation characteristic of Fragile X syndrome. However, more recent molecular data demonstrate that the moderately expanded CGG repeat region of the gene that occurs in premutation alleles, results in both elevated FMR1 mRNA levels and slight to moderate reductions in FMRP levels (Tassone et al., 2000a; Tassone et al., 2000b). The high prevalence of the Fragile X premutation in the general population, estimated to be 1 in 259 females (Rousseau et al., 1995) and 1 in 813 males (Dombrowski et al., 2002), highlights the necessity of investigating the effect of this condition on cognitive development and functioning. Importantly, there is some evidence that clinically affected carriers display some of the same physical and emotional features typically associated with Fragile X syndrome (Hagerman and Hagerman, 2002; Johnston et al., 2001), albeit with far less severity.
One example of the emerging phenotype in carriers of Fragile X syndrome is the identification of a novel neurodegenerative disorder, the Fragile X-associated tremor/ataxia syndrome (FXTAS), recently identified within a subgroup of older male carriers (> 50 years) (Hagerman et al., 2001; Leehey et al., 2003). FXTAS is specifically associated with the Fragile X carrier status and results in striking neuropathology that include generalized brain atrophy; white matter disease, particularly associated with the middle cerebellar peduncles (MCPs) (Brunberg et al., 2002) as well as eosinophilic intranuclear inclusion bodies in neurons and astroglial cells in broad distribution throughout the CNS (Greco et al., 2002; Hagerman et al., 2003). It is important to note that FXTAS is associated only with carriers of Fragile X and then only within a subset of carrier males aged 50 and over. To date, there is no evidence to suggest that FXTAS is associated with the Fragile X full-mutation.
The pathogenic mechanism of FXTAS is believed to be a toxic “gain of function” resulting from the elevated, abnormally large FMR1 mRNA. Specifically it is thought to result from sequestration of increased amounts of proteins that normally bind to the FMR1 mRNA (Hagerman and Hagerman, 2004). The pathologic mechanisms differ between fragile X syndrome and the premutation condition. Clinical involvement in the premutation condition might arise from two sources, lowered FMRP levels and elevated mRNA levels, with potentially dissociable effects on cognitive functioning. In contrast, clinical involvement in fragile X syndrome arises from the lack of FMRP.
To date, few studies exist that have explicitly examined the pattern of cognitive deficit that may be present in individuals with the fragile X premutation and fewer still that have delineated the premutation male cognitive phenotype (Cornish et al., 2005; Loesch et al., 2003a; Loesch et al., 2003b). Preliminary findings from these studies suggest a neuropsychological profile that includes impairment of social cognition (Cornish et al., 2005), alongside deficits in planning of goal-directed behaviour and in executive functions (Loesch et al., 2003a; Loesch et al., 2003b). In stark contrast, the profile of adult males with the FMR1 full mutation, i.e., Fragile X syndrome, is now well documented with substantial evidence supporting the idea that this condition is not simply characterized by global mental retardation. Rather, the Fragile X neuropsychological profile can be described as comprising uneven abilities within and across cognitive domains that remain stable into adulthood. Relative strengths in vocabulary (Abbeduto et al., 2003), verbal working memory (Jakala et al., 1997), and long-term memory for meaningful and learned information (Cornish et al., 2001; Freund and Reiss, 1991) are accompanied by relative weaknesses in executive control (Cornish et al., 2001; Loesch et al., 2003a), selective and sustained attention (Cornish et al., 2001), visual working memory and visual-motor processing (Crowe and Hay, 1990; Kogan et al., 2004).
The extent to which this profile - or some variation thereof - is present in the carriers of fragile X syndrome is the focus of the present study. As discussed, previous research has demonstrated an association between ataxia/tremor and premutation carriers in older men in the form of FXTAS (Hagerman and Hagerman, 2004; Jacquemont et al., 2003). Previous publications have also reported many cases of mild to severe dementia in older males carriers of the premutation whether these patients were ascertained through neurology clinics (Van Esch et al., 2005), or through family studies of Fragile X syndrome (Hagerman et al., 2001). Here, we examine aspects of cognition known to be affected in full-mutation Fragile X males, through a cross-section of the lifespan of premutation males. We raise the possibility that – as with motor control – there is a trajectory of specific and clinically important cognitive deficits that while initially subtle, progress to culminate in significant functional impairments with increasing age. We also address the extent to which potential FXTAS symptoms impact upon cognitive performance and whether there are specific weaknesses across or within cognitive domains that precede the onset of ataxia/tremor. We also explore whether individuals with the premutation allele may vary in their risk of developing more serious effects of CGG repeat expansion in proportion to the number of repeats they possess. Thus, the aim of the research presented here is to provide new information about the specific cognitive deficits in the premutation population so that clinicians will have a reliable tool to identify patients who may need intervention and who may be at risk for developing FXTAS.
