Abstract
The etiology of Parkinson’s disease (PD) is complex and most likely involves numerous environmental and heritable risk factors. Interestingly, many genetic variants, which have been linked to familial forms of PD or identified as strong risk factors, also play a critical role in modulating inflammatory responses. There has been considerable debate in the field as to whether inflammation is a driving force in neurodegeneration or simply represents a response to neuronal death. One emerging hypothesis is that inflammation plays a critical role in the early phases of neurodegeneration. In this review, we will discuss emerging aspects of both innate and adaptive immunity in the context of the pathogenesis of PD. We will highlight recent data from genetic and functional studies that strongly support the theory that genetic susceptibility plays an important role in modulating immune pathways and inflammatory reactions, which may precede and initiate neuronal dysfunction and subsequent neurodegeneration. A detailed understanding of such cellular and molecular inflammatory pathways is crucial to uncover pathogenic mechanisms linking sporadic and hereditary PD and devise tailored neuroprotective interventions.
Keywords: Parkinson’s disease, Immune system, Neurogenetics, Neuroinflammation, Glial cells
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the preferential loss of dopaminergic (DA) neurons in the substantia nigra (SN) pars compacta, and the widespread presence of α-synuclein (α-syn) aggregates, termed Lewy bodies (LB) and Lewy neurites (LN) [1]. PD pathology is not limited to the SN, but distributed throughout the central nervous system (CNS) from the lower brainstem through the midbrain and cerebral cortex [2, 3]. Aggregates of α-syn and LB are also found in the autonomic nervous system (ANS) in patients and experimental models of PD [4–6]. The majority of PD cases are sporadic, and it is generally thought that the interaction between genetic susceptibility and environmental factors contributes to the disease development [7]. Such environmental factors include ageing, neurotoxins, brain trauma, and infections. However, several genes have been identified as causes or risk factors for PD [8]. To date, at least 18 genetic loci, PARK1–18, have been linked to PD and 9 loci have been confirmed as genetic causes and linked to monogenic PD [8]. Some variants of the same gene (SNCA, LRRK2) are also risk factors for sporadic disease. The identification of genes linked to PD susceptibility is therefore crucial for understanding mechanisms of neuronal death in both inherited and sporadic forms of the disease. Interestingly, many of such PD-linked genes have relevant roles in regulating inflammatory responses. It is now clear that inflammation contributes to the pathophysiology and aetiology of neurodegenerative disorders. Triggering factors of neuroinflammation may be pathogens (bacterial or viral infections), the dysregulation of inflammatory pathways (e.g., immune alterations associated with aging, autoimmune disorders, genetic vulnerability), protein aggregates (amyloid β peptides, α-syn) and primary inflammatory responses in neurons. Several lines of evidence, including the association of neurodegenerative diseases with the human leukocyte antigen (HLA) region [9], indicate a role for genetic susceptibility to inflammation in the actual pathogenesis, and thus initiation of neuronal dysfunction. Interestingly, LRRK2 variants, the most common genetic cause of PD, have been recently associated with Crohn’s disease (CD) and leprosy, a chronic infectious disease caused by Mycobacterium leprae [10–12]. These findings suggest that chronic inflammatory diseases, infectious diseases and PD may share common genetic, molecular and cellular pathological pathways. Here, we describe how inflammation occurs at multiple levels in both sporadic and familial PD, and discuss recent data from epidemiological, genetic and functional studies supporting the key role of inflammatory responses in the initiation and progression of disease pathology.
The immune system in neurodegenerative diseases
It is widely recognized that the immune privilege of the CNS is relative rather than absolute [13]. Both innate and adaptive immune responses occur in CNS disorders and contribute to disease pathogenesis.
The innate immune system (phagocytes, NK cells and the complement system) is involved in early inflammatory reactions that promote adaptive immune responses (T- and B-lymphocytes) [14]. Microglia are the resident innate immune cells of the CNS and account for approximately 5–15 % of the brain cells [15]. They play key roles in tissue repair and cellular homeostasis. In response to neuronal injury, microglia become activated, acquire phagocytic properties, and eliminate cellular debris and damaged neurons. Microglial responses can have neuroprotective as well as harmful consequences [16]. Activated microglia produce neurotoxic molecules including cytokines, chemokines, complement proteins, and nitric oxide (NO) [17]. Neuro-immune interactions involving the expression of surface molecules on both microglia and neurons tightly regulate the phagocytic properties of microglial cells. Such molecules comprise CD200/CD200R, CD47/CD172a, CX3CL1/CX3CR, and the complement regulatory proteins, C1q and C3 [18–24]. Genetic factors can also influence microglia activation. Genetic differences in ApoE4 genotype, which have been associated to late-onset Alzheimer’s disease (AD), regulate both systemic and brain inflammatory responses [25]. Two independent studies have recently shown that rare variants in TREM2 confer susceptibility to late-onset AD [26, 27]. TREM2 encodes a membrane protein that regulates the activation of macrophages and dendritic cells. Impaired function of TREM2, therefore, may be the cause of the inefficient clearing of amyloid plaques by microglia and subsequent inflammatory processes, which lead to cognitive decline.
In many neurodegenerative disorders, microglia activation is accompanied by chronic astrogliosis. Even though the role of astrocytes as antigen-presenting cells (APCs) is still a matter of debate [28], it is clear that upon activation astrocytes produce several pro-inflammatory cytokines (TGF-β1, IL-1β, IL-6), chemokines (CCL2, CXCL1, CXCL10, CXCL12), and complement mediators [29], which contribute to neuroinflammation. Interestingly, genetic mutations linked to neurodegeneretive diseases such as amyotrophic lateral sclerosis trigger primary astrocytic dysfunction and subsequent immune reactions [30–32].
Recruitment of peripheral T- and B-lymphocytes with effector and memory functions, infiltration of blood-derived myeloid cells within the damaged CNS, and increased levels of peripheral cytokines and chemokines are often observed in neurodegenerative conditions. While infiltrating T cells and macrophages are crucial in the pathogenesis of CNS inflammatory diseases such as multiple sclerosis (MS), the role of the adaptive immune system and blood-derived cells in the pathogenesis of neurodegenerative disorders is still poorly understood.
Cell-autonomous immune mechanisms in neurons may also play a role in neurodegeneration (reviewed in [33]). For a long time, glial cells have been thought to be the main contributor to cytokine and chemokine production and the presence of immune receptors in the brain. However, there is increasing evidence that neurons can actively initiate and perpetuate innate immune responses [34]. Neurons express molecules originally thought to be specific to the immune system such as Toll-like receptors (TLRs), major histocompatibility complex (MHC) and complement molecules [35]. Although these immune receptors are mainly known to function in the development and organization of neuronal networks and synapses, they also modulate the innate immune responses in neurons; indeed, neurons appear to be capable of sensing and responding to viral infections even in the absence of immune cells [34]. TLRs, the most studied neuronal immune molecules, are a family of evolutionarily conserved innate immune receptors, mainly located on APCs such as B cells, dendritic cells, monocytes/macrophages, and microglia. Interestingly endothelial cells, astrocytes, oligodendrocytes, and neurons also express high levels of TLRs [36]. TLRs recognize pathogen-associated molecules originating from bacteria, viruses, fungi, and parasites during infections. Following ligand binding, TLRs initiate immune responses and cytokine production for host defense. TLRs are also involved in CNS disorders including stroke, demyelinating and neurodegenerative diseases [37]. Host-endogenous ligands associated with damaged cells and tissues (heat shock proteins, fibrinogen, fibronectin, double strand RNA) have been shown to activate TLRs [37]. In “sterile inflammation”, molecules associated with aging and neuronal death may therefore activate neuronal and glial TLRs and initiate protective or harmful signaling cascades.
