Abstract
The production of proteins from mRNAs localized at the synapse ultimately controls the strength of synaptic transmission, thereby affecting behavior and cognitive functions. The regulated transcription, processing, and transport of mRNAs provide dynamic control of the dendritic transcriptome, which includes thousands of messengers encoding multiple cellular functions. Translation is locally modulated by synaptic activity through a complex network of RNA-binding proteins (RBPs) and various types of non-coding RNAs (ncRNAs) including BC-RNAs, microRNAs, piwi-interacting RNAs, and small interference RNAs. The RBPs FMRP and CPEB play a well-established role in synaptic translation, and additional regulatory factors are emerging. The mRNA repressors Smaug, Nanos, and Pumilio define a novel pathway for local translational control that affects dendritic branching and spines in both flies and mammals. Recent findings support a role for processing bodies and related synaptic mRNA-silencing foci (SyAS-foci) in the modulation of synaptic plasticity and memory formation. The SyAS-foci respond to different stimuli with changes in their integrity thus enabling regulated mRNA release followed by translation. CPEB, Pumilio, TDP-43, and FUS/TLS form multimers through low-complexity regions related to prion domains or polyQ expansions. The oligomerization of these repressor RBPs is mechanistically linked to the aggregation of abnormal proteins commonly associated with neurodegeneration. Here, we summarize the current knowledge on how specificity in mRNA translation is achieved through the concerted action of multiple pathways that involve regulatory ncRNAs and RBPs, the modification of translation factors, and mRNA-silencing foci dynamics.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1506-y) contains supplementary material, which is available to authorized users.
Keywords: mTOR, NMDAR, ARC/Arg3.1, Abnormal protein aggregation, Stress granules, EJC
Introduction
Neurons typically have thousands of synapses, whose composition and strength are dynamically controlled by their history of impulse transmission. Synaptic stimulation activates multiple signaling pathways that ultimately lead to post-translational modifications of key proteins and to the regulated production of vastly diverse molecules. All these processes modulate the strength of synaptic transmission and allow for memory formation. Long-lasting memories require durable changes in synaptic strength, either reinforcement or debilitation, which are known as long-term potentiation and long-term depression, respectively. Both forms of synaptic plasticity largely depend on the coordinated translation of messengers localized at the post-synapse that encode multiple receptor subunits, scaffold proteins, adhesion molecules, signaling molecules, and others [1–11]. Previous reports demonstrate through multiple experimental approaches that neuron activity modulates protein synthesis in dendrites and synapses. Balanced translation is key to synaptic homeostasis, and the dysregulation of local protein synthesis may provoke malfunctions or pathogenic conditions [2, 10, 12–21]. Both transcript-specific and global effects were identified through the incorporation of precursor amino-acids or puromycin-derivatives and the use of fluorescent proteins, among other strategies. A clear outcome of the stimulation of brain-derived neurotrophic factor (BDNF) or metabotropic receptors is an increase in overall protein synthesis, which is accompanied by an enhancement in the translation of a number of messengers and the repression of others [13, 22–25]. In contrast, N-methyl-d-aspartate (NMDA) receptor stimulation decreases the local rate of protein synthesis and, at the same time, activates the translation of a number of messengers [2, 6, 10, 15, 26–29]. Response diversity involves multiple molecular mechanisms in which non-coding RNAs and a growing number of RNA binding proteins (RBPs) play a pivotal role [8, 14, 22, 29–34]. An expanding concept is that mRNA repression implies the formation of specific cytoplasmic bodies collectively known as mRNA-silencing foci, which are non-membranous, microscopically visible assemblies of repressed mRNAs and associated proteins [35, 36]. Neuron-specific mRNA-silencing foci related transport as well as the ubiquitous processing bodies (PBs) are present in dendrites and synapses, and their significance for local translation is just beginning to be understood [35, 37–41]. Among other novel regulatory molecules, Pumilio and Smaug reversibly aggregate and form dendritic foci that contribute to the modulation of translation upon synaptic stimulation [14, 42–44].
Translational regulation at the synapse is an important aspect of gene expression control and it is followed by protein modification and degradation, which occur locally and under the control of neuronal activity [9, 11, 45, 46]. Calcium/calmodulin-dependent protein kinase II alpha (CaMKII alpha) is a key protein required for synaptic plasticity under the control of multiple regulatory pathways. Smaug 1 foci, cytoplasmic mRNA polyadenylation and miRNA-mediated silencing as well as post-translational mechanisms locally regulate CaMKII upon synaptic stimulation [14, 47, 48]; reviewed in [49]. The activity-regulated cytoskeleton-associated protein (ARC/Arg3.1) is another example of multi-step regulation. ARC/Arg3.1 transcription is activated by synaptic stimulation. ARC/Arg3.1 mRNA is transported along dendrites in association with granules that contain specific RBPs that trigger ARC/Arg3.1 mRNA decay upon ectopic translation outside stimulated synapses [1, 3, 19, 50, 51]; reviewed in [20].
In this work, we provide an overview of the current knowledge on the mechanisms involved in translational regulation in dendrites and synapses, with a focus on specific RBPs and on the dynamics of relevant mRNA-silencing foci. Most of the studies examined here were performed in mammals; the hippocampus is a frequent experimental model due to its known involvement in memory consolidation. Substantial information has also been gathered from invertebrate organisms and is reported here as well.
The synaptic transcriptome: a nuclear screenplay by local storytellers
The presence of ribosomes at dendrites and post-synaptic surroundings has been known for a long time [52], and our knowledge of the identity of the localized transcripts has been progressively growing. Initially limited to a few messengers serendipitously discovered to have a polarized distribution, the list of localized mRNAs has expanded to thousands using microarray analysis [7]. In a comprehensive deep-sequencing analysis, E. Schuman’s lab identified more than 2,000 transcripts localized in hippocampal dendrites. Notably, 86 % of these transcripts overlap with previously identified localized mRNAs, strongly suggesting that the list is now quite complete [53]. Among other cellular functions, localized mRNAs encode glutamate and gamma-aminobutyric acid (GABA) receptors, voltage-gated ion channels, post-synaptic density (PSD) components and scaffolds; cytoskeleton components and regulators; kinases, phosphatases, and signaling molecules of the calcium and phosphatidylinositol pathways; RBPs that regulate translation, transport, and localization; and molecules involved in protein synthesis, processing, and ubiquitination. Although axons are mostly devoid of mRNAs and polysomes, the translation of specific transcripts is known to occur at specific domains with important consequences for axonal growth, survival, and regeneration; these findings are comprehensively reviewed in [54, 55].
The transcriptome at the synapse comprises nearly 50 % of the mRNA species present in a mature hippocampal neuron, implying that a large number of mRNA molecules are actively transported along relatively long distances—up to hundreds of micrometers—from the cell nucleus [53]. RNA transport has been intensely investigated. Recent articles systematically review how cis-acting elements and specific RBPs dictate the packaging of messengers in large RNA transport granules. These granules move by the action of molecular motors along microtubules and microfilaments [56–61]. Neuron activity influences RNA motility, thus ensuring the efficient delivery of selected transcripts to stimulated synapses and the response at the synapse may involve granule remodeling and mRNA release [37, 38, 52, 56, 61–64]. A number of key RBP components of RNA transport granules are nuclear; the recent discovery of a novel pathway for nuclear mRNA export has renewed the field. Speese and colleagues discovered that several transcripts encoding post-synaptic components of the fly neuromuscular junction leave the nucleus packaged in granules [65]. These granules are relatively large and cannot pass through the nuclear pore; rather, they exit the nucleus through the budding of the nuclear envelope in a mechanism that parallels the export of viral particles (Fig. 1). In a first step, the inner nuclear membrane ensheaths the RNA granule in a vesicle and delivers it to the perinuclear space. A subsequent fusion with the outer nuclear membrane releases the RNA granule into the cytoplasm [65]. The composition of these granules is only partially known. The presence of a C-terminal fragment of the wingless-type MMTV integration site family member 1 (Wnt1) receptor that carries a PDZ-binding motif may help granule targeting to the post-synapse, where PDZ-containing proteins concentrate [65]. An intriguing speculation is that neuron activity enhances the exit of nuclear RNA granules. This might be particularly relevant for ARC/Arg3.1 mRNA, whose transcription increases dramatically upon synaptic stimulation; mRNA floods the cytoplasm and dendrites in relatively short time, potentially involving a massive export pathway such as nuclear envelope budding [3, 20].
Fig. 1.
The dendritic transcriptome and its regulation. The transport of mRNAs to dendrites depends on cis-acting elements and may involve alternative UTRs that contribute to localization and translational regulation. Synaptic activity affects both nuclear and cytoplasmic mRNA processing, which involves specific factors that are depicted in association with the mRNAs in several colors. Two independent mechanisms for the nuclear export of mRNAs directed to the synapse are known: the exit of mRNPs through the nuclear pore and the exit of nuclear RNA granules (red spheres) through budding and fusion of the nuclear membrane. Once in the cytoplasm, the RNA granules (green and red spheres) are transported to the post-synaptic regions by the action of microtubule-dependent molecular motors. Translational regulation at the post-synapse involves multiple mechanisms and molecules: the control of RNA granule integrity; the activation and inactivation of translation factors by mTOR and other pathways; the calpain-mediated decay of the PABP inhibitor PAIP2; non-coding RNAs that are locally modulated at several levels, including biogenesis and stability; and a great variety of RBPs whose aggregation and activity are regulated by synaptic stimulation
Different mRNAs show different localization patterns along proximal and distal dendrite segments, and specific motifs directing their transport have been identified. These RNA signals are preferentially located in the 3′UTR, can be repetitive and redundant, are highly diverse, and include both sequence motifs and structural elements. These properties provide specificity for RBP recognition and diversity of localization patterns [66, 67]; reviewed in [59, 61, 68, 69]. The use of alternative UTRs may affect mRNA subcellular localization; the transcript encoding BDNF, an important neurotrophin linked to several disorders that regulates synapse maturation, is a clear example [70–72]. BDNF is synthesized from several alternatively processed transcripts, and BDNF messengers with long 3′UTRs are transported and translated in the dendrites. The localized production of BDNF is important for dendrite branching and spine growth, and the high levels of neurotrophin synthesized from the BDNF mRNA variants located at the soma cannot meet this requirement [70, 71]. RNA transport is also influenced by RNA editing. The mRNA encoding the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor (AMPAR) is alternatively spliced and edited, thus generating a number of mRNA molecules that localize differentially; for instance, unedited AMPAR transcripts are more efficiently targeted to dendrites [73]. Furthermore, the splice variants termed FLIP are present in dendrites and dendritic spines, whereas the FLOP AMPAR mRNA is largely restricted to the soma.
Finally, cytoplasmic splicing is an emerging regulatory mechanism, and recent work suggests that it may contribute to shaping the local transcriptome (Fig. 1). Intronic sequences are retained in a number of messengers referred to as cytoplasmic intron sequence-retaining transcripts (CIRTs). The mRNAs encoding CaMKII beta, fragile X mental retardation protein (FMRP) and other relevant synaptic molecules are CIRTs. Remarkably, intronic sequences retained in CIRTs have repetitive elements that direct the mRNA to dendrites [74]. It is speculated that CIRTs are spliced locally, and this seems to be the case for the calcium-activated big potassium channel (BKCa) [46]. Cytoplasmic splicing of BKCa mRNA at dendrites allows for the space-restricted production of BKCa protein, thus modulating channel currents in response to local stimulation [46]. The molecular mechanism for cytoplasmic splicing at dendrites is poorly understood but likely involves spliceosome components and/or IRE1-dependent cleavage [75, 76]. Taken together, these findings show that regulated transcription, transport, and local mRNA processing dynamically affect the transcriptome at dendrites and synapses.
Regulation of translation by synaptic activity: multiple subplots make the story
Cis-acting signals present in localized mRNAs direct their transport and trigger multiple mechanisms for translational control that involve non-coding RNAs and a growing number of RBPs. In addition, the stimulation of neurotransmitter or neurotrophin receptors modulates the translational apparatus associated with the post-synapse through several parallel mechanisms that differentially affect different transcripts; the mammalian/mechanistic target of rapamycin (mTOR) pathway has a pivotal role. In this section, we summarize recent advances in these pathways and discuss how translation specificity is achieved through the coordinated regulation of multiple molecular networks.
