Abstract
Double-strand breaks (DSBs) are the most detrimental form of DNA damage. Failure to repair these cytotoxic lesions can result in genome rearrangements conducive to the development of many diseases, including cancer. The DNA damage response (DDR) ensures the rapid detection and repair of DSBs in order to maintain genome integrity. Central to the DDR are the DNA damage checkpoints. When activated by DNA damage, these sophisticated surveillance mechanisms induce transient cell cycle arrests, allowing sufficient time for DNA repair. Since the term “checkpoint” was coined over 20 years ago, our understanding of the molecular mechanisms governing the DNA damage checkpoint has advanced significantly. These pathways are highly conserved from yeast to humans. Thus, significant findings in yeast may be extrapolated to vertebrates, greatly facilitating the molecular dissection of these complex regulatory networks. This review focuses on the cellular response to DSBs in Saccharomyces cerevisiae, providing a comprehensive overview of how these signalling pathways function to orchestrate the cellular response to DNA damage and preserve genome stability in eukaryotic cells.
Keywords: Cancer, Checkpoint, DNA damage, Double-strand break, Yeast, Genome instability
Introduction
The genomic integrity of an organism is constantly being challenged by DNA insults arising from exogenous sources, including ionising radiation (IR), ultraviolet (UV) radiation and carcinogenic agents, as well as endogenous stresses resulting from DNA replication errors and by-products of cellular metabolism, such as reactive oxygen species [1]. The capacity to deal effectively with spontaneous- or environmentally-induced DNA damage is crucial for cellular survival and the maintenance of genomic stability, as inaccuracies in these processes can lead to chromosomal aberrations and cancer [2].
Consequently, cells have evolved sophisticated surveillance mechanisms termed DNA damage checkpoints [3, 4] that monitor the successful completion of cell cycle events and initiate a coordinated cellular response when DNA damage is detected [5–7]. Activation of the DNA damage checkpoint results in cell cycle arrest, activation of transcriptional programmes, initiation of DNA repair or, if the damage is too severe, cellular senescence or programmed cell death [8]. These mechanisms act in concert to preserve genomic integrity and thus the fidelity of cell propagation. Once repair is completed, the DNA damage checkpoint response is down-regulated and cells re-enter the cell cycle in a process known as recovery. Alternatively, if the lesion is irreparable, cells may undergo adaptation and eventually re-enter the cell cycle in the continued presence of DNA damage [9].
DNA double-strand breaks (DSBs) constitute one of the most cytotoxic forms of DNA damage and pose a significant threat to cell viability, survival and homeostasis. DSBs have the potential to promote gross chromosomal rearrangements (GCRs) and potentially deleterious mutations that promote tumourigenesis [10]. Indeed, defects in many proteins with known roles in the DNA damage response (DDR) have been implicated not only in cancer development but also in neurodegenerative disorders, developmental defects, immune deficiency disorders and sterility [11]. Therefore, understanding the molecular mechanisms governing the cellular response to DSBs will not only serve to further our knowledge of the complex biology underpinning these intricate signalling networks but it may also identify exciting avenues for the development of novel and targeted mechanism-based therapeutics with the potential to significantly impact human health. This review focuses on DNA damage checkpoint activation in response to DSBs with particular focus on the budding yeast Saccharomyces cerevisiae. We describe the currently proposed models for how these lesions are detected and discuss the intricate signal transduction mechanisms underlying the DDR. These DNA damage checkpoints are evolutionarily conserved, and therefore reported similarities between S. cerevisiae and vertebrates will also be discussed in this context.
Overview of the DNA damage response in S. cerevisiae
The unicellular eukaryotic budding yeast, S. cerevisiae, is a well-characterised and versatile model organism and has been instrumental in initial discoveries related to cell cycle control and the DDR [4, 12–14]. The DDR in S. cerevisiae relies on several classes of checkpoint and repair genes which are evolutionarily conserved in vertebrates [15]. The principal proteins in S. cerevisiae involved in the early steps of DSB checkpoint activation are listed in Table 1 along with their Schizosaccharomyces pombe and human homologues.
Table 1.
Homologues of the central components of the DNA damage checkpoint in eukaryotes. DNA damage checkpoint proteins and protein complexes involved in the early steps of double-strand break DNA damage checkpoint activation in S. cerevisiae, and their structural or functional homologues in S. pombe and H. sapiens
| Class of protein | S. cerevisiae | Proposed function(s) | S. pombe | H. sapiens |
|---|---|---|---|---|
| Sensors | Mec1-Ddc2 | Principal PIKK complex involved in sensing DNA damage and transducing the checkpoint signal. Mec1 binds Ddc2, which mediates its recruitment to sites of damage | Rad3-Rad26 | ATR-ATRIP |
| Tel1 | PIKK primarily involved in telomere maintenance. Partial overlap with Mec1 in the DNA damage response. | Tel1 | ATM | |
| Mre11-Rad50-Xrs2 | Initial sensing and signalling of DSBs. Involved in DSB repair via HR and NHEJ. | Rad32-Rad50-Nbs1 | MRE11-RAD50-NBS1 | |
| Rad24-Rfc2-5(RFC-like complex) | Loading of Ddc1-Rad17-Mec3 (9-1-1) complex onto DNA. Role in DNA damage signalling. | Rad17-Rfc2-5 | RAD17-RFC2-5 | |
| Ddc1-Rad17-Mec3(PCNA-like complex) | Heterotrimeric complex, structurally related to PCNA, also called 9-1-1. DNA damage signal transduction and recruitment of other checkpoint proteins. | Rad9-Rad1-Hus1 | RAD9-RAD1-HUS1 | |
| Dpb11 | Replication initiation protein and checkpoint sensor recruited to sites of damage by 9-1-1 complex, where it activates Mec1 | Cut5 | TopBP1 | |
| DSB processing | Sae2 | Endonuclease that functions with MRX complex in the first step of DNA DSB resection. | Ctp1 | CtIP |
| Exo1 | 5′–3′ exonuclease involved in recombination and DNA repair. | Exo1 | EXO1 | |
| Sgs1 | RecQ-like helicase that functions with Dna2 to process DSBs. | Rqh1 | BLM | |
| Dna2 | 5′ flap endonuclease/helicase that functions with Sgs1 to resect DSBs. | Dna2 | DNA2 | |
| Adaptors/mediators | Rad9 | Molecular adaptor/mediator required for DNA damage signal transduction and DSB repair. Required for Rad53 and Chk1 activation. | Crb2 | 53BP1; BRCA1; MDC1 |
| Mrc1 | Molecular adaptor required for S phase checkpoint activation. Required for Rad53 activation in response to replication stress. | Mrc1 | Claspin | |
| Effectors | Rad53 | Effector kinase in replication and DNA damage checkpoints. | Cds1 | CHK2 |
| Chk1 | Effector kinase involved in signal transduction. | Chk1 | CHK1 |
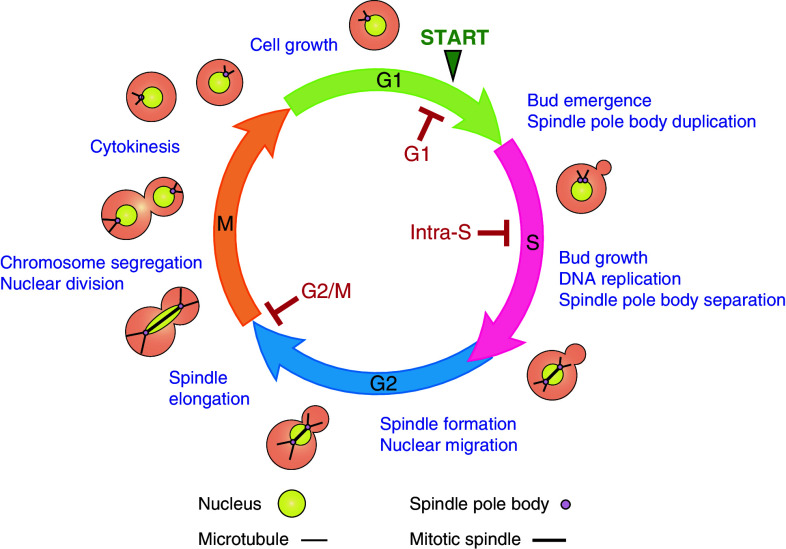
Faithful replication and propagation of genetic information is paramount for cell viability and genomic stability. Hence, cells have evolved a complex regulatory network termed the cell cycle control system which coordinates the order and timing of cell cycle events [16]. The S. cerevisiae mitotic cell cycle (Fig. 1), like all eukaryotes, consists of four phases [17]. The first phase, Gap phase 1 (G1), is the main phase of growth and activation of transcriptional pathways required for the subsequent S phase [18]. In late G1, once cells have reached the critical size and sufficient nutrients are available, they pass through START (termed the restriction point in vertebrates) and enter S phase where the genome is replicated. Following S phase, cells enter Gap phase 2 (G2) and prepare for entry into mitosis (M), the phase in which the duplicated chromosomes are segregated and the cell divides in two. The major processes of the cell cycle occur in S phase where chromosomes must be accurately duplicated and in M phase where they must be properly segregated. It is vital that cells exit mitosis and proceed through cytokinesis only after chromosome segregation is complete. Thus, there are two main transition points in the cell cycle: one at the G1/S transition and the other at the G2/M phase boundaries [16]. It is important to note that, unlike S. pombe and vertebrates, the G2/M transition is not well defined in S. cerevisiae and events traditionally considered as mitotic, such as spindle pole body duplication and mitotic spindle formation, are initiated during S phase in order to facilitate bud formation and nuclear migration [19, 20]. Consequently, it is the metaphase to anaphase transition rather than the G2 to M transition that is regulated by the G2/M DNA damage checkpoint in S. cerevisiae [21]. The key regulators of the cell cycle are a family of serine/threonine kinases known as cyclin-dependent kinases (CDKs) which phosphorylate numerous substrates [22, 23] that play pivotal roles in major cell cycle events, including DNA replication and mitosis [24]. In S. cerevisiae, all cell cycle events are controlled by a single essential CDK known as Cdk1/Cdc28 [14, 25, 26].