Methods
The study enrolled a total of 107 participants, of whom 40 were pre-mutation males. Recruitment was conducted through the UK Clinical Genetics Services and the UK Fragile X Society. Participants’ ages ranged from 20 to 69 years with a mean age of 46.88 years (SD 14.50). The comparison group comprised 67 non-affected adult males with normal FMR1 alleles and were matched individually on the basis of age to the premutation group. Ages ranged from 20 to 68 years, with a mean age of 45.33 years (SD 14.87). The group means did not differ on socio-economic status or occupational status. The entire sample was Caucasian (indigenous white British). Ethics approval to conduct the study was obtained from regional and local ethics committees across the United Kingdom.
Fragile X DNA testing
Direct PCR was carried out using primers F5’CACGACGTTGTAAAACGACACGGAGGCGCCGCTGCCAGG3’, R5’GAGAGGTGGGCTGCGGGCGCT3’, modified from Wang, Green, Bobrow, and Mathew (1995) at 0.5 pmol final concentration. Conditions were as follows: Final concentration 1mM MgCl2, dATP,dCTP & dTTP at 0.2 mM, 7-deazaGTP (AmershamPhamaciaBiotech) at 0.4 mM supplemented with 5% DMSO in a total volume of 20 μl (Wang et al., 1995). Cycling conditions were 32 cycles at 67°C annealing. Products were separated on PAGE gels and visualized by silver staining according to standard protocols. Where the premutation was visible on PCR the repeat size was calculated according to size markers and by electropheresing the products in size order and aligning the stutter bands. Southern blotting was carried out according to standard protocols on genomic DNA using a double digest of EcoR1 (NEB) and the methylation sensitive enzyme Eag1 (NEB) and probed with Ox1.9 (Knight et al., 1993).
Sizing was relative to a female control of known repeat size. Where possible, repeat sizes derived from single bands (SB) were compared to those obtained from direct PCR. Repeat sizes of those individuals who gave a result on direct PCR and on SB were congruent. Blots were over-exposed to detect any evidence of mosaicism against a known mosaic control. A premutation is defined here as an allele between 55 CGG repeats up to approximately 200 repeats without any evidence of abnormal methylation. Mosaicism was considered present when there was evidence of a methylated cell line as well as an unmethylated premutation cell line.
Neuropsychological Evaluation
Intellectual level was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999), which produced a composite IQ score based on four subtests tapping both verbal and performance domains.
A comprehensive battery of neuropsychological tests was administered at study visits. We examined performance within specific cognitive domains known to show impairment in males with the Fragile X full mutation. In particular, we evaluated the following abilities: response inhibition, selective attention, sustained attention, visual-spatial functioning, and visual working memory. Descriptions of both the specific cognitive domain as well as the tests administered for the purpose of evaluating each domain are provided below.
Response inhibition
From a neuropsychological perspective, successful performance on tasks of response inhibition require coordination of several cognitive processes in order to withhold a response that has been strongly triggered by a particular context or cue. Functional imaging studies have generally found increased prefrontal activation during performance on these tasks including activation in the right inferior frontal cortex, right dorso-lateral prefrontal cortex and right inferior parietal cortex [e.g. (Aron et al., 2003; Garavan et al., 2002)]. The following tests were administered:
(1) The Hayling Sentence Completion Test (Burgess and Shallice, 1997). In this two part task, participants had to complete a sentence with a single word, which was either congruous (Section A) or totally incongruous (Section B) with the overall meaning of the sentence. In each case the word is strongly cued by the sentence content with a total of 15 sentences in each section. An error score was obtained which represented the number of inappropriate sentence completion items where the response word is either the word most strongly cued by sentence content or semantically related (see Burgess and Shallice, 1997).
(2) The Stroop Color-Word Test (Trenerry et al., 1989). Participants are presented with lists of color names printed in colored ink. The color name is incongruent with the color of the ink in which it is printed. In the first part, participants must read aloud 112 color words (red, blue, green, tan) within a 2 minute time limit. In the second part, the 112 color words are again presented but this time the participant must say the color of the ink and not the color word itself (e.g., with the word ‘green’ written in blue ink, the participant should say blue). The score is represented by the number of errors made on the interference condition.
Attention
Selective attention, as assessed here, refers to a speeded search in a visually noisy environment for targets defined by a certain feature. Such visual selection has been argued to stem from interactions between regions involved in basic visual processing and those involved in storing representations of the target in memory that exert a ‘top-down’ influence on the system. Animal models and human functional imaging studies suggest that a network linking dorso-lateral and ventro-lateral prefrontal cortex and inferior parietal regions are involved in this process (Duncan, 2006). In terms of sustained attention, the processes of actively maintaining attention to tasks that lack inherent interest or reward have also been linked to networks that incorporate the prefrontal (i.e., right dorso-lateral) and parietal cortices (Pardo et al., 1991). The following tests were administered:
(1) For selective attention, The Test of Everyday Attention- Map Search Task (Robertson et al., 1994). This timed visual search task requires participants to identify target symbols (e.g. a knife and fork sign representing restaurant facilities) from competing and irrelevant distracters on a large colour map of Philadelphia. The score is the total number of correctly identified symbols across two timed conditions.