Non-coding RNAs: great performances by tiny actors
The rodent BC1 RNA and its primate counterpart BC200 were among the first non-coding RNAs identified to have a clear role in translation regulation at synapses (reviewed in [77]). These regulatory RNAs evolved from independent retrotransposition events. The BC1 RNA is a 152-nt transcript that derives from tRNA (Ala), and BC200 is a 200-nt RNA that derives from the signal recognition particle RNA (reviewed in [77]). Both BC RNAs repress translation by interacting with eIF4A and eIF4B through a conserved motif at the 3′ end, thus affecting the helicase activity of eIF4A and the recruitment of the small ribosomal subunit. Although the identity of the target transcripts remains mostly unknown, work with the BC1 KO mouse confirms a role in translation at the post-synapse.
In addition to the BC RNAs, neurons contain a growing number of regulatory small non-coding RNAs; the dysregulation of non-coding RNA-mediated silencing is an emerging cause of several neurodevelopmental pathologies, including Fragile X and Rett syndromes, as well as other complex disorders such as depression, drug addiction, and experimental models of schizophrenia (reviewed in [32, 33, 78–80]). A number of miRNA families are specific to neurons, and comprehensive reviews have been presented on their relevance in neuron development and function in Aplysia, Drosophila, and mammals [32, 33, 78–80]. Synaptic activity largely depends on multiple miRNAs. Several messengers are locally controlled at dendrites and synapses by miRNAs, including transcripts that encode proteins linked to actin dynamics, translation regulation, and numerous other cellular networks. In turn, miRNA-mediated silencing is modulated by synaptic activity and neurotrophins [8, 22, 30, 81–83].
The molecular mechanisms underlying miRNA-mediated silencing have been a matter of intense debate. An integrative model proposes that the miRNA-induced silencing complex (miRISC) affects translation at several levels, and it may involve deadenylation eventually followed by decapping and decay [84, 85]. Messenger RNA degradation is an appealing mechanism for long-lasting synaptic changes as this provides a durable modification of the local transcriptome. Supporting a role for the miRNA pathway in the regulated decay of dendritic transcripts, recent observations indicate that miRISC activity is required for the degradation of the potassium/chloride cotransporter KCC2 and the K channel Kv1.1 mRNAs upon BDNF stimulation [22]. In contrast, translational repression by miRNAs without changes in mRNA levels may enable a quick recovery of expression as is the case of CaMKII alpha mRNA and other transcripts [22, 47, 86]. The miRNA pathway is linked to PB formation, and the miRISC proteins Argonaute 2 (Ago2) and glycine(G)-tryptophan(W) 182 kDa/trinucleotide repeat-containing six proteins (GW182/TNR6C) colocalize in foci proximal to dendritic spines in association with specific mRNAs [36, 37, 85, 87]. Recent work in non-neuronal cells has identified a GW182/TNRC6 splicing variant termed GW220/TNGW1 that stabilizes miRNA targets and directs the formation of a specific type of PB linked to mRNA storage. These PBs contain miRISC but lacks Hedls, a coactivator of decapping [88] and intriguingly, PBs that exclude decapping molecules are present in dendrites. The relevance of GW220/TNGW1 in transient repression by miRNAs at the post-synapse is an open question.
Work in several cell systems indicates that miRNA-mediated silencing is modulated by a number of RBPs. In neurons, FMRP interacts with Ago2 and contributes to the regulation of the post-synaptic component PSD95 by miR-125a [83]. In addition to FMRP and other modulatory proteins, the miRNA pathway frequently involves a network of competitive endogenous RNAs (Fig. 2). These RNAs are coding or non-coding transcripts containing miRNA-recognition motifs that compete with the miRNA targets. A recently discovered circular RNA acts as a sponge for miR-7 and affects neuron development [89]. Work by two independent groups links the topoisomerase Top3β to synaptic defects in both vertebrates and invertebrates. Top3β is found in a multiprotein complex that includes FMRP and RNA and affects FMRP targets. An interesting possibility is that topoisomerase activity affects circular sponge RNAs (reviewed in [90]). Neuronal activity affects the miRNA pathway through additional mechanisms. MicroRNA precursors (pre-miRNA) and the processing enzyme DICER are present in dendrites and isolated synapses, where they provide local regulation of miRNA biogenesis. In addition, the degradation of a number of pre-miRNAs and RISC proteins facilitates the rapid reactivation of selected targets upon specific stimulation [22, 30, 81, 86] (Fig. 2).
Fig. 2.
Mechanisms for translational regulation at the synapse. Several RBPs and non-coding RNAs coordinate the translation of localized mRNAs. The miRNA pathway (upper left) regulates numerous transcripts and is locally controlled at several levels by synaptic activity. MicroRNA biogenesis is stimulated through the activation of DICER by HIV-TAR binding protein (TRBP), and pre-miRNA decay is stimulated by the Lin28/Dis3l2 pathway. MicroRNA-mediated silencing is modulated by FMRP and Pumilio (double arrows) and by PrPc in association with multivesicular bodies (MVB). The miRNA pathway is antagonized by competitive endogenous RNAs that act as miRNA sponges to neutralize specific miRNAs. FMRP (upper right) is an important disease-associated repressor that affects the translation of hundreds of transcripts carrying specific motifs via multiple pathways. Dysregulation of the FMRP pathway is causative of Fragil X mental retardation syndrome (FSMRS) and autism spectrum disorders (ASD). CPEB (bottom right) binds the specific motif termed CPE and recruits the deadenylase PARN and the poly(A) polymerase GLD2. CPEB bidirectionally regulates the deadenylation or cytoplasmic polyadenylation of several transcripts. Smaug, Nanos, and Pumilio (bottom left) define a novel regulatory network conserved in mammals and invertebrates. Mammalian Smaug1/Samd4a controls synaptic CaMKII alpha mRNA and potentially several other transcripts carrying SREs, including Nanos 1 mRNA. Drosophila Smaug regulates Nanos, which together with Pumilio and Brat affects the translation of a number of Drosophila neuronal transcripts. The effect of Pumilio on paralytic/Scn1a, Dlg, and 4E mRNAs has been demonstrated in both Drosophila and mammalian models, and the repression of AChE mRNA by Pumilio 2 has been shown in the mammalian neuromuscular junction. Mammalian miR-134 regulates Pumilio 2 upon synaptic activity. A potential functional interaction between Pumilio and CPEB is indicated by double arrows
It has been suggested that miRNA-mediated silencing occurs in endosomes and multivesicular bodies (MVBs), and more recently autophagy has been proposed to play an important role in regulating DICER activity [91–93]. MicroRNA-mediated silencing is considered to be a cell-autonomous process, but the participation of MVBs and endosomes leads to the speculation that extracellular molecules may be incorporated in the pathway. Cumulative evidence from various experimental systems supports the notion that miRNAs are secreted and then incorporated by distant cells via the endocytic pathway [91–93]. Emerging observations suggest that miRNAs are secreted from the pre-synapse, thus opening the possibility of their endocytosis at the post-synapse. Incipient data supporting this challenging model have been reported, adding a level of complexity to the control of synaptic strength by miRNAs [93, 94].
It is generally accepted that whereas miRNAs act in the cytoplasm to repress their target mRNAs, piRNAs mediate transposon silencing by promoting de novo DNA methylation. In addition, piRNAs and their cognate proteins from the MIWI family are present in dendrites in close proximity to the synapse [31]. The translational regulator Smaug1/Samd4A is involved in synaptic plasticity in mammals [14] and the Drosophila homologue represses fly maternal transcripts with the help of specific piRNAs [95]. However, the evidence for a role of piRNAs on translation at the post-synapse is incipient [31]. A fourth type of regulatory non-coding RNAs is endogenous siRNAs, which are expressed in the brain and show an increase in expression upon training [96]. Whereas the role of BC RNAs and miRNAs in synaptic plasticity is well demonstrated, our understanding of the contribution of piRNA, siRNA and other small non-coding RNAs is just beginning and will require intensive investigation.
RBPs: new plays for old actors
In this section, we summarize the current knowledge on the markedly small number of RBPs with well-known effects on mRNA translation at dendrites and synapses. We briefly discuss the relevance of FMRP and cytoplasmic polyadenylation element (CPE) binding protein (CPEB), two molecules extensively discussed in the literature, and we emphasize novel pathways involving Smaug, Nanos, and Pumilio (Fig. 2).
FMRP and CPEB are among the most studied RBPs, and compelling evidence for their important role in synaptic translation arises from multiple independent studies [5, 21, 34, 48, 64, 97–109]. Fragile X syndrome (FXS) and several forms of autism are associated with mutations in the genes encoding FMRP or FMRP-associated proteins [97, 108]. FMRP is produced at the post-synapse upon group 1 mGluR (mGluR1/5) activation and acts as a translational brake. This balance is extremely important, and a loss of FMRP function provokes serious neurological defects in human diseases and animal models [21, 25, 97, 109, 110]. FMRP mRNA, numerous transcripts encoding synaptic components, general translation factors including mTOR and molecules linked to autism are regulated at the translational level by FMRP or related proteins. This protein family binds secondary structures such as “kissing complexes” and G-quadruplexes. Additional sequence motifs have been identified, and it is expected that additional targets will be discovered [21, 34, 97, 99, 108, 111]. FMRP forms dendritic RNA granules, and a large body of evidence indicates that FMRP associates with polysomes. FMRP affects translation initiation and/or elongation with the help of specific miRNAs in a number of cases [83, 99]. FMRP-mediated mRNA repression is regulated by methylation and phosphorylation and may involve ubiquitination followed by FMRP decay [83, 99]. FMRP is a versatile protein, and in addition to RNA transport, translation, and stability, FMRP modulates the nuclear editing of transcripts encoding important synaptic functions, which may affect their trafficking (Fig. 2) [73]. The dysregulation of RNA editing is associated with neuropsychiatric and neurodevelopmental conditions, further positioning FMRP at the center of neuron health.
Members of the CPEB protein family are expressed in different cell types, where they control mRNA translation by regulating the length of the polyA tail and additional mechanisms that directly affect translation [21, 34, 48, 100, 102, 104, 106, 112]. Like its Xenopus oocyte counterpart, mammalian CPEB forms a bifunctional activator-repressor complex in the dendrites and synapses that contains both the poly(A) polymerase GLD2 and the deadenylase poly(A)-specific ribonuclease (PARN) [98]. Thus, CPEB provides a switch that alternatively activates or inactivates translation (Fig. 2). NMDAR stimulation induces the ubiquitination and phosphorylation of CPEB and provokes the exit of PARN and the activation of GLD2 [48, 98, 101]. More than 100 mRNA transcripts appear to be affected by this dual activity complex, including CaMKII alpha and the NMDAR subunit NR2A, which are also regulated by miRNA and FMRP; the AMPA receptor subunits GluA1 and GluA2; the RBP Hu protein D (HuD); and other signaling molecules that contribute to synaptic plasticity and learning [98, 101]. CPEB forms dendritic granules, and later in this review, we discuss how the regulated oligomerization of CPEB by specific protein domains is emerging as an evolutionarily conserved feature relevant to memory formation and persistence [102–104, 106, 112].
Among other proteins recently linked to translational regulation in dendrites, the RBP eIF4AIII forms dendritic RNA granules and contributes to the spatial restriction of ARC/Arg3.1 translation by a novel mechanism [50]. eIF4AIII is a component of the exon-junction complex (EJC), which is a multiprotein label of exon–exon junctions that is removed during the pioneer translation round. The EJC associated with an exon–exon junction located at the 3′UTR of ARC/Arg3.1 mRNA directs the transcript to the non-sense-mediated decay (NMD) pathway, which is a housekeeping mechanism that dictates the degradation of messengers with premature stop codons located upstream of exon–exon junctions. The NMD pathway requires the interaction of the EJC with translation factors, and it is proposed that ARC/Arg3.1 mRNA is degraded when translated in improper locations, whereas ARC/Arg3.1 mRNA is selectively protected from NMD at activated synapses [20, 50]. NMD affects the fate of additional transcripts encoding several synaptic proteins, and this may involve a trans-acting factor termed Nova. Nova is present in the somatodendritic compartment, where it mediates the transport of specific messengers. It is also present in the nucleus, where it represses splicing modes that ultimately trigger NMD. The Nova-NMD pathway is regulated by neuron activity. Interestingly, a number of synaptic components regulated by Nova at the mRNA level are linked to familial epilepsy and related animal disease models [45]. Another RBP connected to neuronal diseases with an emerging role in dendritic translation is TAR DNA binding protein of 43 kDa (TDP-43). TDP-43 is typically localized to the nucleus in most cell types but forms dendritic RNA granules directly or indirectly linked to translation regulation upon synapse stimulation (reviewed in [113]).