Fig. 1.
Saccharomyces cerevisiae cell cycle and the DNA damage checkpoints. The budding yeast cell cycle, illustrating the specific cellular morphologies at each cell cycle stage. Also depicted are the three DNA damage checkpoints; the G1 checkpoint which arrests cells prior to START, the intra-S phase checkpoint which slows the rate of DNA replication and the G2/M checkpoint which arrests cells at the metaphase/anaphase transition. Once damage is repaired, the checkpoint is down-regulated and cells resume cycling
In S. cerevisiae, DNA damage checkpoints operate at three distinct stages in the cell cycle (Fig. 1). The G1 checkpoint arrests cells at the G1/S transition prior to START [27–29] before cells irreversibly commit to the next cell cycle. This transient arrest delays bud emergence, spindle pole body duplication and S phase entry, allowing time for DNA lesions to be repaired before the onset of DNA replication [27, 28, 30]. However, certain DNA aberrations such as alkylated DNA do not activate the G1 checkpoint and instead cells pass through START. Essentially, these lesions elicit a checkpoint response during S phase since they need to be converted to secondary lesions during DNA replication before being recognised by the checkpoint machinery [31]. The intra-S phase checkpoint slows the rate of replication in response to DNA damage [32], coordinating fork repair mechanisms and cell cycle progression to ensure the fidelity and completion of replication before cells enter mitosis. As discussed above, the G2/M checkpoint arrests cells at the metaphase to anaphase transition, preventing cells from progressing through mitosis in the presence of DNA damage [4].
If DNA damage can be mended efficiently, the lesion is rapidly repaired without induction of cell cycle arrest [33]. However, when the damage cannot be repaired quickly or persists, the DNA damage checkpoint is activated [34]. The apical protein kinases in this evolutionarily conserved signal transduction cascade are members of a family of phosphoinositide 3-kinase-related kinases (PIKKs) which include S. cerevisiae Tel1 and Mec1, S. pombe Tel1 and Rad3, and mammalian ATM (ataxia telangiectasia mutated), ATR (ATM and Rad3-related), and DNA-PKcs (DNA-dependent protein kinase catalytic subunit) [6, 35]. A homologue of DNA-PKcs has not been identified in S. cerevisiae or S. pombe [36]. In S. cerevisiae, Mec1 is often considered to be the principal PIKK since mec1 mutants are severely sensitive to DNA damage [34] and Tel1 function is mostly apparent when several DSBs are generated [80]. In reality, both Tel1 and Mec1 have important roles in DSB signalling, mostly similar to their vertebrate homologues: ATM (scTel1) functions in response to DSBs [37], while ATR (scMec1) is activated via its recruitment to replication protein A (RPA)-coated ssDNA [38, 39] which occurs at stalled replication forks and also at DSBs which have undergone resection, a process which is dependent upon prior activation of ATM [40–42]. These PIKKs promote the activation of downstream effector kinases (scChk1 and Rad53; spChk1 and Cds1; human CHK1 and CHK2), which function to target downstream components of the DDR as well as amplifying the initial DDR signal [43]. In S. cerevisiae, Mec1 activates both Rad53 and Chk1 [44] while in vertebrate cells ATM primarily activates CHK2 (scRad53) and ATR activates CHK1 (scChk1) [43]. PIKK-dependent activation of the effector kinases is regulated by mediator proteins, which include the prototypical mediator protein scRad9 (equivalent to spCrb2; human 53BP1, BRCA1, MDC1) that function as molecular adaptors to recruit proteins to sites of damage and mediate the PIKK-dependent phosphorylation of downstream substrates [8]. Activation of downstream targets is often mediated by phosphorylation events which modulate the transcription level of repair genes and regulate cell cycle transitions by influencing the stability and/or localisation of proteins involved in cell cycle progression or checkpoint maintenance [45]. A major surprise in recent years has been the discovery that protein kinases other than the classical PIKK and CHK kinases also regulate DDR protein function [46]. Additionally, it is becoming clearer that other post-translational modifications (PTMs) are important for DNA damage checkpoint regulation, particularly those linked to chromatin modulation [47]. Mapping the complex biochemical interplay between these post-translational regulatory mechanisms represents a challenge of significant importance in order to gain a more comprehensive understanding of the DDR. Here, we present the known and most current mechanisms postulated to regulate the DDR.
Mechanisms of DNA damage checkpoint activation in response to double-strand breaks
Of the diverse types of DNA lesion, DSBs are the most cytotoxic form of DNA damage and pose a serious threat to cell viability. In response to even a single DSB, cells must trigger a series of events to promote repair of the lesion in order to survive and restore chromosomal integrity [48]. Ionising radiation, chemical agents, oxidative stress, and collapsed replication forks are thought to all lead to the formation of DSBs. Moreover, DSBs are required for normal cellular processes such as chromosome segregation during meiosis, V(D)J recombination in immunoglobulin genes, which occurs specifically in vertebrates, and mating-type switching in yeast [11, 49]. Much work has elucidated the cellular response to DSBs and identified the regulated recruitment and activation of the checkpoint machinery at sites of damage [15].
Sensing DNA double-strand breaks
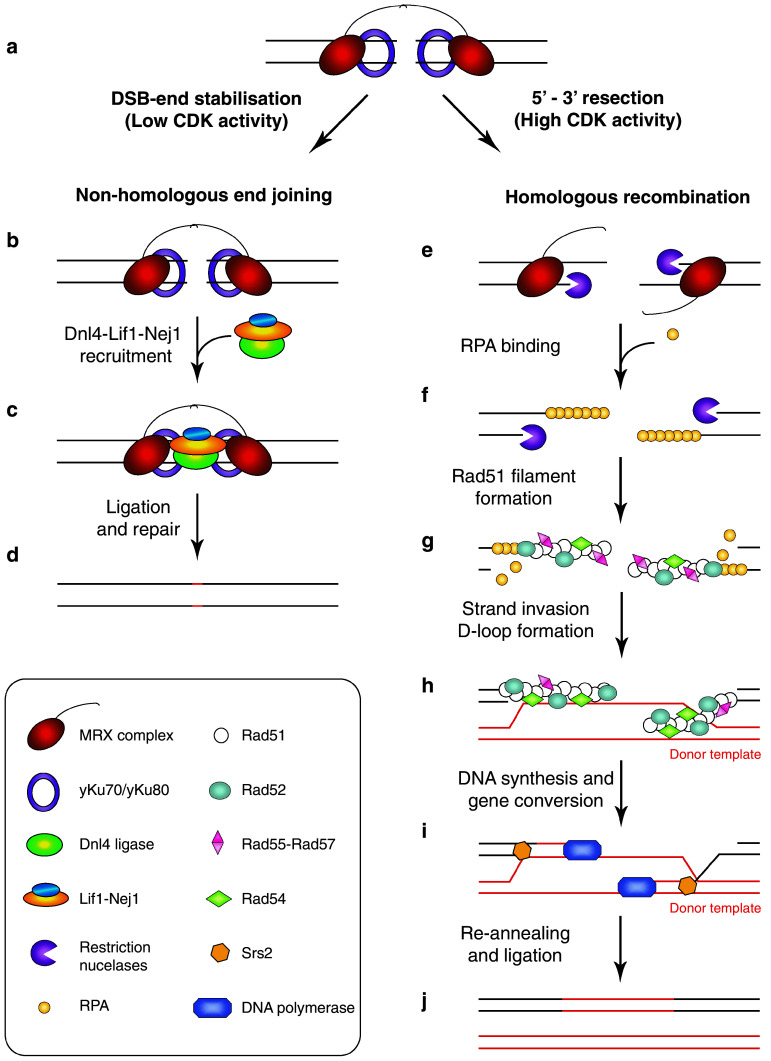
A first step in the activation of the DNA damage checkpoint is sensing of the damaged DNA. Initially thought to be the role of checkpoint proteins, the DSB repair proteins have been found to be important sensors of DNA damage. In S. cerevisiae, DSBs are specifically and independently recognised by both the Ku (yKu70/yKu80) and MRX (Mre11-Rad50-Xrs2) repair complexes (Fig. 2a), which compete for binding to unprocessed DSB ends [50–55]. The Ku complex, normally localised to telomeres, relocates to the site of the DSB and wraps around the dsDNA ends in a sequence-independent manner holding them together to ensure they are properly aligned for rejoining [51, 56, 57]. In vertebrates, the Ku heterodimer recruits DNA-PKcs, forming the DNA-PK holoenzyme, which functions as a DNA end-bridging factor [58–60]. Together with the MRN (MRE11-RAD50-NBS1) complex, they function in tethering the DNA ends together [61]. Although DNA-PKcs is not conserved in S. cerevisiae [62], the MRX complex seems to carry out this function alone as it has robust DNA end-bridging activity [63] and facilitates the bridging of DSB ends via the zinc-hook motifs located in the coiled-coil region of the Rad50 subunit, similar to MRN in vertebrate cells [64–66]. Non-homologous end joining (NHEJ) is the primary DSB repair pathway in G1 phase. In G1, processive resection is significantly reduced due to low CDK activity (Fig. 2b–d) and inhibition by the NHEJ machinery; binding of the Ku complex to DSB ends delays the onset of resection and provides a window of opportunity for DSB repair via NHEJ [53, 67–70]. Conversely, DSB repair via homologous recombination (HR) is restricted to S/G2 phases when CDK activity is high (Fig. 2e–j) and a suitable repair template for HR is available (i.e. the sister chromatid, which is used preferentially as a recombination template over a homolog in G2 diploid cells) [69–72]. Detailed molecular mechanisms for both NHEJ and HR have been recently described in the literature [73, 74].