(2) For sustained attention, The Sustained Attention to Response Test (Manly et al., 2000). This computerized test requires participants to make responses to digits (1 – 9) presented on a screen, except when the critical ‘no-go’ digit (i.e., the number 3) appears. The score includes errors of commission and omission.
Visual Functions
Following on from earlier animal studies, recent human functional imaging studies contrasting visual working memory tasks with suitable control conditions have reported increased activation in dorso-lateral and ventro-lateral regions of the right prefrontal cortex as well as with increased activation in posterior parietal cortex (Bor et al., 2003; Owen, 2000; Owen et al., 1996). In contrast, basic visual functions, such as those required for visual-spatial processing, are thought to rely more on posterior systems, and in particular, the occipital-parietal region. The following tests were administered for:
Visual spatial function:
(1) The Visual Object and Space Perception Battery-Cube Analysis Task (Warrington and James, 1991). Participants are shown 3-dimensional drawings of patterns of solid blocks and they have to estimate the correct number of blocks used to complete the pattern. The score is the total number of correct items.
(2) The Benton Line Orientation Test (Benton et al., 1978). Participants are shown a booklet that contains two parts: the stimuli on the upper part contains a pair of lines that vary in angular separation; and the stimuli on the lower part contains an array of lines 1.5 inches in length labelled ‘1’ through ‘11’ drawn at 18-degree intervals from the point of origin. Participants are required to identify the numbers of the corresponding lines on the lower whole array. The total score is the number of correct items.
Visual memory function:
(3) The Dot Test of Visuospatial Working Memory (Bollini et al., 2000). Participants are presented with a card in which a dot is present at a specific location. Following a 10-second interval, the stimulus card is removed, and the participant is asked to reproduce the dot at the same location on a blank card. The total score is the number of correct items.
(4) The Wechsler Memory Scale (III)- Spatial Span Forward task (Wechsler, 1997). Participants are required to repeat spatial-temporal sequences performed by the examiner. The sequences are a series of taps on 10 identical blocks laid out in two dimensions. The number of blocks tapped increases by one until the participant fails two consecutive trials with the same number of blocks. The total score is the number of correct items.
Neurology Questionnaire
Participants self-reported neurological symptoms on a questionnaire derived from Jacquemont and colleagues (Jacquemont et al., 2004). The neurology questionnaire comprised 2 domains: Tremor: Questions were asked regarding the presence, characteristics, and time-of-onset of tremors; Gait and lower extremities: Questions were asked related to the onset of balance problems, recent falls, and walking distance. The questionnaire was completed over the phone or in person. For the purpose of the survey, symptoms were scored as present if noticed by the respondent, with clarification of the questions or characterization of the symptoms being provided by the interviewing physician as necessary. The participant gave the final answers. The reliability of this questionnaire was previously evaluated by comparison with blind videotape scoring of matched clinical neurological evaluations and found to be highly congruent (Jacquemont et al., 2004).
Statistical Analysis
Of the 107 participants recruited to our study, 3 had missing values for one or more of the neuropsychological measures, and thus were removed from further analysis. The remaining participants comprised 40 premutation males and 64 comparison males.
In order to obtain a single combinatory measure for each of the five cognitive domains: Inhibition, Selective Attention, Sustained Attention, Visual Working Memory and Visual-Spatial function), separate principle component analyses (PCA) were employed. Within each domain all measures were standardized across all participants. Standard linear regression analysis was then performed with cognitive domain scores as dependent variables, and the premutation status (PM or NC), the participant’s age (Age), and the age and status interaction (Age×Status) as explanatory variables. After a full model was fitted to the data, we then employed a backward stepwise variable elimination procedure. A parsimonious model was then obtained for each cognitive domain score.
Results
Intellectual level
The groups did not differ significantly on IQ (p-value 0.12) with all participants demonstrating IQ’s within the normal population range (pre-mutation group: mean full scale IQ 103.8, verbal IQ 101.0, Performance IQ 105.6; comparison group: mean full scale IQ 110.5, verbal IQ 107.5, Performance IQ 109.9).