In comparison with the large number of localized transcripts, the battery of synaptic proteins that interact with RNA is only partially known. Remarkably, two independent screens aimed to identify RBPs in non-neuronal cell types yielded 300 novel molecules among a total of 800 RBPs [114, 115]. These studies concluded that a high proportion of these proteins are not predictable as RNA binders by current computational methods. In addition, a significant number of identified proteins were shown to be causative in neurological diseases when mutated. These striking observations suggest that novel RBPs with potentially important roles in mRNA translation and stability are expected to be identified among the numerous but poorly described proteins present at the synapse [116].
Smaug, Nanos, and Pumilio: a novel translational regulation network
The RBPs Smaug, Nanos, and Pumilio define a novel pathway for translational regulation that affects synapse formation and dendrite branching in both vertebrates and invertebrates [14, 44, 117–123]. Pumilio family (Puf) proteins are highly conserved throughout evolution and play an important role during early development in several taxa (reviewed in [124]). More recently, independent studies in intact organisms and cell cultures demonstrate that mutations or the knockdown of Pumilio provoke strongly defective neuronal phenotypes by affecting multiple transcripts [34, 117–123, 125] (Fig. 2; Table 1). Moreover, work with animals highlights the relevance of the Pumilio pathway in behavior and memory formation. Pumilio mutant flies show an important defect in learning an odor-based paradigm [126], and mice with disrupted brain expression show increased locomotor activity and impaired nesting behavior, a phenotype linked to autism [122].
Table 1.
Pumilio binding elements
| Transcript | PUM binding element |
|---|---|
| Drosophila Paralytic | uguaaaua |
| Mammalian Scn1a | |
| PBE1 | uguacaua |
| PBE2 | uguaaaua |
| PBE3 | uguagaua |
| Drosophila Dlg1 | |
| PBE1 | uguagaua |
| PBE2 | uguacaua |
| Mammalian Dlg1 | uguauaua |
| Mammalian AchE | uguauaua |
Fly paralytic mRNA contains a functional PBE [125]. Mammalian Scn1a mRNA is bound by Pumilio 2 and contains several PBEs. PBE1 is located within the 3′UTR of human, mouse, and rat Scn1a, and PBE2 is located within the coding or non-coding region of alternatively spliced variants. Nucleotide positions are indicated for the following accession numbers: human Scn1a, NM_001165963, PBE1, 7512 and PBE2, 7025; mouse Scn1a, NM_018733, PBE1, 7664; PBE2 7175 and PBE3, 7316. Rat, NM_030875.1, PBE1, 7659 and 7775; PBE2, 7284. Mammalian AChE transcripts contain a PBE at the 3′UTR near the stop codon at the following positions: human NM_55040.1, 2047; mouse NM_009599.3, 2006 and rat NM_172009.1, 1890. PBEs are apparently absent in Drosophila AChE transcripts
Glutamine and asparagine (QN)-rich regions of different lengths are frequent in RBPs that play key roles in synapse translation. Similar regions present in SG or PB components are important for the assembly of these foci or for the recruitment of specific proteins to them
The number of Pumilio genes is highly variable among species. Flies contain one Pumilio variant, whereas two variants are present in mammals. Pumilio 2 is the major neuronal isoform; it has particularly high expression in the cortex and hippocampus, where it forms dendritic granules speculatively linked to mRNA transport and translation [30, 42, 117, 122]. Several experimental approaches indicate that the optimal sequence recognized by mammalian Pumilio 2 or fly Pumilio is UGUAHAUA [111, 119, 122–125, 127]. Pumilio molecules bind this Pumilio binding element (PBE) by means of a tandem array of eight imperfect repeats known as Pumilio homology domains (PUM-HD) or PUF domains, wherein each PUM-HD module contacts one nucleotide from the eight-base target RNA motif [124]. Putative PBEs are present in several neuronal mRNAs. Work by several research groups has demonstrated that a number of post-synaptic transcripts are controlled by Pumilio in insect and mammalian neurons and muscles [44, 117, 119, 121, 122, 125]. Drosophila Pumilio and rodent Pumilio 2 regulate the translation of the voltage-gated sodium channel paralytic (para) and the vertebrate orthologue Scn1a [117, 125], which contain conserved PBEs (Table 1). Voltage-gated channels regulate membrane excitability, which is important for synapse remodeling and growth, and the regulation of para/Scn1a channels by mammalian or fly Pumilio affects synapse morphology and function [44, 117, 128]. Additionally, the translation initiation factor 4E is repressed post-synaptically by Pumilio in flies and mammals, with consequences for local protein synthesis and synapse formation [44, 117]. A third type of transcript contributing to synapse regulation by Pumilio encodes post-synaptic scaffold proteins. The messenger for fly discs large 1 (dlg1), the orthologue of vertebrate PSD95/Dlg4, contains a canonical PBE and is regulated by Pumilio. More recently, the vertebrate orthologue Dlg1 was reported to be upregulated in the CA1 region of Pumilio 2-deficient mice [119, 122] (Table 1). Additional Pumilio targets are expected to contribute to synapse formation and function. In an unbiased screen, Pumilio 2 was shown to bind several transcripts linked to Parkinson’s disease [127]. The presence of PBEs in a number of neuronal messengers is dictated by alternative processing modes; the transcripts for Drosophila Ago1 and other molecules involved in RNA regulation have neuron-specific 3′UTRs that contain putative PBEs [72]. Similarly, the 3′UTR of a dendritic, alternatively processed mammalian BDNF mRNA includes predicted PBEs, the relevance of which awaits confirmation [70].
Pumilio 2 is also abundant at the vertebrate neuromuscular post-synapse. Pumilio 2 regulates the expression of acetylcholinesterase (AChE) mRNA, a major transcript at this cellular location. A conserved PBE is present in the 3′UTR of the alternative splice variant encoding the hydrophilic AChE “t” subunit; the PBE is absent from the messenger encoding the hydrophobic AChE “h” isoform. Thus, specific regulation is provided for the soluble form of the enzyme [123] (Fig. 2; Table 1). In addition to synapse-specific transcripts, Drosophila Pumilio and mammalian Pumilio 2 regulate several components of the ERK and p38 pathways by directly binding to PBE and PBE-like sequences [129, 130]. These kinases signal downstream of synapse activation, and their regulation by Pumilio may contribute to synaptic plasticity in both neurons and muscle cells.
Pumilio proteins affect mRNAs by multiple pathways that may involve translational repression as well as deadenylation and decay [124, 127, 131]; reviewed in [34] (Fig. 2). Pumilio activity in fly peripheral neurons involves the translational repressor Nanos and its partner Brain Tumor (Brat) [124]; Pumilio or Nanos mutant flies show similar neuronal defects [44, 118, 120, 121, 125]. Pumilio molecules cooperate with the miRNA pathway through multiple mechanisms. Mammalian Pumilio 2 and its nematode orthologue associate with Ago2 and eEF1A and attenuate translation elongation [131]. Furthermore, bioinformatic analysis reveals that the presence of PBEs correlates with the presence of miRNA target sites in a large number of mammalian transcripts; it has been proposed that Pumilio affects RNA folding and the accessibility of miRNA sites [127]. Finally, Pumilio regulatory activity can be bidirectional and Pumilio molecules activate translation by interacting with CPEB proteins. Thus far, this interaction has unknown consequences for neuronal function [34, 124].
It is anticipated that this important regulatory pathway is tightly controlled by neuron activity. Vertebrate Pumilio 2 mRNA carries an miR-134 site, and synapse activation increases dendritic levels of both miR-134 and Pumilio 2; thus, Pumilio 2 activity is constrained within a narrow range [30, 42]. Independent work indicates that insect or vertebrate neurons with increased or decreased levels of Pumilio or its partner Nanos show defective phenotypes. Thus, the levels of these two components of the pathway must be narrowly controlled to allow normal function (Fig. 2). Nanos is regulated at the RNA level by Smaug, which belongs to a novel family of mRNA regulators. Smaug molecules interact with RNA through a sterile alpha motif (SAM) conserved domain that recognizes a number of related stem-loop motifs, collectively termed Smaug recognition elements (SREs) (reviewed in [132]). The SAM domain accommodates a number of variations of the SREs; SREs may have stems with different sequence and length, and they may have loops of four to seven nucleotides with only three fixed positions [111, 133, 134]. The secondary structure that surrounds the motif may further affect binding. Due to this flexibility, Smaug regulates hundreds of maternal mRNAs in the Drosophila embryo [135–139]. In fly peripheral neurons, Nanos mRNA forms motile granules that are transported along dendrites. The Nanos transcript is speculatively repressed by Smaug, and mutations in the SRE of fly Nanos are deleterious [118, 120, 121]. More recently, mRNA repression by Smaug was reported at the mammalian synapse [140]. Of the two genes present in the vertebrate genome, the Smaug1/Samd4A homologue is expressed in hippocampal neurons at the time of synaptogenesis and is associated with the post-synaptic density. The abrogation of Smaug1/Samd4A by RNAi strategies dramatically affects synaptogenesis and dendritic spine morphology, and this correlates with a seriously hampered synaptic response (Fig. 3a–c and reference [14]).
Fig. 3.
PBs and Smaug1 foci in dendrites and dendritic spines. a Smaug1/Samd4a knockdown in hippocampal neurons provokes the presence of numerous thin dendritic spines. Deconvoluted confocal Z-stack images of ECFP-expressing neurons are shown. Bar 1 μm. b Smaug1/Samd4a forms mRNA-silencing foci in mature hippocampal neurons. c Smaug1 foci are present in approximately 60 % of the dendritic spines, visualized by transient ECFP expression. d–f The PB markers DCP1a, Hedls, and Rck/p54 form dendritic foci. Whereas Hedls and Rck/p54 are mostly restricted to the dendritic shaft, 40 % of the synapses contain DCP1a [14, 35, 39]. Bars in b–f 10 μm; magnifications 1 μm
Messenger RNA repression by Smaug operates at several levels [95, 132, 141, 142]. Drosophila Smaug inhibits translation initiation by recruiting Cup, a 4E-binding protein that interrupts the interaction between 4E and 4G, thus blocking 40S recruitment [141]. Drosophila Smaug is also able to trigger Nanos mRNA deadenylation by recruiting the CCR4-NOT complex and specific piRNAs [95, 132, 135, 143]. Recently, Smibert and colleagues demonstrated that fly Smaug recruits Ago1 in an miRNA-independent manner [142]. In addition, Drosophila Smaug forms a highly stable mRNA, where cap-dependent and cap-independent translation are blocked [132, 144]. These mechanisms are likely to occur in the mammalian counterpart, and all of these mechanisms are compatible with the aggregation of Smaug in specific mRNA-silencing foci, which are present in the dendrites of mammalian neurons and in fly embryos [14, 95]. Like the Drosophila molecule, mammalian Smaug1/Samd4A represses the translation of mRNAs carrying SREs [111, 140] and the CaMKII alpha mRNA has multiple putative SREs conserved in humans, rodents, Xenopus, and zebrafish. Recent evidence indicates that Smaug1/Samd4A regulates CaMKII alpha mRNA at the post-synapse in an NMDA-dependent manner, thus accounting for the defects upon Smaug1/Samd4A knockdown [14]. CaMKII alpha mRNA is translationally activated by cytoplasmic polyadenylation, opening the possibility that its repression by Smaug1/Samd4A involves deadenylation. Many other neuronal mRNAs are expected to be under the control of Smaug1/Samd4A, and their identification will require further intensive study. Among other mammalian candidates, the messenger encoding Nanos 1 is expressed in the central nervous system and contains putative SREs [111, 145]. Taken together, these data suggest a conserved role of the Smaug–Nanos–Pumilio pathway in translational regulation in neurons.