Fig. 2.
Model of DSB repair by NHEJ and HR pathways in S. cerevisiae. Following DSB creation, the DNA ends are tethered by the MRX and Ku complexes (a). During G1 phase, when CDK activity is low, DSBs are primarily repaired via NHEJ. In NHEJ, DSB ends are further stabilised by MRX and yKu70/yKu80 (b). The Dnl4-Lif1-Nej1 ligase complex is then recruited (c), the broken DNA ends are ligated, and the lesion repaired (d). In S/G2 phases, when CDK activity is high, DSBs are repaired by HR. Firstly, DNA-end resection is initiated by Sae2 and the MRX complex. This is followed by a more processive resection catalysed by Exo1 and/or Dna2 in collaboration with the Sgs1 helicase (e). RPA binds to ssDNA (f). RPA-coated ssDNA is a substrate for Rad51 filament formation, involving Rad52, Rad55-Rad57 and Rad54 (g). Rad51 filament homology search and strand invasion lead to the formation of a D-loop (h). This is followed by disassembly of the Rad51 filament mediated by the Srs2 helicase, and subsequent DNA synthesis (i). Resolution of the Holliday junction is followed by re-annealing and ligation of the newly synthesised DNA (j)
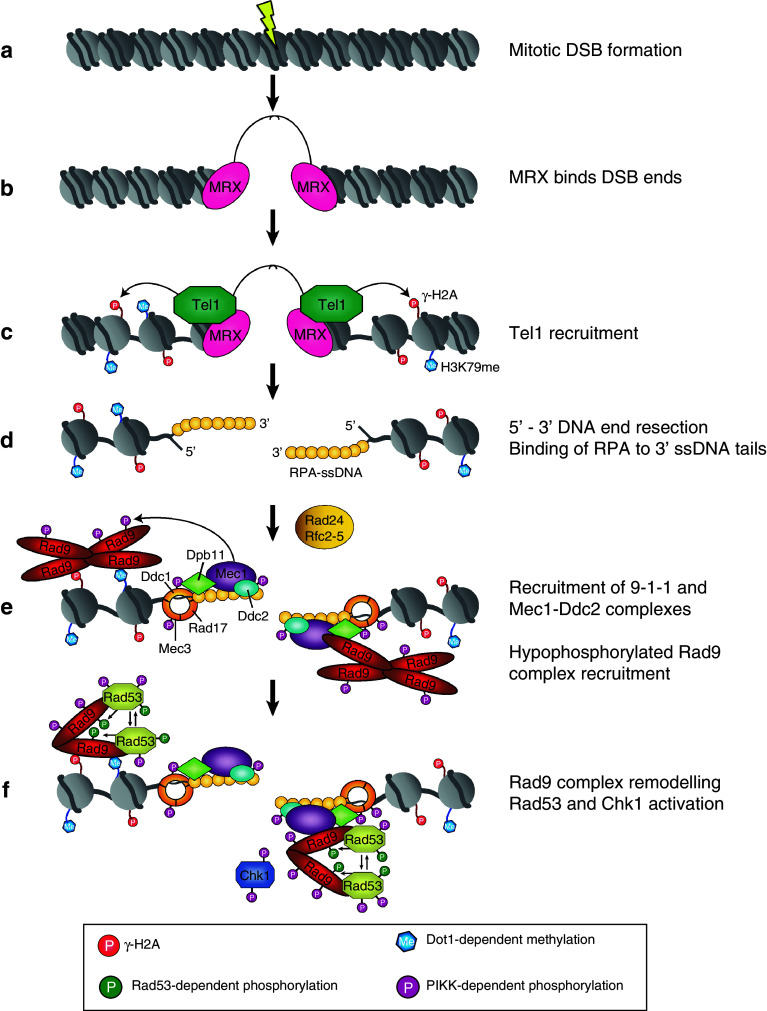
In S. cerevisiae, the MRX complex is the principal sensor required for DSB-induced checkpoint activation [75]. Following DSB formation (Fig. 3a), binding of MRX to DNA ends (Fig. 3b) promotes the recruitment of Tel1 to the DSB (Fig. 3c) via a direct interaction between Tel1 and the C-terminus of Xrs2 [76], leading to Tel1-dependent checkpoint activation prior to DNA end processing [77–79]. In contrast to Mec1, checkpoint-dependent activation of Tel1 has not been extensively studied. One reason for this being that it was long considered to be redundant with Mec1; a tel1Δ mutant is checkpoint proficient and does not exhibit increased sensitivity to genotoxic agents, while additional deletion of TEL1 enhances the sensitivity of mec1 mutants, suggesting some degree of functional redundancy between these genes [80–83]. The apparent minor role of Tel1 in the DDR may be somewhat explained by the capability of yeast to efficiently activate DSB signalling and rapidly convert DSB ends into substrates (i.e. ss-DNA) that preferentially stimulate Mec1 kinase activity. Further insight into Tel1 activation was recently gained from a study showing that Tel1 kinase activity is stimulated by binding of the MRX complex to DNA–protein complexes at DNA ends [84]. This study provides several interesting avenues for future investigation of the exact DNA-end structures that stimulate MRX-dependent Tel1 activation and how MRX specifically modulates Tel1 catalytic activity. Tel1 activity is required for the DNA damage-induced phosphorylation of Xrs2, Mre11 and Sae2, promoting their functions in DNA repair and checkpoint activation [77, 78, 85, 86]. Like its S. cerevisiae homologue, vertebrate ATM (scTel1) also recognises unprocessed DSBs. Unlike Tel1, ATM exists as an inactive dimer that undergoes DNA damage-induced in trans autophosphorylation to form partially active monomers [87]. ATM monomers are recruited to DSBs through an interaction with the NBS1 subunit of the MRN complex and, once recruited, are also activated by MRN bound to DNA [88–90] as well as early processing events induced by MRN [91, 92]. It is proposed that full activation of ATM also requires prior acetylation of ATM by the acetyltransferase Tip60 [93, 94]. Although these activities all contribute to efficient ATM activation, the exact molecular mechanisms of Tel1/ATM activation remain to be elucidated.
Fig. 3.
DSB-induced checkpoint activation in S. cerevisiae. Following DSB formation (a), the DDR signalling cascade is initiated by binding of MRX to DSB ends (b). MRX recruits Tel1, which phosphorylates histone H2A (c). After extensive resection, the ssDNA is rapidly coated with RPA (d). This structure promotes the independent recruitment of the Ddc1-Rad17-Mec3 complex, which is loaded onto DNA by the Rad24-Rfc2-5 complex, and Mec1-Ddc2 (e). Dpb11 is recruited by binding to phosphorylated Ddc1. Subsequently, the hypophosphorylated Rad9 complex is recruited by either binding to γ-H2A and methylated H3K79 or through its association with Dpb11. PIKK-dependent phosphorylation promotes Rad9 complex remodelling. Hyperphosphorylated Rad9 facilitates the PIKK-dependent phosphorylation of Rad53 and Chk1 (f). Activated Rad53 and Chk1 phosphorylate downstream effectors, resulting in activation of the major biological responses to DNA damage, including cell cycle arrest, DNA damage-induced transcription, DNA repair and slowing of DNA replication
Chromatin modulation and the DNA damage response
The phosphorylation of histone H2A on S129 (γ-H2A) by Tel1 is an early event in the activation of the DDR [95–99]. The discovery of this Tel1/Mec1-dependent modification was an important landmark in the DNA damage checkpoint field as it revealed the importance of chromatin context for DNA damage detection and the related downstream DDR processes. Since this discovery, numerous studies have highlighted the role of chromatin manipulation via covalent histone modifications, ATP-dependent chromatin remodelling and the incorporation of histone variants into nucleosomes to induce an efficient DDR [100, 101]. These mechanisms, more complex in vertebrate cells than in yeast due to a higher order of chromatin organisation, are still poorly understood. This field is under extensive investigation and has been recently reviewed elsewhere [102, 103].