Cognitive domains
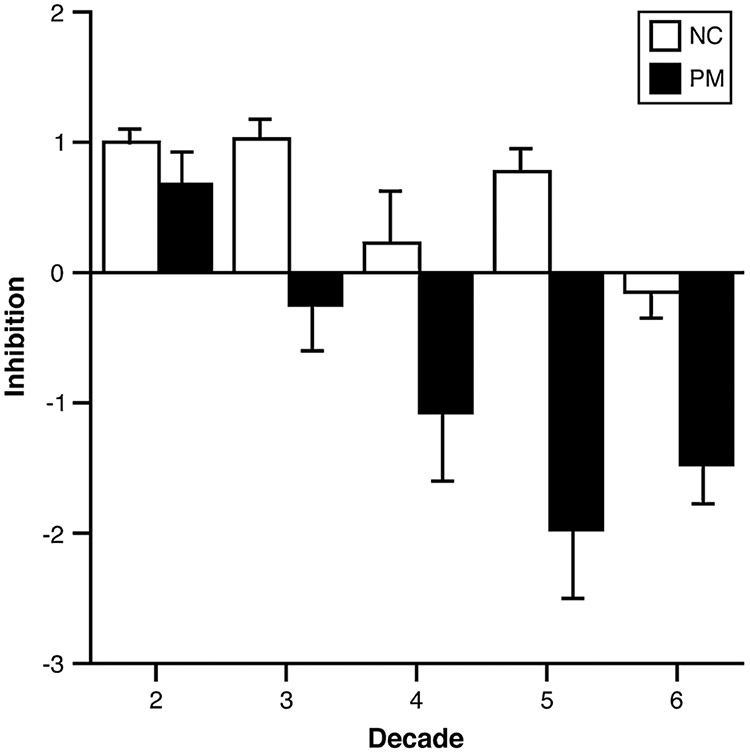
For response inhibition, both the main effect of Age and the Age×Status interaction served as significant explanatory variables (p < 0.001 for both factors). In other words, both response inhibition and differences in response inhibition scores between the premutation and the comparison groups varied with increasing Age. This pattern is clearly seen in Figure 1. Younger male premutation participants differ little from the comparison males on their performance of inhibition tasks, but with increasing age the two groups markedly diverge. Beginning in their 30s, male carriers follow a significantly different trajectory from controls, developing progressively more severe problems in inhibitory control.
Figure 1:
Age trajectories by decade across the cognitive domain of response inhibition in premutation males (PM) versus comparison males (NC). Whereas both groups demonstrate a decline in response inhibition, the PM participants display a relatively disproportionate deterioration in inhibitory functioning with age.
Further analysis to determine the impact of FXTAS related symptoms on performance was examined by using the earliest reported age of onset of diagnosable FXTAS (~ 50 years) (Greco et al., 2002) as a cut-off age and then comparing premutation males (with and without possible FXTAS) to comparison males across the two age ranges (<50 and >50). Given the relatively small sample of premutation males under 50 years who present with FXTAS related symptoms, pair wise comparisons did not include this subgroup. The results are as follows. In males under 50 years, performance was significantly impaired in premutation males without FXTAS symptoms compared to comparison males (p<0.02). In males over 50 years, performance was significantly impaired in premutation males with FXTAS related symptoms compared to comparison males (even when we include those with tremor and gait) (p<0.01). In contrast, premutation males without FXTAS symptoms did not demonstrate a significant difference relative to comparison males (Figure 2).
Figure 2:
Response inhibition is plotted for participants divided according to a cut score of age 50, which represents the mean age of onset for FXTAS. Data for premutation males diagnosed with FXTAS (PM - FXTAS), premutation males without FXTAS (PM), and comparison males (NC) are presented. Data for those premutation individuals with a diagnosis of FXTAS and younger than 50 are not plotted because a small sample size (n=2) obviated inclusion for the statistical analysis. Whereas the PM and NC males show statistically significant differences (p < 0.02) in response inhibition for younger men, this comparison is not significant for older men. Of all the groups, PM males with a diagnosis of FXTAS display the greatest impairment in response inhibition.
The finding of significant interaction of Age X Inhibitory Control prompted us to examine the possibility that one of the contributing factors to this effect was the length of CGG repeats. CGG repeat analysis revealed a significant correlation between severity of inhibitory control deficit and the number of CGG repeats in premutation males such that the larger the repeat size the poorer the performance (r=0.49; p<.001).
For selective attention, the final model retains Age and Status as significant explanatory variables (the p-values are 0.01 and 0.02 respectively). A close examination of Figure 3 reveals that, as Age increases performance declines. Additionally, there is a significant difference between the premutation and the comparison males that remains nearly constant across Age but there is no significant Age × Status interaction.
Figure 3:
Age trajectories by decade across the cognitive domain of selective attention in premutation males (PM) versus comparison males (NC). Both groups display similar rates of decline in selective attention abilities with age. However, the PM group manifests poorer selective attention across all decades tested
For the three remaining cognitive domain scores: sustained attention, visual working memory and visual-spatial function only Age was found to be a significant explanatory variable in the final model.