Evidence for inflammation in PD: triggering factor or consequence of neurodegeneration?
Inflammatory reactions involving microglia, astrocytes, and lymphocytes have been described in several animal models of DA neurodegeneration and PD patients. There has been considerable debate in the field as to whether such events play a role in the prodromal stages of the disease. The emerging hypothesis is that inflammation may even precede the onset of PD. However, it is still poorly understood whether midbrain DA neurons are selectively vulnerable to inflammatory events. More studies addressing the mechanisms underlying such susceptibility in midbrain DA neurons are therefore needed. Here, we summarize evidence from the literature supporting the role of inflammatory events in PD pathology, and highlight novel mechanistic pathways emerging from these studies.
Inflammation in animal models of PD
Activated microglia and astrocytes, increased production of cyclooxygenase-2 (COX-2), pro-inflammatory cytokines and NO, recruitment of CD4+ and CD8+ T cells, and involvement of TLR-dependent pathways have been reported in many toxin-based models of PD [38–43]. Interestingly, the early blockade of neuroinflammation by minocycline, non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 or cytokine inhibitors attenuates the PD-like disease process in these models of DA neuron degeneration, supporting the hypothesis that immune cells and inflammatory molecules are important contributors to the initiation of PD [40, 44–48]. Such experimental models also support the role of astrocytes in PD pathology. Astrocytes effectively endocytose α-synuclein secreted from neurons, and initiate inflammatory responses. Moreover, S100B, a protein highly produced in, and secreted from, astrocytes, has proinflammatory properties and induces neurodegeneration through the RAGE and tumor necrosis factor-alpha (TNF-α) pathway in a mouse model of PD [49]. Neuro-immune interactions also modulate DA neuron degeneration, as shown in mouse models of CX3CR1 and CD200R deficiency [23, 50]. The relationship between inflammation and neurodegeneration remains largely unknown. Transgenic animal models of PD show that inflammation is an early event that can even precede neurodegeneration [51]. Inflammation alone can precipitate PD-like pathology and initiate neurodegenerative events in the nigrostriatal system, which correspond to changes observed in early neurodegeneration. Inflammatory cytokines released from activated microglia and astrocytes alter synaptic transmission and axonal transport and increase neuronal susceptibility to subsequent neurotoxic triggers [47, 48]. Inflammation could therefore prime neurons and immune cells in the brain, rendering neuronal populations vulnerable to degeneration in the face of subsequent insults.
Post-mortem studies in PD patients
The first evidence for inflammatory reactions in PD is shown by the work of McGeer et al. [52]. This study showed for the first time the presence of activated HLA-DR-positive reactive microglia within the SN of PD patients, along with LB and free melanin [52]. Reactive microglial cells can therefore be considered a sensitive index of disease pathology [52]. The presence of microglial activation and reactive astrogliosis was then confirmed by several other post-mortem studies [22, 53–56]. Elevated numbers of microglial cells have been found not only in the SN and putamen but also in other brain regions, including the hippocampus, transentorhinal cortex, cingulate cortex, and temporal cortex [54]. Post-mortem studies also show that several pro-inflammatory cytokines and chemokines (including IL1-β, IL-2, IL-6, TNF-α, TNF-α, IFN-γ, CXCL12, CXCR4, ICAM-1) are elevated in the DA nigrostriatal system and other brain regions outside the SN in PD patients [57–63]. In addition, levels of inducible nitric oxide synthase (iNOS), NFκB, COX-1 and COX-2, and prostaglandin E2 are increased in parkinsonian brains [40, 62, 64].
Reactive astrogliosis is absent or generally described as mild or moderate in PD brains [53, 56, 65]. Studies in animal models show that astrocytes express pro-inflammatory molecules that recruit microglial cells in the early phases of neurodegeneration [66, 67]. However, the exact role of astrocytes in PD pathogenesis is still poorly understood. In PD, the distribution of α-synuclein-positive astrocytes parallels the distribution of LB [68], but no correlation between GFAP staining and disease severity has been found [69]. This finding suggests that astrocytes may also have a potential neuroprotective role. Astrocytes secrete a number of neurotrophic factors for DA neurons, including glial cell-line-derived neurotrophic factor, brain-derived neurotrophic factor [70, 71], and protease-activated receptor-1, and this may have a neuroprotective effect by increasing the activity of glutathione peroxidase [72].
Finally, increased numbers of CD4+ and CD8+ T cells are present in post-mortem human brain specimens of PD patients [42, 52]. The dysfunction of the blood–brain barrier (BBB), which has been described in PD patients [73, 74], may not be sufficient to allow such lymphocyte extravasation. Active mechanisms involving the expression of adhesion molecules on endothelial and glial cells are likely implicated in the regulation of T cell recruitment.
In vivo imaging studies
In vivo PET scan studies with [(11)C](R)-PK11195, a ligand of the peripheral binding site of benzodiazepine, provide evidence for significant microglial activation in the midbrain and putamen of PD patients [75]. Imaging studies have yielded mixed results regarding the correlation between microglial activation and disease severity and duration [75–77]. Such differences are likely due to the fact that microglia are activated early in the disease course. Moreover, there are some limitations associated with the use of 11C-PK11195 due to its high level of non-specific binding, indicating the need for better tracers to follow microglia activation and effects of anti-inflammatory treatments in patients [78].
Peripheral immune activation in PD
Levels of several cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, INF-γ, RANTES) are elevated in the serum of PD patients [79]. Several studies support a role for the adaptive immune system in the aetiology of PD (reviewed in [79]). Peripheral alterations have been reported in the blood of PD patients including a lower ratio of CD4+:CD8+ T lymphocytes, higher expression of MHC II molecules on monocytes in the cerebrospinal fluid (CSF) and peripheral blood, and higher numbers of CD4+CD25+ activated lymphocytes [80–84]. Further evidence in support of the involvement of the adaptive immune system in PD comes from recent studies that show increased levels of autoantibodies against α-syn in serum and CSF in PD patients [85, 86].
Gut inflammation has been recently linked to PD. PD patients show inflammatory reactions within the enteric nervous system (ENS), characterized by increased expression levels of pro-inflammatory cytokines and glial markers [87]. Even though these findings need to be replicated in larger cohorts of patients, studies in animal models of PD further support a link between early inflammatory events in the ENS and PD pathology [88].