A choir of RNA granules sings a varied repertoire: SyAS-foci and self-aggregation motifs
Synaptic activity affects RNA granule motility and integrity, reflecting their role in mRNA transport and repression. The significance of neuronal granules in RNA movement has been reviewed comprehensively [3, 56–61, 68]. In this section, we focus on the relevance of granules in translational regulation and how specific protein domains present on RNA repressors govern their aggregation.
It is an increasingly supported concept that mRNA repression is linked to the formation of mRNA-silencing foci, which harbor the transcripts in association with their repressor RBPs. PBs and stress granules (SGs) were the first mRNA-silencing foci identified. PBs are constitutive, whereas SGs are induced upon acute stress insults [35, 36, 41, 146–148]. SGs contain abortive translation initiation complexes that include a number of translation initiation factors and small ribosomal subunits, and PBs concentrate proteins involved in mRNA decapping and silencing and exclude ribosomes and most translation factors. Polysomes are in close contact with PBs, suggesting that translation occurs in their surroundings [149]. Moreover, PBs dynamically exchange mRNAs with the cytosol and may dissolve, thus releasing the transcripts to allow their translation [35, 36, 41]. Similarly, in vertebrate oocytes, the dissolution of large RNA granules containing dormant mRNAs precedes their translational activation [150]. In the Drosophila oocyte, strongly repressed transcripts that reside in the PB inner core move to the periphery to begin translation [151]. Similarly, when monitoring protein synthesis at the single-molecule level, Carson and coworkers found that mRNA translation occurs in the vicinity of dendritic RNA granules [16].
The relevance of PBs to mRNA repression in dendrites and synapses is emerging. Vertebrate and invertebrate neurons display several types of small dendritic foci. These foci contain subsets of classical PB components, including the decapping protein 1a (DCP1a) and the co-activators Rck/p54, Hedls, and Pat1b (Fig. 3d–f) [22, 35, 37, 39, 47]. Large PBs are also present and are likely formed by the fusion of small foci through homotypic and heterotypic interactions between PB components [22, 35–37, 39, 41, 47, 146, 152–154]. An additional type of foci, which are different from canonical PBs, contain the PB scaffold molecules LSm1 and LSm4, the nuclear cap-binding protein CBP80 and small ribosomal subunits, and exclude 4E and large ribosome subunits. This composition suggests the presence of transcripts stalled at the pioneer translation initiation round and broadens the spectrum of dendritic RNA granules [38].
Independent work from several laboratories has demonstrated that neuron activity regulates the integrity of dendritic PBs. NMDAR activation enhances the exchange of DCP1a between the foci and the cytosol, and prolonged NMDAR stimulation ultimately provokes their dissolution. This activity links NMDAR-dependent translation to DCP1a foci dynamics [37, 39] (Fig. 4). Both insect and vertebrate PBs are connected to miRNA-mediated silencing. Fly dendritic PBs contain Me31B/Rck/p54 and trailer hitch (Tral), another protein linked to the miRNA pathway. Similarly, a number of mammalian miRNAs colocalize with dendritic foci containing Rck/p54, GW182, Ago2, and DCP1a [37, 47, 152]. BDNF stimulation activates the miRNA-mediated silencing of a number of transcripts and enhances the formation of dendritic DCP1a foci, further linking their dynamic assembly to the miRNA pathway [22].
Fig. 4.
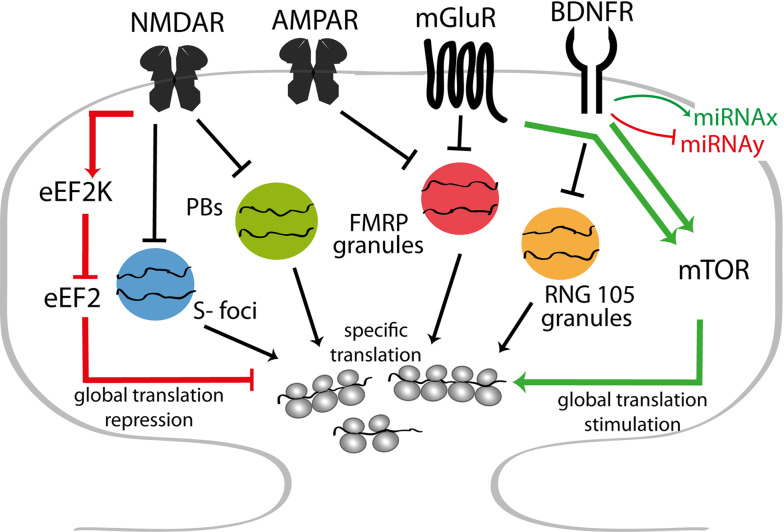
mRNA-silencing foci at the synapse. Translational regulation at the synapse involves multiple mRNA-silencing foci, which are regulated by synaptic activity and thus termed synaptic activity-regulated mRNA-silencing foci (SyAS-foci). Among others, Smaug1 foci (S-foci), a plethora of PBs with various composition and granules containing FMRP or Caprin1/RNG105 are present at the post-synapse. These different SyAS-foci respond to specific stimuli, displaying changes in motility or integrity. Their dissolution allows for the release of mRNA followed by translation. S-foci respond to NMDAR stimulation and remain unaltered upon AMPAR stimulation. AMPAR or mGluR stimulation provokes the dissolution of FMRP granules in the synaptic surroundings, whereas NMDAR activation has no effect. Caprin1/RNG105 granules dissolve upon BDNF Receptor (BDNFR) stimulation [5, 14, 155]. The exchange of mRNPs between PBs and the cytosol increases upon NMDAR activation, which may induce PB disassembly. PBs also respond to BDNF; short exposure increases the number of PBs in dendrites, whereas long exposure triggers their dissolution [22, 37, 39] (not shown). In most cases, the identity of the released transcripts is only partially known. Speculatively, a given mRNA species may associate with multiple SyAS-foci, thus facilitating its regulation upon different stimuli. NMDAR stimulation globally represses translation by the inactivation of eEF2, whereas mGluR and BDNFR stimulation enhances protein synthesis through the mTOR and other pathways
Additional RNA granules containing various mRNA repressors are present in dendrites and synapses. These granules respond to specific stimuli with changes in motility or integrity. Mammalian Smaug1/Samd4A forms specific mRNA-silencing foci associated with synapses, termed S-foci, as they are distinct from PBs and other neuronal RNA granules hitherto described (Fig. 3a–f). As expected if repressed mRNAs are reversibly stored in the S-foci, they disintegrate when mRNAs are captured into polysomes and assemble when the pool of non-translated transcripts increases. Synaptic activity enhances the cycling of mRNAs between the S-foci and polysomes; NMDAR stimulation triggers a rapid and reversible dissolution of the S-foci to release transcripts, thus transiently allowing their translation [14, 63, 140] (Fig. 4). In addition to PBs and S-foci, a few other dendritic RNA granules, including FMRP and Caprin1/RNG105/GPIAP1 granules, also behave as mRNA-silencing foci. These foci respond to synaptic activity, and are collectively termed synaptic-activity regulated silencing foci (SyAS-foci) [5, 34, 63, 107, 155] (Fig. 4).
SyAS-foci formation is mediated by protein self-aggregation, which is a common feature in RBPs linked to translational regulation in dendrites as well as in SG and PB components. A growing concept is that the formation of SGs and abnormal protein aggregates may influence the normal dynamics of RNA granules, thus affecting synaptic plasticity [35, 36, 41, 146, 148]. Multimerization often depends on disordered regions that tend to assemble into ordered aggregates, and a number of self-oligomerization domains display low amino acid diversity, which may include imperfect repeats of 3–4 amino acids, frequently with a high proportion of glutamine (Q) and/or asparagine (N), conferring a prion-like behavior [35, 36, 41, 146, 147, 156, 157] (Table 2). Aplysia CPEB is the first example of the physiological prion-like self-aggregation of an RBP involved in translational regulation. A QN domain specific to the neuronal isoform governs Aplysia CPEB oligomerization upon neuron activation, thereby stimulating the binding to target transcripts [102, 106, 112]. The multimerization of CPEB molecules by QN domains is evolutionarily conserved, and the fly orthologue Oo18 RNA Binding 2 (Orb2) also forms amyloid-like fibers. Orb2 oligomerization temporally correlates with memory formation, and neurons with Orb2 oligomers are thought to be the storage sites of long-lasting memories [103, 104]. Recent work with transgenic flies highlights the complexity of Orb2 regulation by QN-mediated oligomerization. The Orb2 gene generates two isoforms, a less abundant variant termed Orb2A and the more abundant Orb2B, which differentially contribute to memory formation. Persistent learning strictly requires the Orb2A QN domain and the Orb2B RNA binding domain (RBD), whereas the Orb2B QN domain and the Orb2A RBD are dispensable. Genetic and biochemical approaches indicate that the Orb2A QN domain dictates the formation of an oligomer that is highly resistant to physicochemical treatments including boiling, high salt, detergents, and denaturing agents, thus providing a molecular mechanism for enduring effects at the synapse [104]. CPEB oligomerization also occurs in vertebrates. Mammalian CPEB forms dendritic granules through the N-terminal polyQ region and this domain functionally substitutes for the Drosophila Orb2A QN domain in fly-training models [104]. NMDAR stimulation triggers the phosphorylation and monoubiquitination of CPEB3, resulting in the polyadenylation and translation of GluA1 and GluA2 mRNAs, which has important consequences for synaptic plasticity and memory formation [48, 98, 101]. It is an open possibility that the integrity or composition of CPEB granules is affected by these post-translational modifications.
Table 2.
Protein domains that mediate the self-aggregation of neuronal RNA regulators
| Protein | Aggregation domain | References |
|---|---|---|
| Aplysia CPEBa | QN-rich | [102, 106, 112] |
| Drosophila Orb2a | Q-rich | [103] |
| Mammalian CPEB3a | Q-rich | [104] |
| Drosophila Pumiliob | QN-rich | [43] |
| Mammalian Pum2b | QN-rich | [42, 180] |
| Drosophila FMRPc | Q-rich | [110] |
| Mammalian FMRPc | NDF | [105, 181] |
| TDP43d | QGNY-rich | [159, 182, 183] |
| Mammalian FUS/TLSe | QGSY-rich | [156, 159] |
| Mammalian Staufen 1f | dsRBD5 | [162] |
| Drosophila ELAVg | RRM3 | [163] |
| Mammalian HuDg | RRM3 | [184] |
| Caprin1/RNG105h | RGG box | [155, 185] |
| Mammalian Quaking 1i | GSG-domain | [186] |
| hnRNPA1/A2j | GYN-rich | [160] |
aLow-complexity regions rich in QN, Q, or QP are present at the N-terminal of Aplysia, Drosophila, and mammalian CPEB molecules, respectively. Forty-eight percent of the 160 N-terminal aa in the Aplysia molecule are Q or N. A region spanning aa 1–88 in Orb2A and aa 170–240 in Orb2B contain 35 % Q residues and a region spanning aa 10–190 in mammalian CPEB3 contains 39 % Q or P
bBoth Drosophila Pumilio and mammalian Pumilio 2 contain a QN-rich motif in the N-terminal region. The QN domain spans aa 261-550 in mammalian Pumilio 2, and aa 191–286 in the Drosophila molecule
cThe122 aa-long region with 36 % QN at the C-terminal of Drosophila FMRP is absent from the mammalian molecule
dThe QGSY-rich region spans aa 277–414 and is partially conserved in mammals, C. elegans, and Drosophila. Repeats containing aa 331–369 from human TDP43 aggregate in cultured cells
eThe QGSY-rich region spanning aa 1–239 of mammalian FUS/TLS is apparently absent from the invertebrate molecule
fFive dsRBDs are conserved in mammalian, fly, and nematode Staufen molecules. The dsRBD5 and dsRBD2 are respectively major and minor determinants of protein–protein self-association
gThree short sequences in the third RRM mediate homotypic interactions. These sequences are conserved in Drosophila ELAV, Rbp9, and Fne proteins and in the four human members HuR, HuB, HuC, and HuD
hIn addition to the RGG-rich domain spanning aa 577–605, a coiled-coil region at the RNG105 C-terminus is required for granule formation
iHomodimer formation mediated by the GRP33–Sam68–GLD-1 (GSG) domain was shown by biochemical approaches
jA GYN-rich region spanning residues 261–303 in hnRNPA2 and 233–272 in hnRNPA1 mediates aggregation in vitro and shows prionic properties in yeast cells. Point mutations in these motifs lead to pathogenic inclusions
Following a related mechanism, Pumilio molecules aggregate in several species via a conserved QN-rich region (Table 2). Mammalian Pumilio 2 forms dendritic granules and assembles into particles when transfected into non-neuronal cells, and the QN-domain mediates Pumilio 2 recruitment to SGs [42]. Similarly, Drosophila Pumilio forms microscopically visible aggregates when expressed in yeast cells, and the QN domain regulates the synaptic function of fly Pumilio [43]. Whether Pumilio aggregation responds to synaptic activity remains to be investigated. Recent work in vertebrate oocytes demonstrated the relevance of the reversible Pumilio 1 aggregation via the QN domain. Oocyte maturation triggers the translational activation of dormant mRNAs stored in Pumilio 1 granules. This translational activation requires granule disassembly and is impaired when the granules are stabilized by overexpression of the QN domain [150].