Discussion
Our primary aim was to establish whether individuals who are carriers of the Fragile X premutation show subtle impairments on neuropsychological tasks that assess cognitive domains previously demonstrated to be impaired in individuals with full mutation Fragile X syndrome. In addition, we sought to identify the trajectory and specificity of any such impairment – in particular, whether phenotypic effects of the premutation become more apparent and more severe with increasing age. Finally, we sought to address the extent to which a newly identified neurological disorder associated with the Fragile X premutation, FXTAS, may impact on performance in identified domains of cognitive weakness. Our findings are remarkable in three respects.
First, carrier males with the premutation performed significantly worse than males without the premutation on tasks that require inhibitory and executive control in particular. At a cognitive level, problems in attention switching and control have been reported as a fundamental deficit in the Fragile X full mutation from infancy, through childhood and into adulthood (Cornish, Scerif & Karmiloff-Smith 2006; Cornish, Sudhalter and Turk 2004; Cornish, Munir and Cross, 2001). Our findings extend this characteristic profile to include individuals with the Fragile X premutation. Although carrier males appear to possess a less severe deficit as compared to males with the Fragile X full mutation (who will also have mental retardation), the result is nonetheless strongly suggestive of a significant executive dysfunction in these individuals.
Second, we report here the first time, a correlation between CGG repeats length and inhibitory impairment in premutation males. This finding suggests greater neuropathology in carrier males with larger expansions in FMR1 mRNA transcripts. It is therefore possible that expression of FMR1 mRNAs containing repeat expansions approaching the full mutation range (i.e., 200 repeats) produce exceptionally toxic effects to neurons comprising brain circuits critical to inhibitory control. This result is consistent with previous research suggesting that individuals with larger premutations display clinical features approaching the full mutation condition (Hagerman and Hagerman, 2004; Tassone et al., 2000a; Tassone et al., 2000b).
At the brain level, difficulties in inhibitory control have been associated with disruptions to frontal circuits, in particular to the right inferior frontal cortex (IFC). Evidence of this comes from functional imaging of brains of individuals with Attention-Deficit Hyperactivity Disorder (ADHD), a population in which response inhibition is proposed to be a critical endophenotype (Aron and Poldrack, 2005). Similarly, studies of naturally occurring lesions in humans implicate the IFC in inhibitory control (Aron et al., 2003). The tasks employed in the present study evaluated the domains of attention, perception, and response inhibition, all proposed to engage prefrontal cortical areas as well as regions of the parietal lobe. However, the finding of a selective and worsening deficit with age in response inhibition in the premutation males specifically implicates the right IFC, an area exclusively activated in tasks of response inhibition. Interestingly, results of a functional imaging study of fragile X affected females, who display a broad range of functioning dependent on the degree of activation of the affected FMR1 gene, indicate a correlation between activity in the right IFC during a response inhibition task and FMR1 expression levels (Menon et al., 2004). Taken together, data from full mutation males, fragile X affected females, and now premutation males, strengthens the notion that the right IFC has differential susceptibility to alterations in FMR1 gene function.
Third, we report significant differences in response inhibition between younger (i.e., less than 50 years old) premutation and comparison males. This difference, however, is not evident when performance of older (i.e., greater than 50 years old) premutation and comparison males is contrasted. This unusual finding may be explained by differential survival of subgroups of premutation carriers. Young premutation males are much less likely to manifest FXTAS or other related pathology and would therefore be considered healthy even if a subgroup of them harbours incipient yet undetectable pathology. As individuals transition to the older group it is likely that morbidity and mortality rates increase. This may result in a bias in the older group we define here as not displaying symptoms of FXTAS, because a relatively larger proportion of the sample were never going to develop FXTAS in the first place. The category of older premutation males who were to develop FXTAS are more likely to express symptoms at this age and therefore will be accurately categorized as belonging to the premutation males with FXTAS group. A longitudinal study would be able to address this hypothesis and this could be the basis of a future study.
Given that the inhibition deficits appear to have their onset in younger premutation males before the first signs of FXTAS-related symptoms it may therefore point to disruption of inhibitory control as a useful neurological soft sign preceding more generalized and profound effects associated with FXTAS (i.e., brain atrophy, ataxia, peripheral neuropathy, progressive intention tremor, dementia) reported in older patients (>50 years) (Hagerman and Hagerman, 2004). The present study found inhibitory deficits to have a strong co-occurrence in older males with FXTAS-related symptoms but not in older males without FXTAS symptoms. This finding strongly supports the idea that inhibitory deficits are a defining feature of FXTAS and possibly predictive of at-risk patients. It is therefore possible that inhibitory deficits in premutation males are a marker of an early onset of a pathological process affecting prefrontal cortex.