DSB-induced γ-H2A spreads over an approximate 50-kb domain of chromatin surrounding the break [96, 99]. This mark is conserved in mammalian cells where ATM-dependent phosphorylation occurs on S139 of the H2A variant, H2AX (γ-H2AX) [104], spreading over an approximate 2-Mb domain of chromatin surrounding the break [105]. In S. cerevisiae, h2a-S129 mutants exhibit significant sensitivity to DSB-inducing agents as well as impaired DSB repair [96, 106, 107]. Furthermore, h2a-S129 mutants are defective in G1 checkpoint activation in response to DSBs due to impaired recruitment of the Rad9 checkpoint protein to sites of damage, which binds γ-H2A via its tandem BRCT domain [108, 109]. Mammalian cells that are deficient in H2AX are radiosensitive and display elevated genomic instability and defects in sister chromatid recombination [110–113]. In addition, H2AX −/− mouse embryonic fibroblasts are defective in G2-M checkpoint activation following low doses of IR [114].
Several studies have demonstrated that γ-H2A(X) promotes efficient DSB repair by regulating chromatin modulation in response to DNA damage through the recruitment of cohesin, histone modifiers and chromatin remodelling complexes in both yeast and vertebrates [102, 115]. It also facilitates the accumulation and stable retention of checkpoint and repair proteins at the site of damage [116]. This latter function of γ-H2A(X) is key for the activation of a robust checkpoint response as suggested by the fact that co-localisation of checkpoint and repair proteins on either yeast or vertebrate chromatin is sufficient to activate a strong checkpoint response in the absence of DNA damage [117, 118].
Although γ-H2AX is dispensable for the initial recruitment of NBS1 and the putative scRad9 orthologues BRCA1 and 53BP1 to DSBs in mammalian cells [119], their accumulation and stable retention is dependent upon the interaction between MDC1 (another scRad9 orthologue) and γ-H2AX, which promotes further recruitment of ATM to the vicinity of the break leading to the spread of γ-H2AX along chromatin [120, 121]. The localisation of DNA damage mediator proteins of the Rad9 family at sites of damage has been extensively studied in vertebrate cells and has revealed an unexpected importance of ubiquitination in mediating the formation of a complex scaffold of DDR proteins, which is sufficient to trigger the checkpoint cascade [122]. Several ubiquitin ligases have been identified at foci in mammalian cells including RNF8, RNF168, BRCA1, RAD18 and HERC2 [123–130]. Identifying substrates for these ligases, in particular the RNF8/RNF168/BRCA1 pathway, and elucidating how these processes are regulated is an important area for future investigation. In S. cerevisiae, extensive ubiquitination events at sites of DSBs have not been observed and Rad9 accumulation at sites of DSBs seems to be the functional equivalent to the complex focal accumulation of vertebrate DDR proteins.
DNA double-strand break resection
DSB resection is a ssDNA generating process necessary to switch from Tel1/ATM to Mec1/ATR signalling as well as for homologous recombination mechanisms. A significant advancement in our understanding of the molecular mechanisms leading to DSB resection has recently taken place. It is now known that DNA end resection occurs via a two-step mechanism [131, 132]. The first step is initiated by the MRX complex and Sae2 (vertebrate CtIP), which mediate the removal of hairpins, bulky adducts and other aberrant DNA end structures [133–136]. Initial processing by Sae2/CtIP and MRX/MRN results in the removal of approximately 50–100 nucleotides from the 5′ ends of the break, giving rise to short 3′ ssDNA tails [131, 132]. The Ku complex has a low affinity for ssDNA [137], thus resection reduces its binding capability which prevents repair by NHEJ and instead facilitates extensive end processing, an event necessary for HR-mediated DSB repair and robust Mec1/ATR-dependent checkpoint signalling [53].
In the second step, partly reconstituted in vitro [138], the partially resected DNA ends are the substrate for further extensive nucleolytic degradation which is carried out via two redundant pathways. One pathway is dependent on the exonuclease Exo1 while the other is mediated by the helicase-topoisomerase complex Sgs1-Top3-Rmi1 in collaboration with the 5′ flap endonuclease Dna2 [131, 132, 139]. Recent studies in S. cerevisiae have greatly contributed to our understanding of how the transition from initial to long-range resection occurs [138]. MRX and Sae2 act cooperatively to stimulate Exo1-dependent resection [55, 140]. Interestingly, this does not require the nuclease activity of Mre11 nor is it dependent on interactions between MRX or Sae2 and Exo1 [55, 140]. Instead, it is proposed that MRX and Sae2 facilitate Exo1 recruitment partially by suppressing Ku accumulation at DSBs and also through creation of a specific DNA structure (most likely a branched structure) that results in a high-affinity binding site for Exo1 which promotes its recruitment to DNA ends and stimulates its nuclease activity [55, 140, 141]. In addition to this, it has recently been proposed that Exo1 nuclease activity is negatively regulated by 14-3-3 proteins (scBmh1, Bmh2) [142]. Exo1 physically associates with 14-3-3 proteins and it is thought that this interaction modulates Exo1 phosphorylation which could function to prevent Exo1 nuclease activity under certain conditions, for example at stalled replication forks [142]. Both MRX and Top3-Rmi1 also promote DSB resection by Sgs1-Dna2-RPA by facilitating the recruitment of Dna2 to DNA ends and increasing the DNA-binding affinity of Sgs1, which stimulates its helicase activity and promotes DNA unwinding [55, 141, 143, 144]. Resection of DNA ends by Sgs1-Dna2-RPA is confined to 5′-terminated ssDNA as RPA inhibits Dna2-dependent degradation of 3′ ends while stimulating degradation of 5′ ends [143, 144]. This specificity ensures the correct directionality of DNA end processing which is essential for the formation of long 3′ ssDNA overhangs.
Recent in vitro studies have shown that processive resection in vertebrates also occurs via two pathways similar to that described for S. cerevisiae above [145]. In one, BLM (scSgs1) and DNA2 (scDna2) physically interact and function in the 5′–3′ resection of DNA ends, a process dependent on the helicase and nuclease activity of BLM and DNA2, respectively [145]. This process also requires RPA which stimulates the helicase activity of BLM and ensures a strict 5′-3′ polarity of resection by DNA2 [145]. MRN also stimulates BLM–DNA2-mediated resection by recruiting BLM to DNA ends [145]. In a second EXO1 (scExo1)-dependent pathway, MRN, RPA and BLM stimulate resection by promoting the recruitment of EXO1 to DNA ends [145, 146] and, in the case of MRN, by enhancing the processivity of EXO1 [145]. Analogous DSB resection processes have also been reported in Archaea and Bacteria, demonstrating once again the conservation of essential mechanisms required for cell viability and genome stability [138, 147].
Cell cycle regulation of DSB signalling
A characteristic of eukaryotic systems is the CDK-dependent regulation of DSB resection. The discovery of the key role of CDK in regulating DSB resection, checkpoint activation and DNA repair was another major milestone in the understanding of DDR activation [24, 69]. It appeared that CDK control of DSB resection also modulates the balance between NHEJ and HR, ensuring that the cell engages in the most appropriate DSB repair pathway based on its position in the cell cycle [148]. It was first shown that DSB processing is less efficient in G1 phase where cells display a reduced rate and/or processivity of resection due to low CDK activity, in contrast to the robust DNA resection that is initiated in S/G2 phases when CDK activity is high [68, 69, 149]. Interestingly, although resection is less efficient in G1 phase, evidence (including IR-induced Rfa1, Ddc1 and Ddc2 foci formation) suggests that limited processing does occur following IR-induced DSB formation and is confined to regions close to the DNA ends [68, 69, 149]. This limited processing is likely to explain the Mec1-dependency of the G1 checkpoint. Over recent years, CDK targets involved in controlling DSB resection in both G1 and G2 phase have been identified in both yeast and vertebrates. In S. cerevisiae, Sae2 is a major controller of DSB resection in G2 cells and its CDK phosphorylation at a conserved CDK consensus site (S267) promotes DNA end resection [71]. The sae2-S267A mutant exhibits impaired generation of 3′ ssDNA and reduced HR-mediated DSB repair [71]. This CDK phosphorylation site is also conserved in CtIP (T847; analogous to S267 in scSae2) [71]. A second vertebrate-specific CDK site (S327) in CtIP is also phosphorylated in S and G2 phases [150, 151] and promotes the association of CtIP with the putative scRad9 orthologue BRCA1 [152]. The BRCA1-CtIP interaction facilitates BRCA1-mediated ubiquitination of CtIP, which promotes its association with chromatin following DNA damage [153]. Furthermore, interaction of BRCA1 with the MRN complex, with CtIP acting as a bridging factor, is also dependent on CDK activity [150]. Both interactions are important for maintaining the stability of the BRCA1-CtIP-MRN complex at sites of damage, promoting efficient DSB resection [150]. CDK-dependent regulation of DSB resection is highly conserved as mutation of T847 and S327 in CtIP produces a similar phenotype to that observed in S. cerevisiae sae2 mutants [154, 155]. In addition to scSae2, Dna2 has also been recently identified as a direct CDK target [156]. CDK-dependent phosphorylation of Dna2 on several residues (T4, S17 and S237) stimulates its role in DSB resection by promoting its nuclear localisation and recruitment to DSBs. The modest defect in resection observed in a dna2-3A mutant (T4, S17 and S237 mutated to alanine) is greatly augmented by additional deletion of Exo1 indicating that resection in dna2-3A cells is Exo1-dependent and that the Exo1 and Dna2/Sgs1 pathways cooperate to mediate efficient DSB resection [156].