FMRP granules reversibly dissolve upon AMPAR or mGluR stimulation [5, 14, 107], and different domains determine FMRP aggregation in vertebrates and invertebrates. Like CPEB and Pumilio, Drosophila FMRP provides an example of a functionally relevant QN domain, which is present at the C-terminus of the most abundant FMRP isoform. This QN domain mediates protein–protein interactions and is required for short-term memory in flies [110]. The mammalian molecule lacks QN regions and oligomerizes through a specific domain termed NDF (N-terminal domain of FMRP) with yet unknown relevance for translational regulation [105] (Table 2). The mRNA repressor Caprin1/RNG105/GPIAP1 forms dendritic RNA granules that respond to BDNF by dissolving and enabling for the interaction of released mRNAs with the translational apparatus. Caprin1/RNG105/GPIAP1 aggregates through specific domains, including an RGG box and a coiled-coil region that are also required for translational repression [155]. TDP-43 is another important RBP forming dendritic granules that remodel upon neuron depolarization. TDP-43 contains a conserved C-terminal region rich in glycine and uncharged polar amino-acids, mostly asparagine, glutamine, and tyrosine, which facilitates protein aggregation in normal and pathological conditions (Table 2) [113, 148]. Fused in sarcoma/translocated in liposarcoma (FUS/TLS) also forms dendritic RNA granules and joins the growing list of RBPs linked to neurodegeneration [62, 158, 159]. In the FUS/TLS N-terminus, there are 27 imperfect repeats of G/S-Y-G/S surrounded by a high percentage of Q residues. This region defines a protein domain that allows oligomerization in vitro and the recruitment of FUS/TLS to SGs in several cell types [148, 156, 159] (Table 2). The dynamics of FUS/TLS granules upon neuron activity has not been investigated.
Heterogeneous nuclear ribonucleoprotein A2 (hnRNPA2) is involved in mRNA transport and translation in dendrites and forms dendritic granules. A conserved short prion-like region rich in GYN mediates hnRNPA2 aggregation in vitro, and point mutations that exacerbate aggregation lead to pathogenic inclusions [148, 160]. In addition, a protein termed tumor overexpressed gene (TOG) contains multiple hnRNPA2 binding sites that facilitate hnRNPA2 granule formation [161]. Finally, the RBDs of a number of neuronal RBPs, including Staufen, HuD, and the Drosophila orthologue Elav, contribute to oligomerization. Speculatively, interaction with the target mRNA may modulate aggregation [162–164] (Table 2). The molecular determinants of S-foci formation are unknown. The SAM domain is not required and it is expected that novel motifs enabling regulatory diversity will be discovered [14]. In an innovative approach in vitro, McKnight and coworkers found that low-complexity regions present in RBPs govern the spontaneous formation of RNA granules. FUS/TLS, FMR1, TDP-43, and several other RBPs reversibly aggregate into fibers with hydrogel properties that are able to capture native RNPs containing localized transcripts [156, 165]. A preference for homotypic protein interactions and selectivity in heterotypic interactions are observed, thus providing a molecular mechanism for granule diversity in neurons.
In addition to the self-aggregation of translational repressors, RNA–RNA interactions and additional factors such as EJC components and the scaffold protein TOG contribute to mRNA packaging and granule assembly [161, 166, 167]. Whether the aggregation of mRNA-silencing foci directly contributes to mRNA repression is debated. Current evidence favors the notion that SG and PB aggregation follows translation inhibition. The assembly of the various types of mRNA silencing foci that populate dendrites and synapses open important questions and a working model is that translation repression triggers mRNP aggregation, which may facilitate a long-lasting response. RNA granules then facilitate the transport from the cell soma and mRNA storage in the synaptic surroundings. When required, regulated granule dissolution precedes translation activation [63, 150]. It is expected that aggregation is regulated by post-translational modifications triggered by synaptic stimulation, and increasing phosphorylation of the FUS/TLS aggregation domain impairs fiber formation in vitro [165]. An emerging concept is that RNA granules behave as liquid droplets, and their dissolution can be conceptualized as a phase transition event [147, 168]. Thus, in addition to eventual post-translational modifications that directly govern protein–protein interactions, physicochemical factors that influence SGs, as for example calcium levels, pH, or molecular crowding [147], may affect SyAS-foci dynamics and integrity with consequences for mRNA translation.
Resetting the stage for the next scene: how to achieve translational specificity
The overall translational capacity at the post-synapse is modulated by multiple networks, which in concert with the control of RNA granule motility and integrity, help non-coding regulatory RNAs and RBPs to fine-tune translation. Relevantly, the dendritic translational apparatus subtly differs from that at the cell body, providing a basis for specific regulation. A number of ribosomal proteins are encoded by multiple genes, and the mRNAs for specific variants concentrate up to tenfold in the dendrites of Aplysia neurons [169]. Ribosome heterogeneity is common in several cell types of vertebrates and invertebrates and is a source of translation specificity [170]. Additional mechanisms for spatial control include the interaction of the translational apparatus with membrane receptors. The cytoplasmic tail of the netrin receptor DCC associates with eIF4E and ribosome subunits, which locally blocks their activity eventually releasing them upon ligand binding. This mechanism limits the activation of the translational apparatus and allows for synapse-specific responses [18]. Furthermore, initiation and elongation factors as well as ribosomal proteins are locally regulated by post-translational modifications and additional mechanisms dictated by multiple signaling pathways. All these processes have different effects on different transcripts, which commonly display different initiation and elongation rates and form polysomes of varied sizes [16].
The mTOR kinase is a major regulator activated upon multiple synaptic stimuli and differentially affects the translation, repression or stability of several transcripts [12, 29, 81, 171–174]; reviewed in [175, 176]. mTOR modulates translation initiation and elongation through various parallel mechanisms that involve the eIF4E binding protein (4EBP) and S6 kinase (S6K) (Fig. 5). mTOR phosphorylates and inactivates 4EBP, thus releasing eIF4E and facilitating the translation of transcripts with 5′ terminal oligopyrimidine tracts (TOP mRNAs) or related motifs [171, 175] (Fig. 5). Several translation factors are encoded by TOP mRNAs, and multiple studies have demonstrated that the translation of eukaryotic elongation factor 1A (eEF1A) mRNA is stimulated in dendrites upon activation of the mTOR pathway by synaptic activity [172, 173]. Simultaneously, mTOR activates S6K, which in turn differentially affects the activity of three important translation factors: eukaryotic initiation factor 4B (eIF4B), eukaryotic elongation factor 2 (eEF2) kinase (eEF2 K) and the ribosomal protein S6 (reviewed in [175]) (Fig. 5). The phosphorylation of eIF4B and eEF2K results in stimulated initiation and elongation with different effects on different transcripts [12, 81, 171] (Fig. 5). Although the effect of S6 phosphorylation is poorly understood, it is clear that BDNF stimulation triggers S6 phosphorylation and increases dendritic protein synthesis [12, 175]. It is also known that the mTOR/S6K pathway preferentially stimulates the translation of newly processed transcripts through the direct recruitment of activated S6K to the EJC by an S6K target protein termed S6K1 Aly/REF-like target (SKAR) (reviewed in [175]). ARC/Arg3.1 mRNA and other transcripts carrying EJCs in the 3′UTR and messengers before the pioneer translation round associated with CBP80 are expected to be translationally activated upon local mTOR activation [38, 50] (Fig. 5). Finally, mTOR positively affects the stability of specific transcripts, namely, CaMKII alpha, Homer, and GAP43 mRNAs, by yet unknown mechanisms [29]. In addition to its significance for mRNA translation and turnover, mTOR is a central regulator of major cellular networks connecting autophagy, lipid biogenesis, cytoskeleton remodeling, energy, and growth balance. All these networks undoubtedly contribute to the serious neurological defects associated with the dysregulation of the mTOR pathway in human diseases and animal models [171, 175].
Fig. 5.
The mTOR pathway is a major regulator of multiple cellular networks and responds to synaptic stimulation. The activation of the translation regulatory complex mTORC1 is directed by the upstream kinases PI3K and AKT. mTOR activation in non-neuronal cells depends on its localization to the lysosome membrane and a complex mechanism that involves several regulatory proteins (not shown) [176]. The role of lysosomes or functionally related membrane organelles in the regulation of mTOR upon synaptic stimulation is unknown. The inactivation of 4EBP by mTOR facilitates the translation of TOP and TOP-like mRNAs, and eEF1A and several other molecules functionally linked to translation are regulated by this pathway. The phosphorylation of eIF4B by mTOR-activated S6K enhances the helicase activity of the eIF4A subunit, thereby facilitating translation initiation. S6K inactivates eEF2K, thus limiting the phosphorylation and inactivation of eEF2 and stimulating elongation. The translation of transcripts carrying EJCs is stimulated by S6K through SKAR. This pathway may affect the pioneer translation round and transcripts with EJCs at the 3′UTR, which are abundant in neurons [50]. Upon NMDAR activation, mTOR stabilizes the HuD targets CaMKII alpha, Homer, and GAP43 mRNAs [29] by unknown mechanisms; as a result, HuD availability is reduced and this facilitates Kv.v1 mRNA repression by miR-129. The mTOR pathway inhibits autophagy, which in turn affects miRNA-mediated silencing and further connects mTOR with mRNA regulation
Synaptic activity governs the translational activation or repression of specific mRNAs through the coordination of multiple pathways that affect translation factors, regulatory molecules and SyAS integrity. Pioneer studies using biochemical manipulations of synaptoneurosomes and more recent analyses by in situ visualization of protein synthesis using the FUNCAT (fluorescent non-canonical amino acid tagging) strategy have demonstrated that NMDAR stimulation rapidly reduces the local production of proteins [13–15, 27, 177]. This massive translational silencing involves the activation of eEF2K upon calcium entry, followed by eEF2 inactivation and impaired elongation [26]. This mechanism allows poorly initiated transcripts to compete for initiation factors more successfully; as a result, the translation of CaMKII alpha and ARC/Arg3.1 mRNAs increases [2, 19, 20, 27]. The translation of CaMKII alpha mRNA upon NMDAR stimulation is additionally facilitated by the dissolution of the S-foci, which is triggered by calcium entry and PI3K and likely involves mTOR signaling [14]. Calcium entry upon NMDAR stimulation also activates calpain, a protease that degrades the PABP inhibitor PABP interacting protein 2A (PAIP2). Together with the elongation of the polyA tail of specific transcripts, PAIP2 degradation enhances PABP recruitment, thereby facilitating translation [178]. NMDA-specific translation is further assisted by the inhibition of the miRNA-mediated repression of specific transcripts. NMDAR stimulation dictates the degradation of MOV10, a protein linked to the miRNA pathway, which facilitates the translation of CaMKII alpha mRNA and other miRNA targets [81]. At the same time, the mRNA encoding the voltage-gated potassium channel Kv1.1 is translationally repressed upon NMDAR stimulation through a novel network that involves miR-129, the RBP HuD and the mTOR pathway. In this mechanism, Kv1.1 mRNA repression by miR-129 is antagonized by HuD, which is titrated by a number of HuD target transcripts upon mTOR activation [17, 22, 29] (Fig. 5).