As a parallel to our findings, early neuropsychological markers have demonstrated clinical utility in identifying patients with so-called mild cognitive impairment (MCI), a precursor condition to Alzheimer’s dementia (AD). Neuropsychological tests of episodic memory, which employ a visual-spatial learning task prove to be good predictors of those patients with MCI who will go on to develop to AD (Blackwell et al., 2004; Swainson et al., 2001). Identification of MCI patients with a specific neuropsychological profile allows for earlier intervention and in turn, a more prolonged period without the onset of frank dementia. The selectivity of the inhibitory deficits identified in the present study of PM males, supports the use of measures of inhibitory control as potential predictors of risk for later development of FXTAS cognitive symptoms. Although there are no known medications to prevent FXTAS, those such as Venlafaxine can be helpful in improving inhibitory deficits.
Performance in other cognitive domains also provides a window to explore the premutation phenotype. The relatively normal performance and age trajectory on tests of visual working memory, visuo-spatial functions and sustained attention also suggest a rather specific effect of the FMR1 premutation within cognitive function. Given previous evidence, the presence of a disproportionate aging effect may itself form a useful marker of functions that are closely related to the premutation condition. In this respect, selective attention performance, while at a lower rate than comparison males overall, showed no such increase with age. This suggests that there are two effects of the premutation on cognition, one present throughout life and another that increases in severity with age. The latter effect is most likely related to the toxic mRNA effect that can cause cell death and brain atrophy over time, and eventually the full clinical picture of FXTAS. The former effect may be related to a mild reduction in FMRP level which results in a subtle yet measurable Fragile X phenotype including attentional problems. Given that the specific difficulties in inhibitory control exhibited in the premutation males appear to become worse with age, it is likely that these are related to the mRNA toxic effect that is known to accrue with age.
Taken together, these findings highlight an important trajectory of cognitive deficit in premutation carrier males of Fragile X syndrome that appears to begin early in adulthood and become progressively more severe across developmental time. Pieces of the puzzle still need to be explored. For example, a careful investigation of the childhood profile is essential and is hitherto unexplored. Yet precursors of cognitive decline may present even earlier than the thirties having their roots in late childhood.
Overall, the pattern of results highlights a distinct inhibitory ‘signature’ in premutation males that corresponds to the well-documented attentional control impairment in full mutation males with Fragile X. Furthermore, our finding that CGG repeat length correlates with the degree of impairment suggests that increasingly large mRNA transcripts of the FMR1 gene damage susceptible neural networks, in particular, those associated with the right IFC.
Acknowledgments
This research was supported by grants from the Wellcome Trust, NICHD, NIEHS, and NINDS. We express our thanks to the all the regional genetics centres that took part in the study and to the fragile X Society for their support in recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABBEDUTO L, MURPHY MM, CAWTHON SW, RICHMOND EK, WEISSMAN MD, KARADOTTIR S, and O'BRIEN A. Receptive language skills of adolescents and young adults with down or fragile x syndrome. American Journal of Mental Retardation, 108: 149–60, 2003. [DOI] [PubMed] [Google Scholar]
- ARON AR, FLETCHER PC, BULLMORE ET, SAHAKIAN BJ, and ROBBINS TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6: 115–6, 2003. [DOI] [PubMed] [Google Scholar]
- ARON AR and POLDRACK RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry, 57: 1285–92, 2005. [DOI] [PubMed] [Google Scholar]
- BENTON AL, VARNEY NR, and HAMSHER KD. Visuospatial judgment. A clinical test. Archives of Neurology, 35: 364–7, 1978. [DOI] [PubMed] [Google Scholar]
- BLACKWELL AD, SAHAKIAN BJ, VESEY R, SEMPLE JM, ROBBINS TW, and HODGES JR. Detecting dementia: Novel neuropsychological markers of preclinical alzheimer's disease. Dementia and Geriatric Cognitive Disorders, 17: 42–8, 2004. [DOI] [PubMed] [Google Scholar]