The nervous and the immune system influence each other even at long distances in physiological and pathological conditions. The vagus nerve, which originates in the brainstem and innervates visceral organs, controls the immune function via the “inflammatory reflex” [89]. The stimulation of the vagus nerve inhibits cytokine release and attenuates inflammatory reactions in septic shock and post-stroke immunosuppression by activating acetylcholine-producing T cells [90, 91]. Since PD patients show early autonomic dysfunction and the involvement of the vagus nerve, further studies addressing the role of such neuroimmune interactions in neurodegeneration are needed. In principle, it would be possible to target peripheral inflammation and prevent neurodegeneration without affecting the host immune system.
Epidemiological studies
Initial epidemiological data show that chronic use of NSAIDs lowers the risk of development of sporadic PD [92–94]. A large-scale meta-analysis revealed conflicting results regarding the use of NSAIDs and PD risk, indicating that only ibuprofen provides significant protection [95]. Based on a recent Cochrane review, however, there is no evidence for the use of NSAIDs in the prevention of PD [96]. Further studies are therefore needed.
Genetic linkage between inflammation and PD
Polymorphisms in immune-related genes and PD risk
Several genetic studies have investigated the link between polymorphisms in immune-related genes and PD (Table 1).
Genome-wide association studies (GWAS) have identified an association of genetic variation in the HLA region with sporadic PD [9, 97, 98].
A pathway analysis of GWA data revealed that the T cell receptor signaling pathway is a susceptibility factor for PD and genetic variability in genes regulating inflammatory pathways differentiates PD patients from controls [99, 100].
Gene polymorphisms of several inflammatory cytokines, including TNF-α, IL-1β, and CD14 monocyte receptor, might be a risk factor for PD [101, 102].
Three single nucleotide polymorphisms in the CARD15 gene, previously associated with CD, a chronic inflammatory bowel disease, have been shown to be a risk factor for PD [103].
Table 1.
Polymorphisms in immune genes associated with Parkinson’s disease risk
| Gene | Population | Polymorphism ID | Risk | References |
|---|---|---|---|---|
| IL-10 | Sweden | rs1800896 (-1082A/G) | Increased | [191] |
| IL-18 | China | rs1946518 (-607C/A) | Increased | [171] |
| IL-1A | Taiwan | rs1800587 (-889) | Decreased | [173] |
| China | rs180058 (-889) | Decreased | [200] | |
| IL-1B | Finland | IL1B_AvaI (rs16944/rs3087258) | Increased | [185] |
| Canada | IL1B_AvaI (rs16944/rs3087258) | Increased | [184] | |
| Germany | IL1B_AvaI (rs16944/rs3087258) | Increased | [176] | |
| USA | rs16944 (-511) | Increased | [101] | |
| Japan | rs1143634 (3953C/T) | Increased | [182] | |
| Taiwan | IL1B_AvaI (rs16944/rs3087258) | Increased | [173] | |
| IL-6 | Sweden | rs13447446 (174G/C) | Increased | [192] |
| IL-8 | Ireland | rs4073 (T251A) | Increased | [178] |
| TNF-alpha | Poland | rs1800629 (-308A/G) | Increased | [197] |
| Germany | rs1800629 (-308A/G) | Increased | [190] | |
| USA | rs1800629 (-308A/G) | Increased | [101] | |
| Japan | rs4647198 (-1031T/C) | Increased | [183] | |
| Taiwan | rs4647198 (-1031T/C) | Increased | [172] | |
| CD14 | Taiwan | rs2569190 (-260T/C) | Increased | [102] |
| HLA-DQB1 | Germany | HLA-DQB1 | Increased | [189] |
| HLA-DRA | USA (NGRC-GWAS) | rs3129882 | Increased | [9] |
| Ireland/Poland/USA | rs3129882 | Increased | [180] | |
| China | rs3129882 | Increased | [193] | |
| HLA-DRB1 | UK | HLA-DRB1 | Increased | [177] |
| HLA-DRB5 | USA/Europe | chr6:32588205 (hg18) | Increased | [97] |
All data were obtained from systematic meta-analyses at http://www.pdgene.org/ [187]
However, the exact role of these polymorphisms in PD pathogenesis still needs to be elucidated and the frequency of these risk alleles strongly depends on the heterogeneity of the population [104].
The role of PD-genes in immune responses
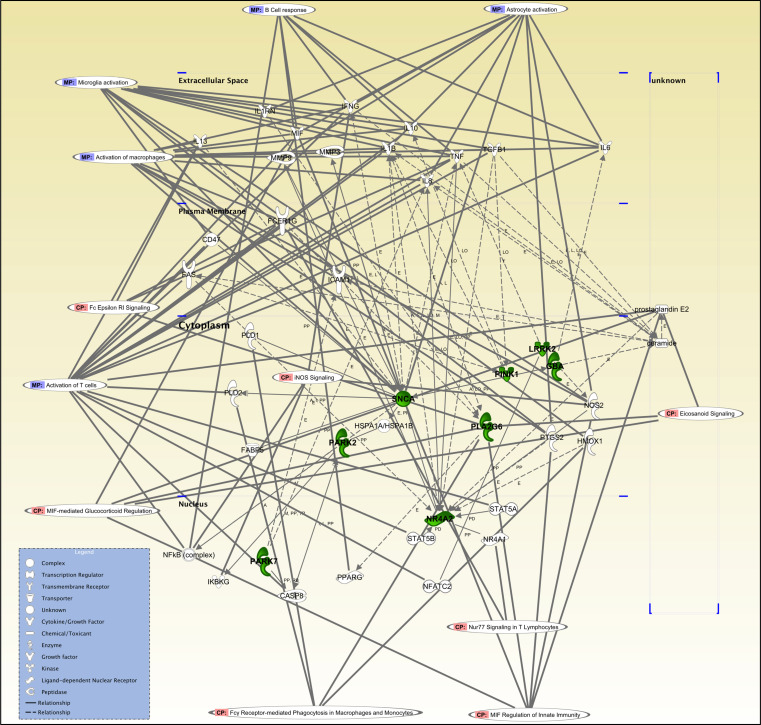
Recent research into the functional genomics of PD has identified PD-genes as regulators of key processes in innate and adaptive immunity (Table 2; Figs. 1, 2). PD proteins may contribute to PD pathogenesis by directly triggering dysfunction in immune cells (e.g., LRRK2, PLA2G6, GBA) or promoting protein misfolding/aggregation and reactive inflammatory responses (e.g., α-syn). As revealed by the pathway analysis shown in Fig. 2, PD-genes are directly or indirectly involved in several immune biological processes including T cell activation (Th1 and Th2 inflammatory responses), B cell responses, and innate immunity responses, such as activation of macrophages, microglia, and astrocytes.
Table 2.