In contrast to the global inhibitory effect of NMDAR stimulation, the activation of group I mGluR globally induces protein synthesis at the post-synapse. Two important pathways, the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) and PI3K/Akt/mTOR, converge to stimulate Cap-dependent initiation upon group I mGluR stimulation. In addition, eEF2K is activated via calcium-calmodulin, thereby slowing elongation. This facilitates ARC/Arg3.1 translation and promotes a relative increase in monosome-associated translation [16, 20, 24, 25, 97, 173]. The translational activation by group I mGluRs is limited below deleterious levels by FMRP. This pathway involves FMRP autoregulation and reversible changes in FMRP granule motility and integrity. The regulation of additional networks contributes to DHPG-specific translation [19, 20, 83, 107, 158].
BDNF strongly stimulates local protein synthesis through the activation of the mTOR pathway, and translation specificity upon BDNFR stimulation is assisted by a dual effect on miRNA-mediated silencing [12, 22]. Compelling evidence was recently presented in which BDNF induces the maturation and the decay of distinct pools of miRNAs. MicroRNA regulation by BDNFR stimulation involves the simultaneous activation of DICER and a protein factor termed Lin28a, which mediates pre-miRNA decay. BDNF activates the MAPK/ERK pathway, which conveys the signal to the HIV-1 TAR RBP (TRBP), a subunit of the DICER complex that enhances its activity [22]. As a consequence, pre-miRNA processing is globally enhanced, a large proportion of the 195 miRNAs present in hippocampal dendrites increase their levels and active miRNA repression correlates with PB assembly [22]. Simultaneously, BDNF post-transcriptionally induces the expression of Lin28a, which facilitates the 3′ polyuridylation of the Let-7 family of pre-miRNAs. This modification triggers the rapid turnover of Let-7 pre-miRNAs by the 3′–5′ exoribonuclease DIS3l2 [179]. As a result, BDNF stimulation dramatically changes the miRNA repertoire and a number of localized transcripts show enhanced translation, whereas many others are repressed. Among other targets, the messengers for CaMKII alpha, Homer 2, and GluA1 are repressed by miRNAs of the Let-7 family and translationally stimulated by BDNF in a Lin28a-dependent manner [22]. It is a common theme that the signaling downstream of neuron stimulation affects a combination of translation factors, regulatory ncRNAs and RBPs as well as RNA granule dynamics, all of which contribute to translational specificity at the post-synapse.
Concluding remarks
Regulated translation at the synapse contributes to the vast computational capacity of the neuronal network by locally affecting the strength of synaptic transmission. A comprehensive analysis of the local transcriptome now seems possible by the almost unlimited power of deep-sequencing techniques and is only restricted by the feasibility of sampling the appropriate tissues or subcellular fractions [53]. The study of non-coding regulatory RNAs has the same possibilities and limitations. The analysis of RBPs and RBP binding elements will be facilitated by high-throughput strategies recently developed to investigate the mRNA interactomes of non-neuronal cells, in which hundreds of novel RBPs were found [111, 114, 115]. Once all these components are mapped, mechanistic insights will require substantial effort to be placed on functional studies.
The dysregulation of localized mRNAs is linked to neurodegeneration. Several RBPs and regulatory RNAs, as well as several pathways that affect their expression, are causative of a number of pathologies and disorders [32, 53, 68, 79, 108, 159, 174]. FMRP, Smaug1/Samd4A, Pumilio, CPEB, Staufen, and Caprin1/RNG105 affect spine morphology and excitability in cultured neurons and model organisms and provoke serious defects paralleling those observed in several human conditions. Several of these important RBPs form dendritic mRNA-silencing foci, collectively termed SyAS-foci, and future studies are pending on how synaptic activity controls their aggregation by modifying specific protein domains or the physicochemical milieu. Multiple mechanisms involving mRNA-silencing foci, RBPs, non-coding RNAs, and the modulation of the translational apparatus concertedly regulate the translational activation or repression of thousands of localized messengers with high spatial and temporal precision. Future work will contribute to understanding the relevance of these multiple pathways in neuron activity and homeostasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 6598 kb) Caption to suggested cover figure. The mRNA repressor Smaug1/Samd4a affects dendritic spines. Dendritic arbors of Smaug1-knockdown hippocampal neurons (yellow) or of untreated cells (white) show the presence of numerous and thin spines provoked by the loss of Smaug1. Deconvoluted confocal Z-stack images of two ECFP-expressing neurons are overlaid
Acknowledgments
We are grateful to MV Baez for kindly providing figure panel 3d and to L Benseñor and LJ Martinez Tosar for their critical reading of the manuscript. This work was supported by the following grants: UBACyT X311 from University of Buenos Aires, Argentina, to GLB; PIP 205-2011-2013 from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) to MGT; and PICT 2010-2339, PICT 2010-1850 and PICT 2011-1301 from Agencia Nacional de Promoción Científica y Tecnológica, (ANPCyT), Argentina, to MGT and GLB.
Footnotes
M. G. Thomas and M. L. Pascual contributed equally to this work.
References
- 1.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21(4):741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 2.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3(3):211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 3.Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci USA. 2001;98(13):7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eom T, Antar LN, Singer RH, Bassell GJ. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J Neurosci. 2003;23(32):10433–10444. doi: 10.1523/JNEUROSCI.23-32-10433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24(11):2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304(5679):1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 7.Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26(51):13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 9.Cajigas IJ, Will T, Schuman EM. Protein homeostasis and synaptic plasticity. EMBO J. 2010;29(16):2746–2752. doi: 10.1038/emboj.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127(1):49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Hanus C, Schuman EM. Proteostasis in complex dendrites. Nat Rev Neurosci. 2013;14(9):638–648. doi: 10.1038/nrn3546. [DOI] [PubMed] [Google Scholar]
- 12.Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24(44):9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieterich DC, Hodas JJ, Gouzer G, Shadrin IY, Ngo JT, Triller A, Tirrell DA, Schuman EM. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci. 2010;13(7):897–905. doi: 10.1038/nn.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baez MV, Luchelli L, Maschi D, Habif M, Pascual M, Thomas MG, Boccaccio GL. Smaug1 mRNA-silencing foci respond to NMDA and modulate synapse formation. J Cell Biol. 2011;195(7):1141–1157. doi: 10.1083/jcb.201108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leski ML, Steward O. Protein synthesis within dendrites: ionic and neurotransmitter modulation of synthesis of particular polypeptides characterized by gel electrophoresis. Neurochem Res. 1996;21(6):681–690. doi: 10.1007/BF02527725. [DOI] [PubMed] [Google Scholar]
- 16.Tatavarty V, Ifrim MF, Levin M, Korza G, Barbarese E, Yu J, Carson JH. Single-molecule imaging of translational output from individual RNA granules in neurons. Mol Biol Cell. 2012;23(5):918–929. doi: 10.1091/mbc.E11-07-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314(5796):144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 18.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141(4):632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28(46):11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zukin RS, Richter JD, Bagni C. Signals, synapses, and synthesis: how new proteins control plasticity. Front Neural Circuits. 2009;3:14. doi: 10.3389/neuro.04.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YW, Ruiz CR, Eyler EC, Lin K, Meffert MK. Dual regulation of miRNA biogenesis generates target specificity in neurotrophin-induced protein synthesis. Cell. 2012;148(5):933–946. doi: 10.1016/j.cell.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273(5280):1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 24.Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA. 1993;90(15):7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26(8):2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marin P, Nastiuk KL, Daniel N, Girault JA, Czernik AJ, Glowinski J, Nairn AC, Premont J. Glutamate-dependent phosphorylation of elongation factor-2 and inhibition of protein synthesis in neurons. J Neurosci. 1997;17(10):3445–3454. doi: 10.1523/JNEUROSCI.17-10-03445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55(4):648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci. 2006;26(27):7143–7146. doi: 10.1523/JNEUROSCI.1796-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosanya NM, Huang PP, Cacheaux LP, Chen CJ, Nguyen K, Perrone-Bizzozero NI, Raab-Graham KF. Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1. J Cell Biol. 2013;202(1):53–69. doi: 10.1083/jcb.201212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, Greenberg ME, Schratt G. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28(6):697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, Kosik KS. Identification of piRNAs in the central nervous system. RNA. 2011;17(6):1090–1099. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10(12):842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 33.Siegel G, Saba R, Schratt G. microRNAs in neurons: manifold regulatory roles at the synapse. Curr Opin Genet Dev. 2011;21(4):491–497. doi: 10.1016/j.gde.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Darnell JC, Richter JD. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb Perspect Biol. 2012;4(8):a012344. doi: 10.1101/cshperspect.a012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: the good, the bad and the ugly. Cell Signal. 2011;23(2):324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36(6):932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cougot N, Bhattacharyya SN, Tapia-Arancibia L, Bordonne R, Filipowicz W, Bertrand E, Rage F. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J Neurosci. 2008;28(51):13793–13804. doi: 10.1523/JNEUROSCI.4155-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.di Penta A, Mercaldo V, Florenzano F, Munck S, Ciotti MT, Zalfa F, Mercanti D, Molinari M, Bagni C, Achsel T. Dendritic LSm1/CBP80-mRNPs mark the early steps of transport commitment and translational control. J Cell Biol. 2009;184(3):423–435. doi: 10.1083/jcb.200807033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeitelhofer M, Karra D, Macchi P, Tolino M, Thomas S, Schwarz M, Kiebler M, Dahm R. Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons. J Neurosci. 2008;28(30):7555–7562. doi: 10.1523/JNEUROSCI.0104-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradhan SJ, Nesler KR, Rosen SF, Kato Y, Nakamura A, Ramaswami M, Barbee SA. The conserved P body component HPat/Pat1 negatively regulates synaptic terminal growth at the larval Drosophila neuromuscular junction. J Cell Sci. 2012 doi: 10.1242/jcs.113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decker CJ, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. 2012;4(9):a012286. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vessey JP, Vaccani A, Xie Y, Dahm R, Karra D, Kiebler MA, Macchi P. Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci. 2006;26(24):6496–6508. doi: 10.1523/JNEUROSCI.0649-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar AM, Silverman EJ, Menon KP, Zinn K. Regulation of synaptic Pumilio function by an aggregation-prone domain. J Neurosci. 2010;30(2):515–522. doi: 10.1523/JNEUROSCI.2523-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menon KP, Andrews S, Murthy M, Gavis ER, Zinn K. The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition. J Neurosci. 2009;29(17):5558–5572. doi: 10.1523/JNEUROSCI.0520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eom T, Zhang C, Wang H, Lay K, Fak J, Noebels JL, Darnell RB. NOVA-dependent regulation of cryptic NMD exons controls synaptic protein levels after seizure. Elife. 2013;2:e00178. doi: 10.7554/eLife.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell TJ, Miyashiro KY, Sul JY, Buckley PT, Lee MT, McCullough R, Jochems J, Kim J, Cantor CR, Parsons TD, Eberwine JH. Intron retention facilitates splice variant diversity in calcium-activated big potassium channel populations. Proc Natl Acad Sci USA. 2010;107(49):21152–21157. doi: 10.1073/pnas.1015264107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillebrand J, Pan K, Kokaram A, Barbee S, Parker R, Ramaswami M. The Me31B DEAD-Box helicase localizes to postsynaptic foci and regulates expression of a CaMKII reporter mRNA in dendrites of Drosophila olfactory projection neurons. Front Neural Circuits. 2010;4:121. doi: 10.3389/fncir.2010.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang YS, Jung MY, Sarkissian M, Richter JD. N-methyl-d-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and alpha CaMKII mRNA polyadenylation at synapses. EMBO J. 2002;21(9):2139–2148. doi: 10.1093/emboj/21.9.