The Ku proteins and the Rad9 mediator are two factors that negatively regulate DSB resection [53, 68, 157, 158]. In the absence of the Ku complex or the Rad9 mediator, resection and checkpoint activation observed in yku and rad9 mutants are no longer CDK-dependent suggesting that the Ku and Rad9 proteins might be directly regulated by CDK to control DSB processing [53, 159]. In vertebrates, similar regulation of resection exists since the Ku complex has been shown to prevent HR by blocking the access to DSB [160]. Additionally, both ubiquitination and PARylation have been shown to be involved in the regulation of Ku retention at DSBs [160, 161]. The exact mode of regulation of resection by CDK remains to be elucidated and several interesting questions remain to be answered, notably how CDK phosphorylation affects Sae2/CtIP activity, how it potentially mitigates Ku inhibition of DSB resection and how it regulates the Rad9 mediator protein in preventing DSB resection.
DNA damage signal transduction
Processive resection coincides with the dissociation of Sae2, MRX and Tel1 from the DSB [15] and concomitant binding of RPA to the resultant 3′ ssDNA tails (Fig. 3d) [52]. The appearance of RPA-coated ssDNA provides increased loading sites for Mec1 [38, 158], with extensive resection of the DSB ends likely promoting the transition from Tel1/ATM-dependent to robust Mec1/ATR-dependent checkpoint signalling [80, 92]. Mec1 associates with Ddc2 (hATRIP) [162–164], which recognises and binds RPA-coated ssDNA thereby localising the Mec1-Ddc2/ATR-ATRIP complex to sites of damage [38, 165–168]. In addition, the 9-1-1 checkpoint clamp (scDdc1-Rad17-Mec3; human RAD9-RAD1-HUS1) and clamp loader (scRad24-Rfc2-5; human RAD17-RFC2-5) are recruited to RPA-coated ssDNA independently of the Mec1-Ddc2/ATR-ATRIP complex (Fig. 3e) and are required to initiate Mec1/ATR-dependent checkpoint signalling in both G1 and G2 phases [163, 166, 167, 169]. The 9-1-1 checkpoint clamp is structurally related to the replication clamp PCNA [170, 171], while the Rad24-Rfc2-5 complex is similar to the PCNA loader except that Rad24 replaces the Rfc1 subunit [172]. Studies have shown that the structure that facilitates Mec1 recruitment on ssDNA is a 5′ ssDNA/dsDNA junction since RPA restricts the loading of the 9-1-1 clamp by the Rad24-Rfc2-5 complex specifically on this type of junction [173–175]. Evidence suggests that both the presence of 5′ ss/dsDNA junctions and the amount of ssDNA control ATR checkpoint activation [176]. In G1 phase, slow accumulation of these types of structures could explain the requirement of the Mec1 signalling pathway in this phase of the cell cycle.
In S. cerevisiae, co-localisation of Mec1-Ddc2 and 9-1-1 complexes at sites of damage facilitates a stable association between Mec1-Ddc2 and Ddc1 [118, 163, 177]. The 9-1-1 complex promotes Mec1-dependent phosphorylation of its targets [162, 163, 178–180] and stimulates Mec1 kinase activity via a direct interaction with Ddc1 both in vitro and in vivo leading to activation of the G1 checkpoint [181]. Unlike S. cerevisiae, evidence demonstrating that the 9-1-1 clamp in vertebrates can similarly directly stimulate ATR kinase activity is lacking despite the fact that this would be structurally possible [182]. Rather, the 9-1-1 clamp functions to recruit TopBP1 (scDpb11) to sites of damage via an interaction between phosphorylated RAD9 (scDdc1) and TopBP1 [183, 184]. Casein kinase 2 (CK2) has been identified as a RAD9 kinase that could stimulate this interaction [185, 186]. This interaction facilitates the association of TopBP1 with ATRIP which stimulates ATR kinase activity [187, 188]. This mechanism of Mec1/ATR activation is also conserved in S. cerevisiae (Figs. 3e and 4) in G2/M phase, and is dependent on the interaction of the replication protein Dpb11 with Ddc1, which promotes Mec1 kinase activity [177, 189–191]. A recent study has identified two specific residues in the C-terminal tail of Dpb11 (W700 and Y735) as being required for activation of Mec1 in vitro, and these residues significantly contribute to activation of the DNA damage checkpoint in G2/M [181]. Subsequently, Mec1/ATR phosphorylates and activates Rad9 (Fig. 3e), a key adaptor protein in the DNA damage checkpoint [81, 192, 193].
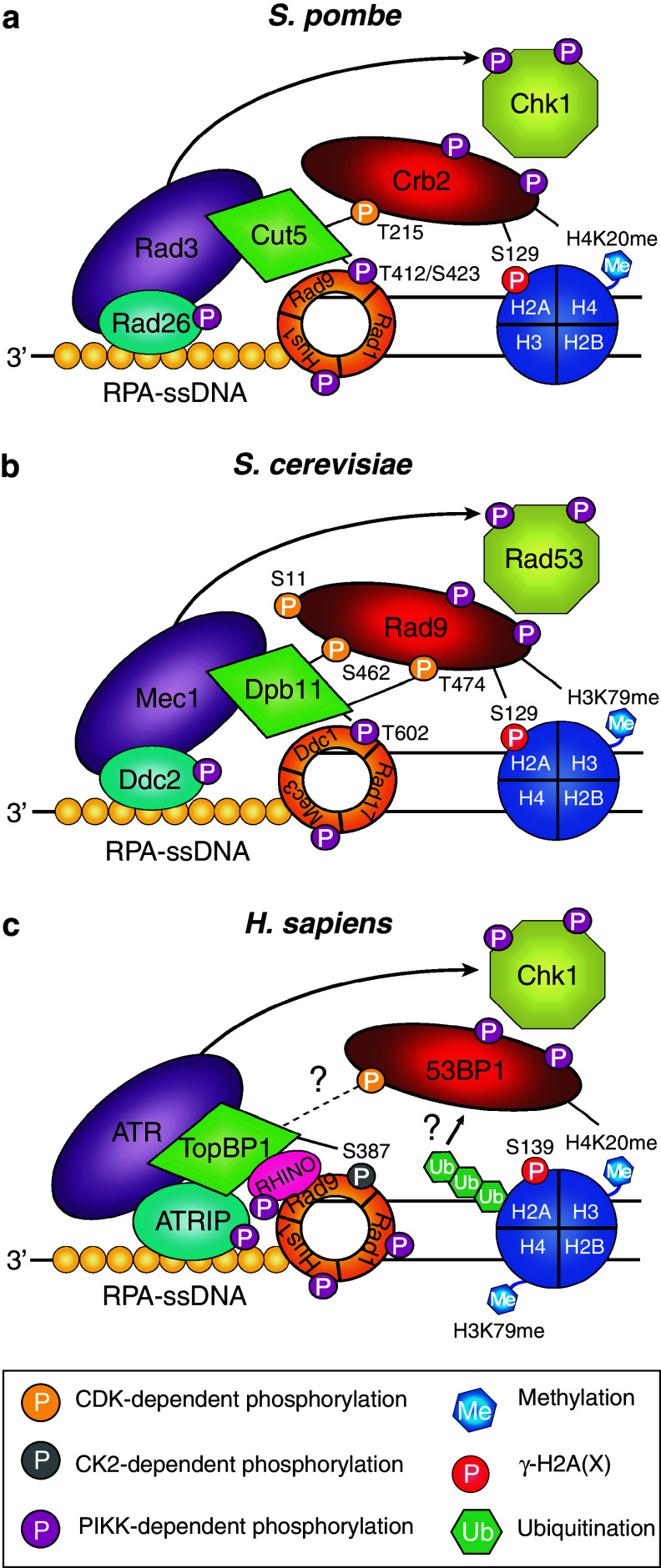
Fig. 4.
Two parallel pathways operate to recruit spCrb2 and scRad9 to sites of damage. Histone modification-dependent and -independent pathways promote the recruitment of spCrb2 and scRad9 to sites of damage. a In S. pombe, Crb2 is recruited to chromatin via binding of its Tudor and BRCT domains to methylated H4K20 and γ-H2A, respectively. Crb2 is also recruited via an interaction between the phosphorylated T215 residue of Crb2 and the two N-terminal BRCT domains of Cut5. b Similar mechanisms exist in S. cerevisiae, where recruitment of Rad9 to chromatin requires binding of its Tudor and BRCT domains to methylated H3K79 and γ-H2A, respectively. Rad9 can also be recruited by an alternative pathway, which requires CDK-dependent phosphorylation of Rad9. Recruitment of Rad9 to sites of damage occurs through a direct interaction between the phosphorylated S462 and T474 residues of Rad9 and the two N-terminal BRCT domains of Dpb11. CDK-dependent phosphorylation of Rad9 S11 may indirectly promote the Dpb11-Rad9 interaction, however the exact mechanism by which this residue contributes remains to be determined. c A similar pathway may exist in vertebrates. 53BP1 is recruited to chromatin via binding of its Tudor domain to methylated H3K79 and/or H4K20. The first two BRCT domains of TopBP1 interact with the phosphorylated S387 residue of RAD9. Whether CDK-dependent phosphorylation of 53BP1 promotes its association with TopBP1 at sites of damage remains to be determined
Rad9 recruitment to sites of damage by histone-dependent and -independent pathways
In undamaged cells, hypophosphorylated Rad9 (so named as to distinguish the cell cycle modified forms from DNA damage-induced hyperphosphorylated Rad9 [81, 193]) exists as a large complex (≥850 kDa) containing the Ssa1 and/or Ssa2 chaperone proteins that promote the stability of the complex [194, 195]. It is proposed that Ssa1/Ssa2 facilitate Mec1-dependent remodelling of the Rad9 complex although this remains to be formally tested [194]. A small proportion of hypophosphorylated Rad9 is associated with chromatin in G1 and G2/M phases and it is suggested that this dynamic association could enhance the speed and efficiency of the Rad9-dependent DNA damage checkpoint response [108, 196]. Following DNA damage, two parallel pathways operate to recruit Rad9 to chromatin (Figs. 3e and 4); one is dependent on histone modifications (γ-H2A and H3K79 methylation) [108, 197, 198], while the other (which is important in G2 phase) is independent of histone modifications and instead requires an interaction between Ddc1 and Dpb11 [199].