Parkinson’s disease genes with a role in immune responses
| Gene | Disorder/Inheritance | Role in immune responses | References |
|---|---|---|---|
| SNCA (PARK1-4) | EOPD/AD | Activation of microglia | [146], [150], [199], [136], [137], [138], [149], [147], [151], [148], [152], [140], [153], [141], [142], [143], [144] |
| Activation of astrocytes | [145], [139] | ||
| Parkin (PARK2) | EOPD/AR | Inhibition of immune responses | [194], [195], [175] |
| Increased expression in astrocytes in unfolded protein stress conditions | [188] | ||
| PINK1 (PARK6) | EOPD/AR | Regulation of cytokine production | [191], [170], [144] |
| Increased levels in reactive astrocytes | [174] | ||
| DJ-1 (PARK7) | EOPD/AR | Activation of astrocytes | [126], [127], [129], [196] |
| Suppression of inflammatory responses | [130] | ||
| LRRK2 (PARK8) | Classical PD/AD | High expression in microglia, monocytes and B cells | [105], [106], [108] |
| Activation of microglia | [109], [110], [201] | ||
| Association with IBD | [10], [113] | ||
| Association with leprosy | [11], [12] | ||
| Immune responses to pathogens | [107], [108] | ||
| PLA2G6 (PARK14) | EO dystonia-parkinsonism/AR | Synthesis of leukotrienes and prostaglandins | [198] |
| GBA1 | Risk factor | Activation of macrophages and cytokine production | [115], [116], [117], [118] |
| Predisposition to infections | [186], [179] | ||
| Thymic regulation | [121], [122] | ||
| Activation of microglia and astrocytes | [119], [120] |
Confirmed monogenic forms and risk variants for PD and their role in the immune system are illustrated. Evidence for a role in inflammatory responses in Parkinson’s disease patients or experimental models of dopaminergic neuron degeneration is in bold
AD, autosomal dominant; AR, autosomal recessive; EOPD, early onset Parkinson’s disease
Fig. 1.

The function of PD genes in the immune system. The main Parkinson’s disease-associated genes have a critical role in the innate and adaptive immune system. Such functions include a activation of glial cells (microglia and astrocytes), b regulation of the release of immunomodulatory molecules (cytokines, chemokines, ROS, NO, VIP), and c differentiation and activation of peripheral immune cells (hematopoietic precursors, macrophages, T cells, and B cells). Individuals carrying certain PD-linked genetic traits are therefore prone to develop aberrant inflammatory reactions which over time confer an higher susceptibility to neurodegeneration
Fig. 2.
Pathway analysis of the function of Parkinson’s disease genes in immune responses. Pathway analysis using ingenuity pathway analysis (IPA) shows direct and indirect associations between gene candidates. Using IPA software analysis, we have identified relevant biological and molecular immune functions linked to PD-genes, including regulation of leukocyte activation, Th1, and Th2 inflammatory responses. Bold lines indicate direct link, dotted lines indicate indirect link
The expression of many PD-genes is not restricted to neurons but also found at high levels in cells of the immune system. In this respect, LRRK2 is highly expressed in APCs including microglia, monocytes and B cells and is involved in the immune responses to pathogens [105–108]. Increasing evidence is accumulating that LRRK2 is involved in innate and adaptive immune responses. Aberrant activation of microglia and kidney inflammatory reactions have been observed in LRRK2-deficient mice [109–112]. LRRK2 negatively regulates the transcription factor NFAT and its deficiency in mice triggers hyperactive immune responses and greater susceptibility to inflammatory bowel disease [113]. These data support the hypothesis that LRRK2 mutations are linked to excessive and poorly controlled immune responses, which may cause neuronal dysfunction and increase neuronal vulnerability to degeneration. The association of LRRK2 with CD and bowel inflammation suggests that peripheral inflammation may be involved in the prodromal stages of the disease.
Heterozygous mutations in the GBA1 gene, which encodes acid β-glucosidase (glucocerebrosidase, GCase), represent the strongest genetic risk factor for PD [114]. The role of GBA mutations in the pathogenesis of PD and other synucleinopathies is not clear. Homozygous mutations in the GBA1 gene cause Gaucher’s disease (GD), the most common lysosomal storage disorder. GD and PD were initially associated because of the observation of Parkinsonism and LB pathology in patients with GD. GBA is highly expressed in cells of the mononuclear phagocyte lineage and GCase deficiency results in the accumulation of glucosylceramide (an intermediate of the sphingolipid pathway) in macrophages and subsequent release of several pro-inflammatory molecules [115]. The onset and progression of GD are in fact associated with a sustained systemic inflammatory reaction and increased levels of pro-inflammatory molecules [115–118]. In the neuronopathic forms of GD, neuronal loss is also accompanied by astrocytosis and microgliosis [119, 120]. Interestingly, GCase deficiency, even in the absence of large amounts of sphingolipid storage, which may mimic the condition observed in PD, triggers inflammatory reactions [116]. Increasing evidence suggests that GBA directly modulates immune cells other than macrophages, including T and B cells [121, 122].
It is not known whether PD patients carrying heterozygous GBA mutations also display such immune phenotype. Interestingly, non-motor symptoms, such as dementia and neuropsychiatric disturbances, are very common in GBA-PD patients [123]. Since inflammation has been associated with cognitive decline [124], further immunological investigations are needed both in PD patients and asymptomatic mutation carriers.
Given the crucial role of LRRK2 and GBA in autophagy and lysosomal function, it is likely that the dysfunction of these enzymes not only affects the degradation of misfolded proteins in neurons [125] but also the phagocytic properties of microglial cells with subsequent inflammatory reactions.
Astrocytes also play an important role in protein clearance in the SNC. Interestingly, many PD-genes are expressed at high levels in astrocytes. Among them, DJ-1 is expressed mainly by astrocytes in the normal human brain [126], and is strongly up-regulated in reactive astrocytes in PD brain, MS inflammatory lesions, and stroke [126–129]. DJ-1 plays a key role in the suppression of inflammatory responses in astrocytes by regulating NO and pro-inflammatory cytokines production [130]. In PD patients with DJ-1 mutations, a non-cell autonomous dysfunction of astrocytes and neuroinflammation can therefore be the driving force of neurodegeneration.
α-syn is found at high levels within astrocytes and α-syn-positive inclusions in astroglia have been described in PD and other α-synucleinopathies [68, 131–135]. TLRs likely mediate the clearance of α-syn by microglial cells and astrocytes [136–138]. The current theory is that α-syn released by neuronal terminals is internalized by adjacent astrocytes, where it forms aggregates [68]. Recent evidence confirmed the transfer of α-syn from neurons to astrocytes [67], and an additional study has shown that the accumulation of α-syn within astrocytes initiates DA neuronal death, suggesting a non–cell autonomous component in the pathogenesis of the disease [139]. Early α-syn accumulation in glial cells may therefore trigger inflammatory reactions and the release of pro-inflammatory molecules, which in turn damage vulnerable neurons [51, 66, 140–144]. Extracellular forms of α-syn and its modified forms can in fact activate glial cells promoting harmful inflammatory reactions including the release of inflammatory cytokines, NADPH oxidase and ROS production [145–151]. The pro-inflammatory properties of wild-type and mutant forms of α-syn have been further confirmed in several experimental models of PD [148, 152]. Importantly, neuroinflammation precedes neuronal death in these models [51].