- BOLLINI AM, ARNOLD MC, and KEEFE RS. Test-retest reliability of the dot test of visuospatial working memory in patients with schizophrenia and controls. Schizophrenic Research, 45: 169–73, 2000. [DOI] [PubMed] [Google Scholar]
- BOR D, DUNCAN J, WISEMAN RJ, and OWEN AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron, 37: 361–7, 2003. [DOI] [PubMed] [Google Scholar]
- BRUNBERG JA, JACQUEMONT S, HAGERMAN RJ, BERRY-KRAVIS EM, GRIGSBY J, LEEHEY MA, TASSONE F, BROWN WT, GRECO CM, and HAGERMAN PJ. Fragile x premutation carriers: Characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. American Journal of Neuroradiology, 23: 1757–66, 2002. [PMC free article] [PubMed] [Google Scholar]
- BURGESS P and SHALLICE T. The Hayling and Brixton tests. England: Thames Valley Test Company, 1997. [Google Scholar]
- CORNISH K, KOGAN C, TURK J, MANLY T, JAMES N, MILLS A, and DALTON A. The emerging fragile x premutation phenotype: Evidence from the domain of social cognition. Brain and Cognition, 57: 53–60, 2005. [DOI] [PubMed] [Google Scholar]
- CORNISH KM, MUNIR F, and CROSS G. Differential impact of the fmr-1 full mutation on memory and attention functioning : A neuropsychological perspective. Journal of Cognitive Neuroscience, 13: 144–50, 2001. [DOI] [PubMed] [Google Scholar]
- CROWE SF and HAY DA. Neuropsychological dimensions of the fragile x syndrome: Support for a non-dominant hemisphere dysfunction hypothesis. Neuropsychologia, 28: 9–16, 1990. [DOI] [PubMed] [Google Scholar]
- DOMBROWSKI C, LEVESQUE S, MOREL ML, ROUILLARD P, MORGAN K, and ROUSSEAU F. Premutation and intermediate-size fmr1 alleles in 10572 males from the general population: Loss of an agg interruption is a late event in the generation of fragile x syndrome alleles. Human Molecular Genetics, 11: 371–8, 2002. [DOI] [PubMed] [Google Scholar]
- FREUND LS and REISS AL. Cognitive profiles associated with the fra(x) syndrome in males and females. American Journal of Medical Genetics, 38: 542–7, 1991. [DOI] [PubMed] [Google Scholar]
- GARAVAN H, ROSS TJ, MURPHY K, ROCHE RA, and STEIN EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage, 17: 1820–9, 2002. [DOI] [PubMed] [Google Scholar]
- GRECO CM, HAGERMAN RJ, TASSONE F, CHUDLEY AE, DEL BIGIO MR, JACQUEMONT S, LEEHEY M, and HAGERMAN PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile x carriers. Brain, 125: 1760–71, 2002. [DOI] [PubMed] [Google Scholar]
- HAGERMAN PJ, GRECO CM, and HAGERMAN RJ. A cerebellar tremor/ataxia syndrome among fragile x premutation carriers. Cytogenetics and Genome Research, 100: 206–12, 2003. [DOI] [PubMed] [Google Scholar]
- HAGERMAN PJ and HAGERMAN RJ. Fragile x-associated tremor/ataxia syndrome (fxtas). Mental Retardation and Developmental Disabilities Research Reviews, 10: 25–30, 2004. [DOI] [PubMed] [Google Scholar]
- HAGERMAN RJ and HAGERMAN PJ. The fragile x premutation: Into the phenotypic fold. Current Opinions in Genetic Development, 12: 278–83, 2002. [DOI] [PubMed] [Google Scholar]
- HAGERMAN RJ, LEEHEY M, HEINRICHS W, TASSONE F, WILSON R, HILLS J, GRIGSBY J, GAGE B, and HAGERMAN PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile x. Neurology, 57: 127–30, 2001. [DOI] [PubMed] [Google Scholar]
- JACQUEMONT S, FARZIN F, HALL D, LEEHEY M, TASSONE F, GANE L, ZHANG L, GRIGSBY J, JARDINI T, LEWIN F, BERRY-KRAVIS E, HAGERMAN PJ, and HAGERMAN RJ. Aging in individuals with the fmr1 mutation. American Journal of Mental Retardation, 109: 154–64, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACQUEMONT S, HAGERMAN RJ, LEEHEY M, GRIGSBY J, ZHANG L, BRUNBERG JA, GRECO C, DES PORTESV, JARDINI T, LEVINE R, BERRY-KRAVIS E, BROWN WT, SCHAEFFER S, KISSEL J, TASSONE F, and HAGERMAN PJ. Fragile x premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. American Journal of Human Genetics, 72: 869–78, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKALA P, HANNINEN T, RYYNANEN M, LAAKSO M, PARTANEN K, MANNERMAA A, and SOININEN H. Fragile-x: Neuropsychological test performance, cgg triplet repeat lengths, and hippocampal volumes. Journal of Clinical Investigation, 100: 331–8, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON C, ELIEZ S, DYER-FRIEDMAN J, HESSL D, GLASER B, BLASEY C, TAYLOR A, and REISS A. Neurobehavioral phenotype in carriers of the fragile x premutation. American Journal of Medical Genetics, 103: 314–9, 2001. [PubMed] [Google Scholar]
- KNIGHT SJ, FLANNERY AV, HIRST MC, CAMPBELL L, CHRISTODOULOU Z, PHELPS SR, POINTON J, MIDDLETON-PRICE HR, BARNICOAT A, PEMBREY ME, and et al. Trinucleotide repeat amplification and hypermethylation of a cpg island in FRAXE mental retardation. Cell, 74: 127–34, 1993. [DOI] [PubMed] [Google Scholar]