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130(1):179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 51.Dynes JL, Steward O. Arc mRNA docks precisely at the base of individual dendritic spines indicating the existence of a specialized microdomain for synapse-specific mRNA translation. J Comp Neurol. 2012;520(14):3105–3119. doi: 10.1002/cne.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35(3):535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 53.Cajigas IJ, Tushev G, Will TJ, tom Dieck S, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74(3):453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13(5):308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shigeoka T, Lu B, Holt CE. Cell biology in neuroscience: RNA-based mechanisms underlying axon guidance. J Cell Biol. 2013;202(7):991–999. doi: 10.1083/jcb.201305139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doyle M, Kiebler MA. Mechanisms of dendritic mRNA transport and its role in synaptic tagging. EMBO J. 2011;30(17):3540–3552. doi: 10.1038/emboj.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macdonald PM. mRNA localization: assembly of transport complexes and their incorporation into particles. Curr Opin Genet Dev. 2011;21(4):407–413. doi: 10.1016/j.gde.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326(5957):1212–1216. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136(4):719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meignin C, Davis I. Transmitting the message: intracellular mRNA localization. Curr Opin Cell Biol. 2010;22(1):112–119. doi: 10.1016/j.ceb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Shahbabian K, Chartrand P. Control of cytoplasmic mRNA localization. Cell Mol Life Sci. 2012;69(4):535–552. doi: 10.1007/s00018-011-0814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshimura A, Fujii R, Watanabe Y, Okabe S, Fukui K, Takumi T. Myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr Biol. 2006;16(23):2345–2351. doi: 10.1016/j.cub.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 63.Pascual ML, Luchelli L, Habif M, Boccaccio GL. Synaptic activity regulated mRNA-silencing foci for the fine tuning of local protein synthesis at the synapse. Commun Integr Biol. 2012;5(4):388–392. doi: 10.4161/cib.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bianco A, Dienstbier M, Salter HK, Gatto G, Bullock SL. Bicaudal-D regulates fragile X mental retardation protein levels, motility, and function during neuronal morphogenesis. Curr Biol. 2010;20(16):1487–1492. doi: 10.1016/j.cub.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, Moore MJ, Budnik V. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149(4):832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao Y, Tatavarty V, Korza G, Levin MK, Carson JH. Multiplexed dendritic targeting of alpha calcium calmodulin-dependent protein kinase II, neurogranin, and activity-regulated cytoskeleton-associated protein RNAs by the A2 pathway. Mol Biol Cell. 2008;19(5):2311–2327. doi: 10.1091/mbc.E07-09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muslimov IA, Patel MV, Rose A, Tiedge H. Spatial code recognition in neuronal RNA targeting: role of RNA-hnRNP A2 interactions. J Cell Biol. 2011;194(3):441–457. doi: 10.1083/jcb.201010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xing L, Bassell GJ. mRNA localization: an orchestration of assembly, traffic and synthesis. Traffic. 2013;14(1):2–14. doi: 10.1111/tra.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pratt CA, Mowry KL. Taking a cellular road-trip: mRNA transport and anchoring. Curr Opin Cell Biol. 2013;25(1):99–106. doi: 10.1016/j.ceb.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134(1):175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baj G, Leone E, Chao MV, Tongiorgi E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci USA. 2011;108(40):16813–16818. doi: 10.1073/pnas.1014168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hilgers V, Perry MW, Hendrix D, Stark A, Levine M, Haley B. Neural-specific elongation of 3′ UTRs during Drosophila development. Proc Natl Acad Sci USA. 2011;108(38):15864–15869. doi: 10.1073/pnas.1112672108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.La Via L, Bonini D, Russo I, Orlandi C, Barlati S, Barbon A. Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing. Nucleic Acids Res. 2013;41(1):617–631. doi: 10.1093/nar/gks1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckley PT, Lee MT, Sul JY, Miyashiro KY, Bell TJ, Fisher SA, Kim J, Eberwine J. Cytoplasmic intron sequence-retaining transcripts can be dendritically targeted via ID element retrotransposons. Neuron. 2011;69(5):877–884. doi: 10.1016/j.neuron.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayashi A, Kasahara T, Iwamoto K, Ishiwata M, Kametani M, Kakiuchi C, Furuichi T, Kato T. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J Biol Chem. 2007;282(47):34525–34534. doi: 10.1074/jbc.M704300200. [DOI] [PubMed] [Google Scholar]
- 76.Glanzer J, Miyashiro KY, Sul JY, Barrett L, Belt B, Haydon P, Eberwine J. RNA splicing capability of live neuronal dendrites. Proc Natl Acad Sci USA. 2005;102(46):16859–16864. doi: 10.1073/pnas.0503783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iacoangeli A, Tiedge H. Translational control at the synapse: role of RNA regulators. Trends Biochem Sci. 2013;38(1):47–55. doi: 10.1016/j.tibs.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McNeill E, Van Vactor D. MicroRNAs shape the neuronal landscape. Neuron. 2012;75(3):363–379. doi: 10.1016/j.neuron.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35(5):325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salta E, De Strooper B. Non-coding RNAs with essential roles in neurodegenerative disorders. Lancet Neurol. 2012;11(2):189–200. doi: 10.1016/S1474-4422(11)70286-1. [DOI] [PubMed] [Google Scholar]
- 81.Banerjee S, Neveu P, Kosik KS. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64(6):871–884. doi: 10.1016/j.neuron.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 82.McCann C, Holohan EE, Das S, Dervan A, Larkin A, Lee JA, Rodrigues V, Parker R, Ramaswami M. The Ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc Natl Acad Sci USA. 2011;108(36):E655–E662. doi: 10.1073/pnas.1107198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 2011;42(5):673–688. doi: 10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336(6078):237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izaurralde E. Elucidating the temporal order of silencing. EMBO Rep. 2012;13(8):662–663. doi: 10.1038/embor.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Duebel J, Bicker S, Fehling HJ, Schubeler D, Oertner TG, Schratt G, Bibel M, Roska B, Filipowicz W. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141(4):618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 87.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 88.Castilla-Llorente V, Spraggon L, Okamura M, Naseeruddin S, Adamow M, Qamar S, Liu J. Mammalian GW220/TNGW1 is essential for the formation of GW/P bodies containing miRISC. J Cell Biol. 2012;198(4):529–544. doi: 10.1083/jcb.201201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 90.Nott A, Tsai LH. The Top3beta way to untangle RNA. Nat Neurosci. 2013;16(9):1163–1164. doi: 10.1038/nn.3506. [DOI] [PubMed] [Google Scholar]
- 91.Gibbings D, Mostowy S, Jay F, Schwab Y, Cossart P, Voinnet O. Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nat Cell Biol. 2012;14(12):1314–1321. doi: 10.1038/ncb2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, Li X, Lubell K, Lim do H, Cho IS, Nakahara K, Preall JB, Sontheimer EJ, Carthew RW. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11(9):1150–1156. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 94.Xu J, Chen Q, Zen K, Zhang C, Zhang Q. Synaptosomes secrete and uptake functionally active microRNAs via exocytosis and endocytosis pathways. J Neurochem. 2013;124(1):15–25. doi: 10.1111/jnc.12057. [DOI] [PubMed] [Google Scholar]
- 95.Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, Lai EC, Pelisson A, Simonelig M. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467(7319):1128–1132. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smalheiser NR, Lugli G, Thimmapuram J, Cook EH, Larson J. Endogenous siRNAs and noncoding RNA-derived small RNAs are expressed in adult mouse hippocampus and are up-regulated in olfactory discrimination training. RNA. 2011;17(1):166–181. doi: 10.1261/rna.2123811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012;122(12):4314–4322. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Udagawa T, Swanger SA, Takeuchi K, Kim JH, Nalavadi V, Shin J, Lorenz LJ, Zukin RS, Bassell GJ, Richter JD. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol Cell. 2012;47(2):253–266. doi: 10.1016/j.molcel.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Villalba A, Coll O, Gebauer F. Cytoplasmic polyadenylation and translational control. Curr Opin Genet Dev. 2011;21(4):452–457. doi: 10.1016/j.gde.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Pavlopoulos E, Trifilieff P, Chevaleyre V, Fioriti L, Zairis S, Pagano A, Malleret G, Kandel ER. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell. 2011;147(6):1369–1383. doi: 10.1016/j.cell.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115(7):879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- 103.Majumdar A, Cesario WC, White-Grindley E, Jiang H, Ren F, Khan MR, Li L, Choi EM, Kannan K, Guo F, Unruh J, Slaughter B, Si K. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell. 2012;148(3):515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Kruttner S, Stepien B, Noordermeer JN, Mommaas MA, Mechtler K, Dickson BJ, Keleman K. Drosophila CPEB Orb2A mediates memory independent of Its RNA-binding domain. Neuron. 2012;76(2):383–395. doi: 10.1016/j.neuron.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adinolfi S, Ramos A, Martin SR, Dal Piaz F, Pucci P, Bardoni B, Mandel JL, Pastore A. The N-terminus of the fragile X mental retardation protein contains a novel domain involved in dimerization and RNA binding. Biochemistry. 2003;42(35):10437–10444. doi: 10.1021/bi034909g. [DOI] [PubMed] [Google Scholar]
- 106.Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140(3):421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 107.Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ. Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J Neurosci. 2012;32(8):2582–2587. doi: 10.1523/JNEUROSCI.5057-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, Williams Z, Ohler U, Tuschl T. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492(7429):382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang T, Bray SM, Warren ST. New perspectives on the biology of fragile X syndrome. Curr Opin Genet Dev. 2012;22(3):256–263. doi: 10.1016/j.gde.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Banerjee P, Schoenfeld BP, Bell AJ, Choi CH, Bradley MP, Hinchey P, Kollaros M, Park JH, McBride SM, Dockendorff TC. Short- and long-term memory are modulated by multiple isoforms of the fragile X mental retardation protein. J Neurosci. 2010;30(19):6782–6792. doi: 10.1523/JNEUROSCI.6369-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, Gueroussov S, Albu M, Zheng H, Yang A, Na H, Irimia M, Matzat LH, Dale RK, Smith SA, Yarosh CA, Kelly SM, Nabet B, Mecenas D, Li W, Laishram RS, Qiao M, Lipshitz HD, Piano F, Corbett AH, Carstens RP, Frey BJ, Anderson RA, Lynch KW, Penalva LO, Lei EP, Fraser AG, Blencowe BJ, Morris QD, Hughes TR. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499(7457):172–177. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003;115(7):893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- 113.Buratti E, Baralle FE. TDP-43: gumming up neurons through protein–protein and protein-RNA interactions. Trends Biochem Sci. 2012;37(6):237–247. doi: 10.1016/j.tibs.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, Wyler E, Bonneau R, Selbach M, Dieterich C, Landthaler M. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell. 2012;46(5):674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 115.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149(6):1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 116.Schrimpf SP, Meskenaite V, Brunner E, Rutishauser D, Walther P, Eng J, Aebersold R, Sonderegger P. Proteomic analysis of synaptosomes using isotope-coded affinity tags and mass spectrometry. Proteomics. 2005;5(10):2531–2541. doi: 10.1002/pmic.200401198. [DOI] [PubMed] [Google Scholar]