Evidence for a role of genetic variation in the regulation of immune pathways also exists for other rare PD-linked genes (summarized in Table 2). Among these of particular interest is Nurr1 that has been linked to a rare form of familial PD, although such association has not been confirmed after the initial reports [154, 155]. Nurr1 is a transcription factor that regulates expression of the gene encoding tyrosine hydroxylase (TH) [156]. It belongs to the NR4A subfamily (NR4A1/NUR77, NR4A2/NURR1, and NR4A3/NOR1) of orphan NRs, which are key transcriptional regulators of cytokines and growth factors [157]. Nurr1 is also highly expressed in human lymphocytes and macrophages [158]. Interestingly, the rare Nurr1 variants potentially linked to PD affect the transcription of gene that encodes TH and also decrease Nurr1 mRNA level in lymphocytes [154, 159]. In experimental models, the reduction of Nurr1 expression results in exaggerated inflammatory responses in microglial cells that are further intensified by astrocytes, leading to the death of DA neurons [160]. In addition, Nurr1 has an indirect immunomodulatory effect mediated by the regulation of vasoactive intestinal protein [161], which promotes the release of glial-derived trophic factors and inhibits microglia activation [162].
Several transgenic animal models containing mutant or knock-out PD genes do not show overt neuroinflammation. It is unlikely that inflammation alone is the cause of neurodegeneration even in familial forms of PD linked to mutations in “immune genes”. However, it is tempting to speculate that such PD mutations and SNPs in immune genes confer an immune phenotype that contributes to the development of aberrant immune responses upon inflammatory triggers (e.g., ageing, infections, sterile inflammation). Past infections may in fact contribute to cognitive impairment and neurodegeneration [163]. Inflammation can initiate neuronal, axonal, and synaptic dysfunction in susceptible neuronal populations, such as the vulnerable DA neurons, and, over time, increase neuronal vulnerability to subsequent neurodegenerative insults.
It would therefore be interesting to profile the immune responses to selected stimuli (e.g., adjuvants, immune modulators, hormones, cytokines), and to correlate those with differences to the genetic background and clinical features of PD patients, both in manifesting and non-manifesting mutation carriers. The greater homogeneity of these populations would facilitate the identification of immune markers that could eventually be used as disease biomarkers.
Therapeutic considerations
The diagnosis of PD typically occurs when more than 60 % of nigrostriatal DA neurons have been damaged and the cardinal motor symptoms are clearly evident. Even though many DA treatments have been developed for PD, l-dihydroxyphenylalanine (l-dopa) still remains the gold standard. The long-term use of l-dopa is associated with the development of side effects such as motor and non-motor fluctuations and dyskinesia. In addition, the progression of the disease in several brain regions outside the SN and in the peripheral ANS dramatically contributes to the development and progression of motor symptoms, which are poorly responsive to l-dopa, and other debilitating non-motor complications (psychosis, cognitive impairment and dementia, depression, postural instability, autonomic dysfunction, and sleep disorders). Thus, there has been an increasing interest in identifying novel therapeutic targets for the development of disease-modifying drugs that may help prevent or ameliorate PD pathology. Inflammation has been considered an epiphenomenon of neurodegeneration contributing to the progression of many neurodegenerative disorders. As such, anti-inflammatory drugs have been tested late during the disease course and have failed to show neuroprotection [164, 165]. However, it is now clear that the inflammatory reactions are a dynamic process involving complex interactions between different immune cells and neuronal populations at prodromal stages of the disease.
Therefore, immunomodulatory interventions in combination with other neuroprotective agents can be beneficial in preventing PD onset and improving the current management of motor and non-motor parkinsonian symptoms that respond poorly to DA therapy. Immunomodulatory drugs for PD have not yet been rigorously corroborated in clinical trial studies. Minocycline, a semi-synthetic, second-generation tetracycline analog, has been tested in experimental models and PD patients. Minocycline effectively crosses the BBB and, besides its antibacterial properties, shows potent anti-inflammatory and neuroprotective effects [166]. Despite some contradictory results in preclinical studies [167], a double-blind, futility clinical trial in early PD warrants further consideration of minocycline for Phase III clinical trials [168]. Pioglitazone, a synthetic peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, which modulates microglia activation without compromising the host immunity, is currently under investigation in a Phase II placebo-controlled clinical trial for the treatment of early PD.
Activated inflammatory pathways in both sporadic and familial PD, such as the TLR-pathways, pro-inflammatory cytokines, and neuro-immune molecules, may represent opportunities for early therapeutic interventions [169]. In this regard, further studies are needed in order to design molecules that efficiently cross the BBB and do not interfere with the host immune system.
Anti-inflammatory drugs should be tested in the prodromal phase of the disease. However, the absence of disease biomarkers represents the major obstacle to such early therapeutic intervention. Interestingly, the cellular and molecular networks affecting PD disease susceptibility are also involved in mechanisms of immune regulation in both sporadic and inherited forms of the disease. It is therefore likely that individual genetic traits determine an early dysregulation of the immune system that, in combination with other triggers, initiates neuronal dysfunction and progressive neurodegeneration. The role of such inflammatory processes may be more pronounced in PD patients carrying specific genetic traits. With the advent of novel genetic screening, it would be feasible to identify selected individuals carrying PD-linked mutations or genetic susceptibility traits, which predispose to aberrant inflammatory responses (e.g., SNCA or LRRK2 mutations carriers, HLA haplotypes, lymphocyte signaling, cytokine release). Neuroprotective interventions targeting critical inflammatory pathways in preclinical/early stages of disease in these patients or mutation carriers may therefore be an effective strategy for preventing the onset or slowing the progression of PD pathology.
Conclusions
Although PD is a complex multifactorial disorder, increasing evidence supports the role of inflammation as an important driving force of disease pathology. In this review, we have summarized the evidences for inflammation in PD and highlighted how genetic variants may modulate such immune and inflammatory pathways in both sporadic and familial PD. Understanding the link between PD genetic variants and specific immune responses is therefore crucial to identify novel biomarkers of early stages of the disease, and to devise tailored neuroprotective interventions by targeting critical inflammatory pathways.
Acknowledgments
This work was supported by grants from the German Research Council and Marie Curie Career Integration Grant, and by a Research Grant from the Alexander Von Humboldt Foundation.
Conflict of interest
The authors declare no conflicts of interest.