- KOGAN CS, BOUTET I, CORNISH K, ZANGENEHPOUR S, MULLEN KT, HOLDEN JJ, DER KALOUSTIANVM, ANDERMANN E, and CHAUDHURI A. Differential impact of the fmr1 gene on visual processing in fragile x syndrome. Brain, 127: 591–601, 2004. [DOI] [PubMed] [Google Scholar]
- LEEHEY MA, MUNHOZ RP, LANG AE, BRUNBERG JA, GRIGSBY J, GRECO C, JACQUEMONT S, TASSONE F, LOZANO AM, HAGERMAN PJ, and HAGERMAN RJ. The fragile x premutation presenting as essential tremor. Archives of Neurology, 60: 117–21, 2003. [DOI] [PubMed] [Google Scholar]
- LOESCH DZ, BUI QM, GRIGSBY J, BUTLER E, EPSTEIN J, HUGGINS RM, TAYLOR AK, and HAGERMAN RJ. Effect of the fragile x status categories and the fragile x mental retardation protein levels on executive functioning in males and females with fragile x. Neuropsychology, 17: 646–57, 2003a. [DOI] [PubMed] [Google Scholar]
- LOESCH DZ, HUGGINS RM, BUI QM, TAYLOR AK, PRATT C, EPSTEIN J, and HAGERMAN RJ. Effect of fragile x status categories and fmrp deficits on cognitive profiles estimated by robust pedigree analysis. American Journal Medical Genetics A, 122: 13–23, 2003b. [DOI] [PubMed] [Google Scholar]
- MANLY T, DAVISON B, HEUTINK J, GALLOWAY M, and ROBERTSON I. Not enough time or not enough attention?: Speed, error and self-maintained control in the sustained attention to response test (SART). Clinical Neuropsychological Assessment, 3: 167–177, 2000. [Google Scholar]
- MENON V, LEROUX J, WHITE CD, and REISS AL. Frontostriatal deficits in fragile x syndrome: Relation to fmr1 gene expression. Proceedings of the National Academy of Science U S A, 101: 3615–20, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN AM. The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Experimental Brain Research, 133: 33–43, 2000. [DOI] [PubMed] [Google Scholar]
- OWEN AM, MILNER B, PETRIDES M, and EVANS AC. Memory for object features versus memory for object location: A positron-emission tomography study of encoding and retrieval processes. Proceedings of the National Academy of Science U S A, 93: 9212–7, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDO JV, FOX PT, and RAICHLE ME. Localization of a human system for sustained attention by positron emission tomography. Nature, 349: 61–4, 1991. [DOI] [PubMed] [Google Scholar]
- ROBERTSON I, WARD T, RIDGEWAY V, and NIMMO-SMITH I. The test of everyday attention manual. England: The Test of Everyday Attention Manual, 1994. [DOI] [PubMed] [Google Scholar]
- ROUSSEAU F, ROUILLARD P, MOREL ML, KHANDJIAN EW, and MORGAN K. Prevalence of carriers of premutation-size alleles of the fmri gene--and implications for the population genetics of the fragile x syndrome. American Journal of Human Genetics, 57: 1006–18, 1995. [PMC free article] [PubMed] [Google Scholar]
- SWAINSON R, HODGES JR, GALTON CJ, SEMPLE J, MICHAEL A, DUNN BD, IDDON JL, ROBBINS TW, and SAHAKIAN BJ. Early detection and differential diagnosis of alzheimer's disease and depression with neuropsychological tasks. Dementia and Geriatric Cognitive Disorders, 12: 265–80, 2001. [DOI] [PubMed] [Google Scholar]
- TASSONE F, HAGERMAN RJ, LOESCH DZ, LACHIEWICZ A, TAYLOR AK, and HAGERMAN PJ. Fragile x males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. American Journal Medical Genetics, 94: 232–6, 2000a. [DOI] [PubMed] [Google Scholar]
- TASSONE F, HAGERMAN RJ, TAYLOR AK, GANE LW, GODFREY TE, and HAGERMAN PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-x syndrome. American Journal of Human Genetics, 66: 6–15, 2000b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENERRY MR, CROSSON B, DEBOE J, and LEBER WR. Stroop neuropsychological screening test. Odessa, FL: Psychological Assessment Resources, 1989. [Google Scholar]
- VAN ESCH H, DOM R, BEX D, SALDEN I, CAECKEBEKE J, WIBAIL A, BORGHGRAEF M, LEGIUS E, FRYNS JP, and MATTHIJS G. Screening for FMR-1 premutations in 122 older flemish males presenting with ataxia. European Journal of Human Genetics, 13: 121–3, 2005. [DOI] [PubMed] [Google Scholar]
- WANG Q, GREEN E, BOBROW M, and MATHEW CG. A rapid, non-radioactive screening test for fragile x mutations at the FRAXA and FRAXE loci. Journal of Medical Genetics, 32: 170–3, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARRINGTON EK and JAMES M. The visual object and space perception battery. England: Thames Valley Test Company, 1991. [Google Scholar]
- WECHSLER D. Wechsler memory scale memory - 3rd edition. USA: Psychological Press, 1997. [Google Scholar]
- WECHSLER D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation, 1999. [Google Scholar]