In contrast to histone H2A, which is phosphorylated in a DNA damage-dependent manner, methylation of histone H3K79 (H3K79me) is constitutive [200] and is mediated by the conserved histone methyltransferase (HMT) Dot1 [200, 201]. H3K79me is buried in the nucleosome core [202] and is inaccessible until DNA damage-induced chromatin remodelling exposes the mark, providing a docking site for checkpoint proteins at the site of damage [116]. In S. cerevisiae, H3K79me is essential for checkpoint activation in both G1 and S phase; dot1Δ and h3K79A mutants are defective in G1 and intra-S phase checkpoint activation [197, 203, 204]. Furthermore, cells harbouring a mutated Rad9 Tudor domain, which mediates the interaction between Rad9 and H3K79me in vitro [197, 198], are G1 checkpoint-defective and exhibit minor sensitivity to various genotoxic treatments [197, 205]. Moreover, epistasis analyses revealed that the Rad9 Tudor domain and Dot1 operate in the same pathway to mediate resistance to DNA damage [197] and belong to the same epistasis group as H2A-S129 and the Rad9 BRCT domains with respect to checkpoint activation [107, 108]. Collectively, these studies support a model whereby, following DNA damage in G1 phase, Rad9 is recruited to chromatin via binding of its Tudor and BRCT domains to H3K79me and γ-H2A, respectively. This recruitment also occurs in the absence of DSB resection and can be induced by the Tel1 kinase [77, 80]. This is demonstrated by the fact that, in G1-arrested cells lacking Mec1, Rad53 is activated [80]. Therefore, in this condition, Tel1 is also able to activate Rad9 by mechanisms that may be similar to those described for Mec1 [206] but are currently unknown. In wild-type G1 cells, gradual DSB end processing/accumulation of Mec1-activating structures will eventually induce a switch from Tel1 to Mec1 signalling. The molecular events involved in Mec1 signalling in G1 phase are not well understood but they only seem to rely on the histone modification-dependent recruitment of Rad9. In contrast, loss of either histone modification does not significantly perturb G2/M checkpoint activation, indicating that an alternative mechanism for Rad9 recruitment to sites of damage must operate in G2/M phase independently of H3K79me and γ-H2A [197, 203, 204].
Evidence for a parallel pathway operating in G2/M first came from studies in S. pombe where recruitment of Crb2 to sites of damage is mediated by two partially redundant mechanisms [207]. It is worth noting that H3K79me has not been reported in S. pombe [207], but effects on checkpoint activation and repair that resemble those of H3K79me in S. cerevisiae have been shown to be mediated by H4K20me [208]. Conversely, there is no evidence that H4K20me occurs in S. cerevisiae [209, 210] and strains in which H4K20 cannot be methylated are checkpoint proficient [197]. Analogous to that described for scRad9 above, one pathway for Crb2 recruitment is regulated by H4K20me and γ-H2A (Fig. 4a) and is dependent upon the Tudor and BRCT domains of Crb2 which bind H4K20me and γ-H2A, respectively [207, 208, 211–214]. The second pathway is independent of histone modifications and instead requires phosphorylation of a single CDK consensus site (T215) in the N-terminus of Crb2 [207]. Cells harbouring a Crb2 Tudor domain mutation or mutations abolishing either H4K20me or γ-H2A display synergistic genetic interactions with a crb2-T215A mutant, demonstrating that H4K20me, γ-H2A and the Tudor domain of Crb2 act cooperatively with phosphorylated Crb2-T215 to recruit Crb2 to sites of damage [207, 208, 215]. The model for Crb2 recruitment to sites of damage in the absence of histone modifications is as follows: Rad3-dependent phosphorylation of T412/S423 in the C-terminus of the Rad9 (scDdc1) subunit of the 9-1-1 complex promotes its interaction with the replication factor Cut5 (scDpb11; human TopBP1), which binds these phosphorylated residues via its two C-terminal BRCT domains [216]. The Rad9–Cut5 interaction promotes the recruitment of Crb2 to sites of damage through an interaction between the two N-terminal BRCT domains of Cut5 and phosphorylated T215 in the N-terminus of Crb2 [207, 217, 218]. This interaction facilitates the Rad3-dependent phosphorylation of Crb2 and subsequent activation of Chk1 [216, 218].
Recent studies in S. cerevisiae have described a similar histone modification-independent mechanism for the recruitment of Rad9 to sites of damage [191, 196, 199]. It relies on Mec1-dependent signalling that induces phosphorylation of T602 in the C-terminus of Ddc1 providing a docking site for Dpb11 (Fig. 4b), which binds phosphorylated T602 via its two C-terminal BRCT domains [199, 219]. However, a recent study has shown that Dpb11 is proficient for focus formation in zeocin-treated mec1Δ tel1Δ sml1Δ cells, suggesting that PIKK-dependent phosphorylation of Ddc1 is not absolutely required for the association of Dpb11 with the 9-1-1 complex [220]. Similarly, in Xenopus, TopBP1 (scDpb11) can be recruited to chromatin by a mechanism that is dependent on the 9-1-1 complex but independent of RAD9 (scDdc1) S387 phosphorylation [221]. It is likely that the Ddc1-Dpb11 interaction promotes the recruitment of Rad9 to sites of damage via a direct interaction between the N-terminal BRCT domains of Dpb11 and two recently identified CDK phosphorylation sites (S462 and T474) on Rad9 [191, 199]. CDK-dependent phosphorylation of Rad9 S462 and T474 creates a binding site for Dpb11 and the subsequent Dpb11-Rad9 interaction functions to recruit the Rad9 mediator to sites of damage [191]. Phosphorylation of a CDK consensus site in the N-terminus of Rad9 (S11) has also been implicated in promoting the Dpb11-Rad9 interaction albeit indirectly [196]. However, the exact mechanism by which Rad9 S11 phosphorylation contributes to this interaction remains to be determined. Once recruited to sites of damage, Mec1-dependent phosphorylation of Rad9 facilitates the recruitment of Rad53 and promotes its Mec1-dependent activation [192, 195, 206]. The Rad9 protein contains 20 putative sites for phosphorylation by CDK, 9 of which conform to the strict consensus sequence [(S/T)-P-X-(R/K)]. Mass spectrometric analyses identified 15 of these CDK sites to be phosphorylated in vivo [23, 222, 223]. While particular functions have been attributed to specific sites as discussed above, at present the functional roles of the remaining residues remain elusive. The complex CDK-dependent phosphorylation of Rad9 is likely to be very important for all its DDR functions, including its role in the inhibition of DSB resection.
Analogous to scRad9 and spCrb2, mammalian 53BP1 functions as a molecular adaptor at sites of damage, facilitating IR-induced phosphorylation of a subset of ATM targets including CHK2, BRCA1 and SMC1 [114, 224–228]. 53BP1 is recruited to chromatin via binding of the 53BP1 Tudor domain to H4K20me and/or H3K79me [198, 211, 229], which could possibly only be recognised following prior RNF8-RNF168-UBC13-mediated polyubiquitination of histone H2A and H2AX [123–127]. The accumulation and stable retention of 53BP1 in ionising radiation-induced foci (IRIF) is dependent on γ-H2AX and its role in recruiting MDC1 [115, 119, 121, 230, 231]. Similar to the Rad9-Cut5 (scDpb11) interaction described above for S. pombe, the first two BRCT domains of TopBP1 (scDpb11) in vertebrates interact with a C-terminal phosphorylation site (S387) on RAD9 (scDdc1) which is constitutively phosphorylated during the cell cycle [183, 184], possibly by casein kinase 2 [185, 186]. In addition, a novel player in ATR-mediated checkpoint signalling, RHINO (Rad9, Rad1, Hus1 interacting nuclear orphan), has recently been identified [232]. Recruitment of RHINO to sites of damage is dependent on the 9-1-1 complex, and mutants that are unable to bind the 9-1-1 complex are checkpoint defective [232]. RHINO also independently associates with TopBP1 [232]. It is suggested that the RHINO-TopBP1 interaction may function to promote TopBP1 recruitment to the 9-1-1 complex at sites of damage possibly through a mechanism that is independent of RAD9 S387 phosphorylation; however, this remains to be determined [232]. The TopBP1-RAD9 interaction (Fig. 4c) promotes the TopBP1-dependent activation of ATR, which is mediated by the ATR activation domain (AAD) of TopBP1, facilitating the ATR-dependent activation of CHK1 [187, 188]. TopBP1 colocalises with 53BP1 following IR-induced DNA damage and interacts with 53BP1 in vitro [233, 234]. Similar to scRad9, the 53BP1 protein, like BRCA1 and MDC1, is regulated by CDK activity during a normal cell cycle and several of its CDK consensus sites have been shown to be phosphorylated in vivo [235–247]. 53BP1 contains 42 putative CDK phosphorylation sites and mass spectrometric analysis identified 27 of these sites to be phosphorylated in vivo [235–242]. Furthermore, 5 of these sites (S294, S380, S552, S1028, S1114) are phosphorylated during an unperturbed cell cycle [236]. Although there is clear functional similarity between 53BP1 and its yeast orthologue, it remains to be determined whether CDK-dependent phosphorylation of 53BP1 promotes its association with TopBP1 leading to efficient DNA damage checkpoint activation.