Abbreviations
- AD
Alzheimer’s disease
- APC
Antigen-presenting cell
- α-syn
α-Synuclein
- CNS
Central nervous system
- COX
Cyclooxygenase
- CD
Crohn’s disease
- DA
Dopaminergic
- EOPD
Early onset Parkinson’s disease
- FBXO7
F-box protein 7
- GBA
Glucocerebrosidase
- GD
Gaucher’s disease
- GCase
Glucocerebrosidase
- GWAS
Genome wide association study
- HLA
Human leukocyte antigen
- IBD
Inflammatory bowel disease
- IL-1β
Interleukin-1beta
- LB
Lewy bodies
- LN
Lewy neurites
- LPS
Lipopolysaccharide
- LRRK2
Leucine-rich repeat kinase 2
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS
Multiple sclerosis
- NO
Nitric oxide
- NR
Nuclear receptor
- PD
Parkinson’s disease
- PLA2G6
Phospholipase A2 group VI
- PPAR
Peroxisome proliferator-activated receptor
- ROS
Reactive oxygen species
- SN
Substantia nigra
- TH
Tyrosine hydroxylase
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
- VIP
Vasoactive intestinal peptide
References
- 1.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5:75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- 2.Shults CW. Lewy bodies. Proc Natl Acad Sci USA. 2006;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 4.Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology. 1992;42:726–732. doi: 10.1212/wnl.42.4.726. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O. Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis. 2012;47:258–267. doi: 10.1016/j.nbd.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Gasser T, Hardy J, Mizuno Y. Milestones in PD genetics. Mov Disord. 2011;26:1042–1048. doi: 10.1002/mds.23637. [DOI] [PubMed] [Google Scholar]
- 9.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mira MT, Alcais A, Nguyen VT, Moraes MO, Di Flumeri C, et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature. 2004;427:636–640. doi: 10.1038/nature02326. [DOI] [PubMed] [Google Scholar]
- 12.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 13.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 15.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 16.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 17.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 18.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 19.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Depboylu C, Schafer MK, Arias-Carrion O, Oertel WH, Weihe E, et al. Possible involvement of complement factor C1q in the clearance of extracellular neuromelanin from the substantia nigra in Parkinson disease. J Neuropathol Exp Neurol. 2011;70:125–132. doi: 10.1097/NEN.0b013e31820805b9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, et al. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 26.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2012;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, et al. Variant of TREM2 associated with the Risk of Alzheimer’s disease. N Engl J Med. 2012;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- 29.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Investig. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Investig. 2012;122:1156–1163. doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 37.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 39.Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, et al. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- 40.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, et al. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, et al. Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis. 2005;20:360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, et al. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, et al. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deleidi M, Hallett PJ, Koprich JB, Chung CY, Isacson O. The Toll-like receptor-3 agonist polyinosinic:polycytidylic acid triggers nigrostriatal dopaminergic degeneration. J Neurosci. 2010;30:16091–16101. doi: 10.1523/JNEUROSCI.2400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, et al. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain J Neurol. 2012;135:3336–3347. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 51.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 53.Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience. 2000;95:425–432. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- 54.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 55.Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- 56.Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, et al. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 57.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, et al. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 58.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 59.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 60.Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P, et al. Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci Lett. 1995;193:129–132. doi: 10.1016/0304-3940(95)11686-q. [DOI] [PubMed] [Google Scholar]
- 61.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, et al. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile Parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 62.Mogi M, Kondo T, Mizuno Y, Nagatsu T. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett. 2007;414:94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Shimoji M, Pagan F, Healton EB, Mocchetti I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotox Res. 2009;16:318–328. doi: 10.1007/s12640-009-9076-3. [DOI] [PubMed] [Google Scholar]
- 64.Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 65.Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 66.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 67.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 69.Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, et al. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res. 2011;36:1452–1463. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- 70.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 71.Chen PS, Peng GS, Li G, Yang S, Wu X, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 72.Ishida Y, Nagai A, Kobayashi S, Kim SU. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J Neuropathol Exp Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- 73.Haussermann P, Kuhn W, Przuntek H, Muller T. Integrity of the blood-cerebrospinal fluid barrier in early Parkinson’s disease. Neurosci Lett. 2001;300:182–184. doi: 10.1016/s0304-3940(01)01574-9. [DOI] [PubMed] [Google Scholar]
- 74.Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 75.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 76.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, et al. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism Relat Disord. 2010;16:57–59. doi: 10.1016/j.parkreldis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Politis M, Su P, Piccini P. Imaging of microglia in patients with neurodegenerative disorders. Front Pharmacol. 2012;3:96. doi: 10.3389/fphar.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009381. doi: 10.1101/cshperspect.a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiszer U, Mix E, Fredrikson S, Kostulas V, Link H. Parkinson’s disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol Scand. 1994;90:160–166. doi: 10.1111/j.1600-0404.1994.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 81.Fiszer U, Mix E, Fredrikson S, Kostulas V, Olsson T, et al. Gamma delta+ T cells are increased in patients with Parkinson’s disease. J Neurol Sci. 1994;121:39–45. doi: 10.1016/0022-510x(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 82.Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, et al. Lymphocyte populations in Parkinson’s disease and in rat models of Parkinsonism. J Neuroimmunol. 2001;113:146–152. doi: 10.1016/s0165-5728(00)00422-7. [DOI] [PubMed] [Google Scholar]
- 83.Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, et al. Circulating interleukin-15 and RANTES chemokine in Parkinson’s disease. Acta Neurol Scand. 2007;116:374–379. doi: 10.1111/j.1600-0404.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 85.Papachroni KK, Ninkina N, Papapanagiotou A, Hadjigeorgiou GM, Xiromerisiou G, et al. Autoantibodies to alpha-synuclein in inherited Parkinson’s disease. J Neurochem. 2007;101:749–756. doi: 10.1111/j.1471-4159.2006.04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maetzler W, Berg D, Synofzik M, Brockmann K, Godau J, et al. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J Alzheimers Dis. 2011;26:171–179. doi: 10.3233/JAD-2011-110221. [DOI] [PubMed] [Google Scholar]