- 117.Vessey JP, Schoderboeck L, Gingl E, Luzi E, Riefler J, Di Leva F, Karra D, Thomas S, Kiebler MA, Macchi P. Mammalian Pumilio 2 regulates dendrite morphogenesis and synaptic function. Proc Natl Acad Sci USA. 2010;107(7):3222–3227. doi: 10.1073/pnas.0907128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olesnicky EC, Bhogal B, Gavis ER. Combinatorial use of translational co-factors for cell type-specific regulation during neuronal morphogenesis in Drosophila . Dev Biol. 2012;365(1):208–218. doi: 10.1016/j.ydbio.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen G, Li W, Zhang QS, Regulski M, Sinha N, Barditch J, Tully T, Krainer AR, Zhang MQ, Dubnau J. Identification of synaptic targets of Drosophila Pumilio. PLoS Comput Biol. 2008;4(2):e1000026. doi: 10.1371/journal.pcbi.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brechbiel JL, Gavis ER. Spatial regulation of nanos is required for its function in dendrite morphogenesis. Curr Biol. 2008;18(10):745–750. doi: 10.1016/j.cub.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye B, Petritsch C, Clark IE, Gavis ER, Jan LY, Jan YN. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr Biol. 2004;14(4):314–321. doi: 10.1016/j.cub.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 122.Siemen H, Colas D, Heller HC, Brustle O, Pera RA. Pumilio-2 function in the mouse nervous system. PLoS One. 2011;6(10):e25932. doi: 10.1371/journal.pone.0025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marrero E, Rossi SG, Darr A, Tsoulfas P, Rotundo RL. Translational regulation of acetylcholinesterase by the RNA-binding protein Pumilio-2 at the neuromuscular synapse. J Biol Chem. 2011;286(42):36492–36499. doi: 10.1074/jbc.M111.285510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quenault T, Lithgow T, Traven A. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol. 2011;21(2):104–112. doi: 10.1016/j.tcb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 125.Muraro NI, Weston AJ, Gerber AP, Luschnig S, Moffat KG, Baines RA. Pumilio binds para mRNA and requires Nanos and Brat to regulate sodium current in Drosophila motoneurons. J Neurosci. 2008;28(9):2099–2109. doi: 10.1523/JNEUROSCI.5092-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, Broger C, Tully T. The Staufen/Pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13(4):286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 127.Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One. 2008;3(9):e3164. doi: 10.1371/journal.pone.0003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22(5):221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- 129.Kim SY, Kim JY, Malik S, Son W, Kwon KS, Kim C. Negative regulation of EGFR/MAPK pathway by Pumilio in Drosophila melanogaster . PLoS One. 2012;7(4):e34016. doi: 10.1371/journal.pone.0034016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee MH, Hook B, Pan G, Kershner AM, Merritt C, Seydoux G, Thomson JA, Wickens M, Kimble J. Conserved regulation of MAP kinase expression by PUF RNA-binding proteins. PLoS Genet. 2007;3(12):e233. doi: 10.1371/journal.pgen.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Friend K, Campbell ZT, Cooke A, Kroll-Conner P, Wickens MP, Kimble J. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol. 2012;19(2):176–183. doi: 10.1038/nsmb.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pinder BD, Smibert CA. Smaug: An unexpected journey into the mechanisms of post-transcriptional regulation. Fly (Austin) 2013;7(3):142–145. doi: 10.4161/fly.24336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aviv T, Lin Z, Ben-Ari G, Smibert CA, Sicheri F. Sequence-specific recognition of RNA hairpins by the SAM domain of Vts1p. Nat Struct Mol Biol. 2006;13(2):168–176. doi: 10.1038/nsmb1053. [DOI] [PubMed] [Google Scholar]
- 134.Green JB, Gardner CD, Wharton RP, Aggarwal AK. RNA recognition via the SAM domain of Smaug. Mol Cell. 2003;11(6):1537–1548. doi: 10.1016/s1097-2765(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 135.Semotok JL, Luo H, Cooperstock RL, Karaiskakis A, Vari HK, Smibert CA, Lipshitz HD. Drosophila maternal Hsp83 mRNA destabilization is directed by multiple SMAUG recognition elements in the open reading frame. Mol Cell Biol. 2008;28(22):6757–6772. doi: 10.1128/MCB.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Weaver T, Hughes TR, Westwood JT, Smibert CA, Lipshitz HD. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev Cell. 2007;12(1):143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 137.Oberstrass FC, Lee A, Stefl R, Janis M, Chanfreau G, Allain FH. Shape-specific recognition in the structure of the Vts1p SAM domain with RNA. Nat Struct Mol Biol. 2006;13(2):160–167. doi: 10.1038/nsmb1038. [DOI] [PubMed] [Google Scholar]
- 138.Ravindranathan S, Oberstrass FC, Allain FH. Increase in backbone mobility of the VTS1p-SAM domain on binding to SRE-RNA. J Mol Biol. 2010;396(3):732–746. doi: 10.1016/j.jmb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 139.Li X, Quon G, Lipshitz HD, Morris Q. Predicting in vivo binding sites of RNA-binding proteins using mRNA secondary structure. RNA. 2010;16(6):1096–1107. doi: 10.1261/rna.2017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baez MV, Boccaccio GL. Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J Biol Chem. 2005;280(52):43131–43140. doi: 10.1074/jbc.M508374200. [DOI] [PubMed] [Google Scholar]
- 141.Nelson MR, Leidal AM, Smibert CA. Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J. 2004;23(1):150–159. doi: 10.1038/sj.emboj.7600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pinder BD, Smibert CA. microRNA-independent recruitment of Argonaute 1 to nanos mRNA through the Smaug RNA-binding protein. EMBO Rep. 2013;14(1):80–86. doi: 10.1038/embor.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zaessinger S, Busseau I, Simonelig M. Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development. 2006;133(22):4573–4583. doi: 10.1242/dev.02649. [DOI] [PubMed] [Google Scholar]
- 144.Jeske M, Moritz B, Anders A, Wahle E. Smaug assembles an ATP-dependent stable complex repressing nanos mRNA translation at multiple levels. EMBO J. 2010;30(1):90–103. doi: 10.1038/emboj.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haraguchi S, Tsuda M, Kitajima S, Sasaoka Y, Nomura-Kitabayashid A, Kurokawa K, Saga Y. nanos1: a mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mech Dev. 2003;120(6):721–731. doi: 10.1016/s0925-4773(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 146.Franks TM, Lykke-Andersen J. The control of mRNA decapping and P-body formation. Mol Cell. 2008;32(5):605–615. doi: 10.1016/j.molcel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci. 2013 doi: 10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramaswami M, Taylor JP, Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 2013;154(4):727–736. doi: 10.1016/j.cell.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cougot N, Molza AE, Giudice E, Cavalier A, Thomas D, Gillet R. Structural organization of the polysomes adjacent to mammalian processing bodies (P-bodies) RNA Biol. 2013;10(2):314–320. doi: 10.4161/rna.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kotani T, Yasuda K, Ota R, Yamashita M. Cyclin B1 mRNA translation is temporally controlled through formation and disassembly of RNA granules. J Cell Biol. 2013;202(7):1041–1055. doi: 10.1083/jcb.201302139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weil TT, Parton RM, Herpers B, Soetaert J, Veenendaal T, Xanthakis D, Dobbie IM, Halstead JM, Hayashi R, Rabouille C, Davis I. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat Cell Biol. 2012;14(12):1305–1313. doi: 10.1038/ncb2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R, Ramaswami M. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52(6):997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller LC, Blandford V, McAdam R, Sanchez-Carbente MR, Badeaux F, DesGroseillers L, Sossin WS. Combinations of DEAD box proteins distinguish distinct types of RNA: protein complexes in neurons. Mol Cell Neurosci. 2009;40(4):485–495. doi: 10.1016/j.mcn.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 154.Savas JN, Makusky A, Ottosen S, Baillat D, Then F, Krainc D, Shiekhattar R, Markey SP, Tanese N. Huntington’s disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. Proc Natl Acad Sci USA. 2008;105(31):10820–10825. doi: 10.1073/pnas.0800658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shiina N, Shinkura K, Tokunaga M. A novel RNA-binding protein in neuronal RNA granules: regulatory machinery for local translation. J Neurosci. 2005;25(17):4420–4434. doi: 10.1523/JNEUROSCI.0382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, McKnight SL. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149(4):753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reijns MA, Alexander RD, Spiller MP, Beggs JD. A role for Q/N-rich aggregation-prone regions in P-body localization. J Cell Sci. 2008;121(Pt 15):2463–2472. doi: 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fujii R, Okabe S, Urushido T, Inoue K, Yoshimura A, Tachibana T, Nishikawa T, Hicks GG, Takumi T. The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol. 2005;15(6):587–593. doi: 10.1016/j.cub.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 159.Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011;9(4):e1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Carter R, Boylan KB, Wojtas AM, Rademakers R, Pinkus JL, Greenberg SA, Trojanowski JQ, Traynor BJ, Smith BN, Topp S, Gkazi AS, Miller J, Shaw CE, Kottlors M, Kirschner J, Pestronk A, Li YR, Ford AF, Gitler AD, Benatar M, King OD, Kimonis VE, Ross ED, Weihl CC, Shorter J, Taylor JP. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barbarese E, Ifrim MF, Hsieh L, Guo C, Tatavarty V, Maggipinto MJ, Korza G, Tutolo JW, Giampetruzzi A, Le H, Ma XM, Levine E, Bishop B, Kim DO, Kuwada S, Carson JH. Conditional knockout of tumor overexpressed gene in mouse neurons affects RNA granule assembly, granule translation, LTP and short term habituation. PLoS One. 2013;8(8):e69989. doi: 10.1371/journal.pone.0069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martel C, Dugre-Brisson S, Boulay K, Breton B, Lapointe G, Armando S, Trepanier V, Duchaine T, Bouvier M, Desgroseillers L. Multimerization of Staufen1 in live cells. RNA. 2010;16(3):585–597. doi: 10.1261/rna.1664210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Toba G, White K. The third RNA recognition motif of Drosophila ELAV protein has a role in multimerization. Nucleic Acids Res. 2008;36(4):1390–1399. doi: 10.1093/nar/gkm1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tosar LJ, Thomas MG, Baez MV, Ibanez I, Chernomoretz A, Boccaccio GL. Staufen: from embryo polarity to cellular stress and neurodegeneration. Front Biosci (Schol Ed) 2012;4:432–452. doi: 10.2741/s277. [DOI] [PubMed] [Google Scholar]
- 165.Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012;149(4):768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 166.Singh G, Kucukural A, Cenik C, Leszyk JD, Shaffer SA, Weng Z, Moore MJ. The cellular EJC interactome reveals higher-order mRNP structure and an EJC-SR protein nexus. Cell. 2012;151(4):750–764. doi: 10.1016/j.cell.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hartswood E, Brodie J, Vendra G, Davis I, Finnegan DJ. RNA:RNA interaction can enhance RNA localization in Drosophila oocytes. RNA. 2012;18(4):729–737. doi: 10.1261/rna.026674.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell. 2012;149(6):1188–1191. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 169.Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006;127(7):1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol. 2012;13(6):355–369. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485(7396):109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25(24):5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25(31):7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78(3):510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 176.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126(Pt 8):1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 178.Khoutorsky A, Yanagiya A, Gkogkas CG, Fabian MR, Prager-Khoutorsky M, Cao R, Gamache K, Bouthiette F, Parsyan A, Sorge RE, Mogil JS, Nader K, Lacaille JC, Sonenberg N. Control of synaptic plasticity and memory via suppression of poly(A)-binding protein. Neuron. 2013;78(2):298–311. doi: 10.1016/j.neuron.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 179.Chang HM, Triboulet R, Thornton JE, Gregory RI. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature. 2013 doi: 10.1038/nature12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jaruzelska J, Kotecki M, Kusz K, Spik A, Firpo M, Reijo Pera RA. Conservation of a Pumilio-Nanos complex from Drosophila germ plasm to human germ cells. Dev Genes Evol. 2003;213(3):120–126. doi: 10.1007/s00427-003-0303-2. [DOI] [PubMed] [Google Scholar]
- 181.Ramos A, Hollingworth D, Adinolfi S, Castets M, Kelly G, Frenkiel TA, Bardoni B, Pastore A. The structure of the N-terminal domain of the fragile X mental retardation protein: a platform for protein–protein interaction. Structure. 2006;14(1):21–31. doi: 10.1016/j.str.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 182.Budini M, Buratti E, Stuani C, Guarnaccia C, Romano V, De Conti L, Baralle FE. Cellular model of TAR DNA-binding protein 43 (TDP-43) aggregation based on its C-terminal Gln/Asn-rich region. J Biol Chem. 2012;287(10):7512–7525. doi: 10.1074/jbc.M111.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ayala YM, Pantano S, D’Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C. elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348(3):575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 184.Kasashima K, Sakashita E, Saito K, Sakamoto H. Complex formation of the neuron-specific ELAV-like Hu RNA-binding proteins. Nucleic Acids Res. 2002;30(20):4519–4526. doi: 10.1093/nar/gkf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shiina N, Yamaguchi K, Tokunaga M. RNG105 deficiency impairs the dendritic localization of mRNAs for Na+/K+ATPase subunit isoforms and leads to the degeneration of neuronal networks. J Neurosci. 2010;30(38):12816–12830. doi: 10.1523/JNEUROSCI.6386-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen T, Richard S. Structure-function analysis of Qk1: a lethal point mutation in mouse quaking prevents homodimerization. Mol Cell Biol. 1998;18(8):4863–4871. doi: 10.1128/mcb.18.8.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 6598 kb) Caption to suggested cover figure. The mRNA repressor Smaug1/Samd4a affects dendritic spines. Dendritic arbors of Smaug1-knockdown hippocampal neurons (yellow) or of untreated cells (white) show the presence of numerous and thin spines provoked by the loss of Smaug1. Deconvoluted confocal Z-stack images of two ECFP-expressing neurons are overlaid