Activation of Rad53 and Chk1 effector kinases
Once recruited to sites of damage, scRad9 is hyperphosphorylated in a PIKK-dependent manner [81, 193] which promotes remodelling of the large hypophosphorylated complex into a smaller 560-kDa complex comprising hyperphosphorylated Rad9, Ssa1/2 and Rad53 [81, 193–195] (Fig. 3e). Hyperphosphorylated Rad9 acts as a molecular adaptor/scaffold (Fig. 3f) to facilitate amplification of the checkpoint signal [195, 206], and its molecular role in Mec1-dependent Rad53 activation, partially reconstituted in vitro [206], is the best understood. Phosphorylation of several SQ/TQ sites on Rad9 provides a docking site for Rad53, which binds these phosphorylated residues via its two FHA domains [192, 206, 248–250]. Rad9 functions as a molecular adaptor (Fig. 3f) to bring Rad53 into close proximity to Mec1 at sites of damage to facilitate the Mec1-dependent phosphorylation of Rad53 [206]. Rad9 also functions as a molecular scaffold to bring Rad53 molecules into close proximity, thus catalysing Rad53 in trans autophosphorylation [195]. Vertebrate CHK2 is also known to dimerize and trans-autophosphorylate in an ATM-dependent manner, but a molecular role for DNA damage mediators in this activation remains to be investigated [251]. Fully activated Rad53 is then released from the hyperphosphorylated Rad9 complex in an ATP-dependent manner [195]. As well as mediating the interaction between Rad9 and Rad53, phosphorylation of the Rad9 SQ/TQ cluster domain (SCD) promotes Rad9 oligomerisation at sites of damage via an interaction between the BRCT domains of Rad9 and the phosphorylated SCD [252]. Although Rad9 oligomerisation appears to be dispensable for initial Rad53 activation, it is required for the maintenance of Rad53 activation and checkpoint-induced cell cycle arrest [252]. Rad9 oligomerisation promotes its accumulation at sites of damage, allowing amplification of the DNA damage signal and sustained activation of Rad53 [252]. It is also proposed that a negative feedback loop exists in which fully activated Rad53 phosphorylates the Rad9 tandem BRCT domains to attenuate the BRCT-SCD interaction, mediating the turnover of hyperphosphorylated Rad9 by promoting its dissociation from sites of damage and subsequently dampening Rad53 activity [252].
Rad9 also facilitates the DNA damage-induced phosphorylation of the parallel downstream kinase Chk1 (Fig. 3f), where Mec1-dependent Chk1 activation requires the Chk1 activation domain (CAD) of Rad9 [44, 253]. Mechanistic activation of Chk1 by Rad9 remains a mystery to date since the physical interaction between Rad9 and Chk1 demonstrated by yeast two-hybrid analyses [44, 254] has never been corroborated by other biochemical assays. Rad9-dependent activation of Chk1 may occur in a manner analogous to Rad9′s role as a molecular adaptor in the activation of Rad53: Chk1 could bind to the Rad9 CAD domain, promoting its recruitment to sites of damage and subsequent Mec1-dependent phosphorylation. Additionally, the existence of budding yeast chk1 mutants that can be activated in a Rad9-independent manner [255] suggests that Rad9 might facilitate the conformational change shown to be required for Chk1 activation [256]. Once activated, Rad53 and Chk1 phosphorylate several downstream targets that are involved in cell cycle control and transcriptional regulation.
In contrast to S. cerevisiae where Rad53 (CHK2 in humans) is the principal effector kinase, CHK1 is the primary effector of both the DNA damage and replication checkpoints in vertebrates, with CHK2 playing a subsidiary role [43]. CHK1 activation is mediated by ATR and the adaptor protein Claspin (scMrc1) and occurs primarily in response to replication stress and UV-induced DNA damage [251]. In contrast to vertebrates, scMrc1 regulates activation of Rad53 (the main effector kinase in S. cerevisiae) in response to replication stress [257]. Chk1 only becomes activated under these conditions when Mrc1 is absent and the replication stress signal is converted into a DNA damage signal [257, 258], leading to Rad9-mediated activation of both Rad53 and Chk1 [257]. It is proposed that Mrc1 mediates the Mec1-dependent phosphorylation of Rad53 by acting as a molecular adaptor in a manner analogous to the role of Rad9 in responding to DNA damage [259], as Mrc1 physically interacts with Rad53 following MMS-induced DNA damage [260, 261]. In vertebrates, ATR-dependent phosphorylation of S317 and S345 on CHK1 reportedly promotes CHK1 activation by inducing a conformational change that relieves the inhibition of the N-terminal kinase domain by the C-terminal regulatory domain [262–265] and stimulates the release of CHK1 from chromatin [266, 267]. CHK1 activation is also known to be dependent on 53BP1, BRCA1 and MDC1 [43]. Whether this dependency relies on direct molecular mechanisms, rather than the role of the Rad9-family DNA damage mediators in the focal organisation of DDR proteins essential for checkpoint maintenance, is currently unknown. CHK1 phosphorylates several downstream targets, including Cdc25A, Cdc25C and Wee1, which are key regulators of the cell cycle [268–270]. The recent identification of novel putative CHK1 substrates involved not only in the DDR (e.g. KU70, FEN1 and RIF1) but also in other cellular processes including RNA metabolism [271] will provide key insights into the physiological functions of CHK1.
Targets of the DNA damage checkpoints
The G1 checkpoint
The G1 checkpoint induces cell cycle arrest at or prior to START, before cells become irreversibly committed to the next cell cycle (Fig. 1). Rad53 directly phosphorylates the Swi6 regulatory subunit of the SBF [Swi4/6-dependent cell cycle box (SCB) binding factor] transcription factor, which inhibits transcription of the G1/S cyclins (Cln1 and Cln2) [272, 273]. Furthermore, Rad53-dependent checkpoint signalling promotes the cytoplasmic accumulation of the Los1 tRNA export factor, leading to activation of the Gcn4 transcription factor, which contributes to the G1 checkpoint by delaying the accumulation of Cln2 [274]. This reduced level of G1/S cyclin activity prevents the destruction of the B-type cyclin inhibitor Sic1, thus inhibiting progression through the G1/S transition [275, 276]. DNA damage-dependent phosphorylation of Chk1 in G1-arrested cells [79] suggests that it also participates in the G1 checkpoint. However, the mechanistic role of Chk1 in the G1 checkpoint has not been explored.
In vertebrates, the G1 checkpoint is very robust and a two-wave model of checkpoint activation has been proposed [277]. The first response requires ATM-dependent phosphorylation of CHK2 on T68 which facilitates CHK2 homodimerization and in trans autophosphorylation [278–280]. Activated CHK2 phosphorylates the Cdc25A phosphatase targeting it for proteosomal degradation [270, 281, 282]. Cdc25A functions to remove inhibitory phosphorylation on T14/Y15 residues of CDK2 [16], and thus loss of Cdc25A activity prevents dephosphorylation and activation of the CDK2-cyclinE kinase complex which is required for S phase entry [282, 283]. A second response is dependent upon ATM- and CHK2-mediated phosphorylation of the tumour suppressor protein p53 [284–287], which reduces binding of the negative regulator MDM2 to p53 and stimulates p53 activation [285, 286, 288–290]. In addition, phosphorylation of the p53 regulator MDMX by ATM and CHK2 promotes its MDM2-mediated ubiquitination and degradation [291]. Together, these events lead to the stabilization and nuclear accumulation of p53 [292]. p53 promotes the transcriptional induction of the CDK inhibitor p21, which inhibits CDK2-cyclinE activity [293, 294]. Although the p53-dependent response is slower, it is proposed that it functions to maintain the G1 arrest initiated by the Cdc25A pathway [277].
The intra-S phase checkpoint
Two checkpoints operate during S phase to signal the presence of DNA damage (intra-S) or replication stress (replication checkpoint) [295]. Although genetically separable, these checkpoints partially overlap as many checkpoint proteins function in both pathways due to the occurrence of similar or common DNA structures [296]. Faithful replication of the genome is paramount to the maintenance of genomic stability, thus it is not surprising that the multiple checkpoint and repair pathways that act in S phase display considerable complexity and redundancy [297]. Recently, it has been shown that two independent pathways function in the activation of Mec1 in response to replication stress [298]. One pathway is dependent on the 9-1-1 complex and the other requires the leading strand polymerase DNA polymerase epsilon (pol ε) and the replication factors Dpb11 and Sld2 [298]. It is proposed that the 9-1-1 complex, which loads specifically onto 5′ junctions [174, 175], signals replication stress on the lagging strand, while pol ε and the leading strand factors Dpb11 and Sld2 detect replication perturbations on the leading strand, with both pathways independently leading to Mec1 activation [298]. In S. cerevisiae, checkpoint activation in S phase fully depends on the Mec1 and Rad53 kinases [32]. Mec1-dependent activation of Rad53 in response to replication stress or DNA damage is mediated by the adaptor proteins Mrc1 [257] and Rad9 [299], respectively. The critical role of Rad53 and Mec1 in promoting cell viability following DNA damage is to stabilise DNA replication forks [300–302]. In addition, Mec1 and Rad53 activation inhibits firing of late replication origins when replication from already fired origins is stalled due to DNA damage [303, 304], and prevents mitotic entry until DNA replication is complete [305].