- 87.Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 88.Villaran RF, Espinosa-Oliva AM, Sarmiento M, De Pablos RM, Arguelles S, et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease. J Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 89.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 90.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 92.Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 93.Ton TG, Heckbert SR, Longstreth WT, Jr, Rossing MA, Kukull WA, et al. Nonsteroidal anti-inflammatory drugs and risk of Parkinson’s disease. Mov Disord. 2006;21:964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- 94.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- 95.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76:863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rees K, Stowe R, Patel S, Ives N, Breen K, et al. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies. Cochrane Database Syst Rev. 2011;11:CD008454. doi: 10.1002/14651858.CD008454.pub2. [DOI] [PubMed] [Google Scholar]
- 97.Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Srinivasan BS, Doostzadeh J, Absalan F, Mohandessi S, Jalili R, et al. Whole genome survey of coding SNPs reveals a reproducible pathway determinant of Parkinson disease. Hum Mutat. 2009;30:228–238. doi: 10.1002/humu.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, et al. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum Mol Genet. 2012;22:1039–1049. doi: 10.1093/hmg/dds492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- 102.Lin JJ, Chen CH, Yueh KC, Chang CY, Lin SZ. A CD14 monocyte receptor polymorphism and genetic susceptibility to Parkinson’s disease for females. Parkinsonism Relat Disord. 2006;12:9–13. doi: 10.1016/j.parkreldis.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 103.Bialecka M, Kurzawski M, Klodowska-Duda G, Opala G, Juzwiak S, et al. CARD15 variants in patients with sporadic Parkinson’s disease. Neurosci Res. 2007;57:473–476. doi: 10.1016/j.neures.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 104.Sharma M, Ioannidis JP, Aasly JO, Annesi G, Brice A, et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology. 2012;79:659–667. doi: 10.1212/WNL.0b013e318264e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miklossy J, Arai T, Guo JP, Klegeris A, Yu S, et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol. 2006;65:953–963. doi: 10.1097/01.jnen.0000235121.98052.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Melrose HL, Kent CB, Taylor JP, Dachsel JC, Hinkle KM, et al. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience. 2007;147:1047–1058. doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 107.Gardet A, Benita Y, Li C, Sands BE, Ballester I, et al. LRRK2 Is involved in the IFN-{gamma} response and host response to pathogens. J Immunol. 2010;185:5577–5585. doi: 10.4049/jimmunol.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, et al. Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm. 2011;118:795–808. doi: 10.1007/s00702-011-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim B, Yang MS, Choi D, Kim JH, Kim HS, et al. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS ONE. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 111.Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, et al. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci USA. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Z, Lee J, Krummey S, Lu W, Cai H, et al. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol. 2011;12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen MJ, Myer BJ, Khokher AM, Rushton N, Cox TM. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: increased release of interleukin-6 and interleukin-10. Q J Med. 1997;90:19–25. doi: 10.1093/qjmed/90.1.19. [DOI] [PubMed] [Google Scholar]
- 116.Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Investig. 2002;109:1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barak V, Acker M, Nisman B, Kalickman I, Abrahamov A, et al. Cytokines in Gaucher’s disease. Eur Cytokine Netw. 1999;10:205–210. [PubMed] [Google Scholar]
- 118.Hollak CE, Evers L, Aerts JM, van Oers MH. Elevated levels of M-CSF, sCD14 and IL8 in type 1 Gaucher disease. Blood Cells Mol Dis. 1997;23:201–212. doi: 10.1006/bcmd.1997.0137. [DOI] [PubMed] [Google Scholar]
- 119.Farfel-Becker T, Vitner EB, Pressey SN, Eilam R, Cooper JD, et al. Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum Mol Genet. 2011;20:1375–1386. doi: 10.1093/hmg/ddr019. [DOI] [PubMed] [Google Scholar]
- 120.Vitner EB, Farfel-Becker T, Eilam R, Biton I, Futerman AH. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease. Brain J Neurol. 2012;135:1724–1735. doi: 10.1093/brain/aws095. [DOI] [PubMed] [Google Scholar]
- 121.Mistry PK, Liu J, Yang M, Nottoli T, McGrath J, et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci USA. 2010;107:19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu J, Halene S, Yang M, Iqbal J, Yang R, et al. Gaucher disease gene GBA functions in immune regulation. Proc Natl Acad Sci USA. 2012;109:10018–10023. doi: 10.1073/pnas.1200941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77:276–280. doi: 10.1212/WNL.0b013e318225ab77. [DOI] [PubMed] [Google Scholar]
- 124.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain J Neurol. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 127.Neumann M, Muller V, Gorner K, Kretzschmar HA, Haass C, et al. Pathological properties of the Parkinson’s disease-associated protein DJ-1 in alpha-synucleinopathies and tauopathies: relevance for multiple system atrophy and Pick’s disease. Acta Neuropathol. 2004;107:489–496. doi: 10.1007/s00401-004-0834-2. [DOI] [PubMed] [Google Scholar]
- 128.van Horssen J, Drexhage JA, Flor T, Gerritsen W, van der Valk P, et al. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic Biol Med. 2010;49:1283–1289. doi: 10.1016/j.freeradbiomed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Mullett SJ, Hamilton RL, Hinkle DA. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology. 2009;29:125–131. doi: 10.1111/j.1440-1789.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 130.Waak J, Weber SS, Waldenmaier A, Gorner K, Alunni-Fabbroni M, et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- 131.Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. Alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 132.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- 133.Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain J Neurol. 2004;127:2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- 134.Togo T, Dickson DW. Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol. 2002;104:398–402. doi: 10.1007/s00401-002-0569-x. [DOI] [PubMed] [Google Scholar]
- 135.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- 136.Beraud D, Twomey M, Bloom B, Mittereder A, Ton V, et al. Alpha-synuclein alters toll-like receptor expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, et al. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, et al. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2012;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gu XL, Long CX, Sun L, Xie C, Lin X, et al. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, et al. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rabano A, et al. Alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet. 2012;21:3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- 143.Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Watson MB, Richter F, Lee SK, Gabby L, Wu J, et al. Regionally-specific microglial activation in young mice over-expressing human wild-type alpha-synuclein. Exp Neurol. 2012;237:318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, et al. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20:2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 146.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, et al. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 147.Reynolds AD, Kadiu I, Garg SK, Glanzer JG, Nordgren T, et al. Nitrated alpha-synuclein and microglial neuroregulatory activities. J Neuroimmune Pharmacology. 2008;3:59–74. doi: 10.1007/s11481-008-9100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, et al. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Fernandez-Montesinos R, Caro M, et al. Glial innate immunity generated by non-aggregated alpha-synuclein in mouse: differences between wild-type and Parkinson’s disease-linked mutants. PLoS ONE. 2010;5:e13481. doi: 10.1371/journal.pone.0013481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, et al. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 151.Zhang W, Wang T, Pei Z, Miller DS, Wu X, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 152.Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chung CY, Koprich JB, Hallett PJ, Isacson O. Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression. Proc Natl Acad Sci USA. 2009;106:22474–22479. doi: 10.1073/pnas.0912193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Le WD, Xu P, Jankovic J, Jiang H, Appel SH, et al. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33:85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 155.Zheng K, Heydari B, Simon DK. A common NURR1 polymorphism associated with Parkinson disease and diffuse Lewy body disease. Arch Neurol. 2003;60:722–725. doi: 10.1001/archneur.60.5.722. [DOI] [PubMed] [Google Scholar]
- 156.Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126:4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- 157.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 159.Mages HW, Rilke O, Bravo R, Senger G, Kroczek RA. NOT, a human immediate-early response gene closely related to the steroid/thyroid hormone receptor NAK1/TR3. Mol Endocrinol. 1994;8:1583–1591. doi: 10.1210/mend.8.11.7877627. [DOI] [PubMed] [Google Scholar]
- 160.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luo Y, Henricksen LA, Giuliano RE, Prifti L, Callahan LM, et al. VIP is a transcriptional target of Nurr1 in dopaminergic cells. Exp Neurol. 2007;203:221–232. doi: 10.1016/j.expneurol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 162.Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J. 2003;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- 163.Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, et al. Infectious burden and cognitive function: The Northern Manhattan study. Neurology. 2013;80:1209–1215. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 165.Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 166.Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Diguet E, Fernagut PO, Wei X, Du Y, Rouland R, et al. Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci. 2004;19:3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 168.NINDS NET-PT Investigators A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 169.Drouin-Ouellet J, Cicchetti F. Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol Sci. 2012;33:542–551. doi: 10.1016/j.tips.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 170.Akundi RS, Huang Z, Eason J, Pandya JD, Zhi L, Cass WA, Sullivan PG, Bueler, H (2011) Increased mitochondrial calcium sensitivity abnormal expression of innate immunity genes precede dopaminergic defects in Pink1–deficient mice. PLoS ONE 6:e16038 [DOI] [PMC free article] [PubMed]
- 171.Xu X, Li D, He Q, Gao J, Chen B, Xie A. Interleukin–18 promoter polymorphisms risk of Parkinson's disease in a Han Chinese population. Brain Res. 2011;1381:90–94. doi: 10.1016/j.brainres.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 172.Wu YR, Feng IH, Lyu RK, Chang KH, Lin YY, Chan H, Hu FJ, Lee–Chen GJ, Chen CM. Tumor necrosis factor–alpha promoter polymorphism is associated with the risk of Parkinson's disease. Am J Medical Genet B. 2007b;144B:300–304. doi: 10.1002/ajmg.b.30435. [DOI] [PubMed] [Google Scholar]
- 173.Wu YR, Chen CM, Hwang JC, et al. Interleukin–1 alpha polymorphism has influence on late–onset sporadic Parkinson's disease in Taiwan. J Neural Transm. 2007a;114:1173–1177. doi: 10.1007/s00702-007-0726-4. [DOI] [PubMed] [Google Scholar]
- 174.Wilhelmus MM, Pol SM, Jansen Q, Witte ME, Valk P, Rozemuller J, Drukarch B, Vries HE, Horssen J. Association of Parkinson disease–related protein PINK1 with Alzheimer disease multiple sclerosis brain lesions. Free Radic Biol Med. 2011;50:469–476. doi: 10.1016/j.freeradbiomed.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 175.Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee JK, Tansey MG (2011) Lipopolysaccharide tumor necrosis factor regulate Parkin expression via nuclear factor–kappa B. PLoS ONE 6:e23660 [DOI] [PMC free article] [PubMed]
- 176.Schulte T, Schols L, Muller T, Woitalla D, Berger K, Kruger R. Polymorphisms in the interleukin–1 alpha beta genes the risk for Parkinson's disease. Neurosci Lett. 2002;326:70–72. doi: 10.1016/s0304-3940(02)00301-4. [DOI] [PubMed] [Google Scholar]
- 177.Saiki M, Baker A, Williams–Gray CH, et al. Association of the human leucocyte antigen region with susceptibility to Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:890–891. doi: 10.1136/jnnp.2008.162883. [DOI] [PubMed] [Google Scholar]
- 178.Ross OA, O'Neill C, Rea IM, Lynch T, Gosal D, Wallace A, Curran MD, Middleton D, Gibson JM. Functional promoter region polymorphism of the proinflammatory chemokine IL–8 gene associates with Parkinson's disease in the Irish. Hum Immunol. 2004;65:340–346. doi: 10.1016/j.humimm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 179.Rosenbloom BE, Weinreb NJ, Zimran A, Kacena KA, Charrow J, Ward E. Gaucher disease cancer incidence: a study from the Gaucher Registry. Blood. 2005;105:4569–4572. doi: 10.1182/blood-2004-12-4672. [DOI] [PubMed] [Google Scholar]
- 180.Puschmann A, Verbeeck C, Heckman MG, et al. Human leukocyte antigen variation Parkinson's disease. Parkinsonism Related Disord. 2011;17:376–378. doi: 10.1016/j.parkreldis.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kuno S. Influence of interleukin–1beta gene polymorphisms on age–at–onset of sporadic Parkinson's disease. Neurosci Lett. 2000;284:73–76. doi: 10.1016/s0304-3940(00)00991-5. [DOI] [PubMed] [Google Scholar]
- 183.Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, Kuno S. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson's disease. Neurosci Lett. 2001;311:1–4. doi: 10.1016/s0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- 184.McGeer PL, Yasojima K, McGeer EG. Association of interleukin–1 beta polymorphisms with idiopathic Parkinson's disease. Neurosci Lett. 2002;326:67–69. doi: 10.1016/s0304-3940(02)00300-2. [DOI] [PubMed] [Google Scholar]
- 185.Mattila KM, Rinne JO, Lehtimaki T, Roytta M, Ahonen JP, Hurme M. Association of an interleukin 1B gene polymorphism (–511) with Parkinson's disease in Finnish patients. J Med Genet. 2002;39:400–402. doi: 10.1136/jmg.39.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marodi L, Kaposzta R, Toth J, Laszlo A. Impaired microbicidal capacity of mononuclear phagocytes from patients with type I Gaucher disease: partial correction by enzyme replacement therapy. Blood. 1995;86:4645–4649. [PubMed] [Google Scholar]
- 187.Lill CM, Roehr JT, McQueen MB, et al. Comprehensive research synopsis systematic meta–analyses in Parkinson's disease genetics: The PDGene database. PLoS Genet. 2012;8:e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ledesma MD, Galvan C, Hellias B, Dotti C, Jensen PH. Astrocytic but not neuronal increased expression redistribution of parkin during unfolded protein stress. J Neurochem. 2002;83:1431–1440. doi: 10.1046/j.1471-4159.2002.01253.x. [DOI] [PubMed] [Google Scholar]
- 189.Lampe JB, Gossrau G, Herting B, Kempe A, Sommer U, Fussel M, Weber M, Koch R, Reichmann H. HLA typing Parkinson's disease. Eur Neurol. 2003;50:64–68. doi: 10.1159/000072500. [DOI] [PubMed] [Google Scholar]
- 190.Kruger R, Hardt C, Tschentscher F, et al. Genetic analysis of immunomodulating factors in sporadic Parkinson's disease. J Neural Transm. 2000;107:553–562. doi: 10.1007/s007020070078. [DOI] [PubMed] [Google Scholar]
- 191.Hakansson A, Westberg L, Nilsson S, et al. Investigation of genes coding for inflammatory components in Parkinson's disease. Mov Disord. 2005b;20:569–573. doi: 10.1002/mds.20378. [DOI] [PubMed] [Google Scholar]
- 192.Hakansson A, Westberg L, Nilsson S, et al. Interaction of polymorphisms in the genes encoding interleukin–6 estrogen receptor beta on the susceptibility to Parkinson's disease. Am J Med Genet B. 2005a;133B:88–92. doi: 10.1002/ajmg.b.30136. [DOI] [PubMed] [Google Scholar]
- 193.Guo Y, Deng X, Zheng W, et al. HLA rs3129882 variant in Chinese Han patients with late–onset sporadic Parkinson disease. Neurosci Lett. 2011;501:185–187. doi: 10.1016/j.neulet.2011.05.245. [DOI] [PubMed] [Google Scholar]
- 194.Greene JC, Whitworth AJ, Andrews LA, Parker TJ, Pallanck LJ. Genetic genomic studies of Drosophila parkin mutants implicate oxidative stress innate immune responses in pathogenesis. Human Mol Genet. 2005;14:799–811. doi: 10.1093/hmg/ddi074. [DOI] [PubMed] [Google Scholar]
- 195.Frank-Cannon TC, Tran T, Ruhn KA. Parkin deficiency increases vulnerability to inflammation–related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ–1, a cancer– Parkinson's disease–associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bialecka M, Klodowska–Duda G, Kurzawski M, Slawek J, Gorzkowska A, Opala G, Bialecki P, Sagan L, Drozdzik M. Interleukin–10 (IL10) tumor necrosis factor alpha (TNF) gene polymorphisms in Parkinson's disease patients. Parkinsonism Related Disord. 2008;14:636–640. doi: 10.1016/j.parkreldis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 198.Balsinde J, Balboa MA. Cellular regulation proposed biological functions of group VIA calcium–independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 199.Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha–synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou YT, Yang JF, Zhang YL, Wang XY, Chan P. Protective role of interlekin-1 alpha gene polymorphism in Chinese Han population with sporadic Parkinson's disease. Neurosci Lett. 2008;445:23–25. doi: 10.1016/j.neulet.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 201.Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]