The rate of S phase progression is decreased in response to DNA damage partly by regulating the phosphorylation status of RPA [180, 306, 307] and inhibiting DNA polymerase α-primase (pol α-primase) activity [308, 309]. DNA pol α-primase is required for both G1 and S-phase checkpoints, where activated Rad53 inhibits its phosphorylation, thus preventing initiation of DNA synthesis downstream of the lesion [308, 309]. DNA polymerase ε is also required for activation of the S-phase checkpoint. It acts as a sensor of DNA replication and links the DNA replication machinery to the checkpoint [298, 310, 311].
Rad53 also targets components of the CDK- and DDK (Dbf4-dependent kinase)-dependent pathways which are required for the firing of early and late origins of replication [312]. The recent discovery that the replication initiation protein Sld3 and the Cdc7 kinase regulatory subunit Dbf4 are Rad53 substrates and are phosphorylated in a Rad53-dependent manner in response to DNA damage was a significant advancement in our understanding of the molecular mechanisms governing the DDR in S phase [313, 314]. Rad53 directly phosphorylates Sld3 in vitro indicating that it is a direct target of Rad53 in vivo. Rad53-dependent phosphorylation of Sld3, which has been already phosphorylated by Clb5/6-Cdk1 [315, 316], prevents its interaction with the Dpb11 and Cdc45 replication proteins which are both required for the initiation of DNA replication, thus preventing late origin firing [313, 314]. Inhibiting downstream components of the CDK-dependent pathway, and not targeting CDK complexes directly, ensures that CDK activity remains high in S phase in the presence of DNA damage, which is crucial to prevent the re-licensing of early firing origins [313, 314]. Rad53-mediated phosphorylation of Dbf4 also blocks late origin firing, yet how this phosphorylation event inhibits DDK activity remains to be elucidated [313, 314].
Another recently identified target of the intra-S phase checkpoint is Exo1 [317, 318], which is recruited to stalled replication forks [319]. Deletion of EXO1 almost completely suppresses the sensitivity of rad53Δ cells to a range of DNA-damaging agents, suggesting that Exo1 might be a primary target of Rad53 [317]. Indeed, Exo1 is phosphorylated in a Rad53-dependent manner in response to MMS treatment [318, 320]. These data suggest that Rad53-dependent phosphorylation of Exo1 inhibits Exo1-dependent resection events at the fork and subsequent replication fork breakdown by limiting ssDNA accumulation and DNA damage checkpoint activation [317, 318]. Furthermore, in the absence of Rad53, Chk1 also plays a role in the stabilisation of replication forks. This function is independent of its role in inhibiting anaphase entry and only occurs in the absence of Exo1 [317].
Rad53 also targets transcription via Dun1, a protein kinase required for the transcriptional induction of several DNA damage-inducible genes and genes encoding ribonucleotide reductase (RNR) subunits involved in the modulation of dNTP pools [321–323]. Dun1 induces RNR gene transcription by phosphorylating and inhibiting the repressor Crt1, promoting its dissociation from the promoter region of RNR2, RNR3 and RNR4 [324]. Dun1 also phosphorylates the RNR inhibitor Sml1 and targets it for degradation [325]. Furthermore, Dun1 directly phosphorylates Dif1, a protein involved in regulating the nuclear import of the Rnr2-Rnr4 subunits [326]. Dun1-dependent phosphorylation of Dif1 promotes its degradation, allowing Rnr2-Rnr4 to relocalise to the cytoplasm where it binds Rnr1 to form an active complex [326].
In vertebrates, ATR is the primary PIKK activated in response to replication perturbations, with ATM playing a minor role in response to DSBs [327]. The intra-S phase checkpoint functions to suppress origin firing and stabilise stalled replication forks to maintain replication fork integrity [328–331]. ATR-dependent phosphorylation of CHK1 is dependent on the mediator proteins TopBP1 and Claspin. As discussed above, TopBP1 is recruited to sites of damage by the 9-1-1 complex, where it functions to stimulate ATR kinase activity [183, 184, 187, 188]. The replication fork-associated protein Claspin functions to recruit CHK1 to stalled replication forks, facilitating ATR-dependent phosphorylation and activation of CHK1 [332–335]. ATR-CHK1 signalling in response to replication stress also requires the mediator proteins Timeless and Tipin (timeless-interacting protein), and loss of either protein impairs intra-S phase checkpoint activation [336–338]. Tipin binds RPA-coated ssDNA [336, 337] and it is thought that Timeless-Tipin may function to recruit Claspin and CHK1 to stalled replication forks [337, 339]. These interactions likely serve to increase the local concentration of CHK1 at sites of damage, facilitating efficient ATR-mediated phosphorylation of CHK1. Activated CHK1 dissociates from chromatin to phosphorylate its substrates [266], including the CDC25 phosphatases [340, 341]. In response to replication perturbations, the ATM/ATR-CHK2/CHK1-Cdc25A-CDK2 pathway prevents the initiation of DNA replication by inhibiting loading of the replication initiation factor Cdc45 onto replication origins [268, 282, 342]. Furthermore, ATR-CHK1-mediated signalling inhibits an interaction between Cdc45 and the Mcm7 subunit of the MCM helicase complex at origins of replication via a CDK2-independent mechanism [343]. Additionally, a distinct pathway dependent on ATM, NBS1, BRCA1 and SMC1 mediates the ATM-dependent phosphorylation of the SMC1 and SMC3 subunits of the cohesin complex [344–347], which promotes chromosomal repair and enhances cell survival [346, 347]. The exact mechanism by which these phosphorylation events contribute to intra-S phase checkpoint activation remains to be elucidated, although it is proposed that they may function to modulate the rate of DNA synthesis or regulate recombinational repair following DNA damage [345–347].
The G2/M checkpoint
DNA damage-induced G2 arrest is the most prominent checkpoint response in most eukaryotes, where entry into mitosis is prevented via inhibition of CDK activity. In S. pombe and vertebrates, CDK activity is regulated by inhibitory phosphorylation of a conserved tyrosine residue (Y15) of the spCdc2/human CDK1 kinase complexes [348–352]. Additional inhibitory phosphorylation can also occur on the adjacent threonine residue (T14) [348, 349, 353]. The phosphorylation status of these residues is reciprocally regulated by the Wee1 family of kinases (scSwe1; spWee1 and Mik1; human Wee1 and Myt1) and the Cdc25 family of phosphatases (scMih1; spCdc25; human Cdc25A, Cdc25B, Cdc25C) [354]. In S. pombe, Chk1 inhibits Cdc2 activity by targeting both Wee1 and Cdc25 [355]. Chk1-dependent phosphorylation of Wee1 stabilises the protein, ensuring phosphorylation of Cdc2 on Y15 is maintained [355–357], while phosphorylation of Cdc25 by Chk1 promotes the association of Cdc25 with the 14-3-3 protein Rad24, which sequesters Cdc25 in the cytoplasm, thus preventing the dephosphorylation and activation of Cdc2 [358–360]. Either of these mechanisms are sufficient to elicit an efficient G2 DNA damage checkpoint response, preventing entry into mitosis until DNA repair is complete [355]. A similar mechanism exists in mammalian cells [7].
In S. cerevisiae, however, the DNA damage checkpoint does not control cell cycle progression by inhibitory phosphorylation of the equivalent tyrosine residue (Y19) of Cdk1 [361, 362]. Instead, this checkpoint induces a mitotic arrest by inhibiting the metaphase to anaphase transition [363]. Both Rad53 and Chk1 function in the G2/M checkpoint response to inhibit anaphase entry and mitotic exit in the presence of DNA damage [44, 364, 365]. This is accomplished, at least in part, by inhibiting the degradation of Pds1 [366]. Chk1-dependent phosphorylation of Pds1 prevents its degradation by the APCCdc20 complex, thus inhibiting sister chromatid separation and preventing anaphase entry [44, 367, 368]. Rad53 also contributes to the stability of Pds1 by inhibiting the interaction between Pds1 and Cdc20 [368]. In addition to inhibiting mitotic entry, a parallel pathway dependent on the Rad53 kinase acts to prevent mitotic exit by maintaining high levels of mitotic CDK activity [44]. The Cdc5 polo-like kinase is a component of the mitotic exit network (MEN), which initiates mitotic exit by promoting the destruction of the mitotic cyclins [369, 370]. Recent major findings identified Rad53 as a Cdc5 substrate that is phosphorylated in a Cdc5-dependent manner during an unperturbed cell cycle [371–373]. Although the exact mechanism by which Cdc5 promotes checkpoint inactivation by targeting Rad53 is currently unknown, recent findings support a model in which Cdc5-dependent phosphorylation of Rad53 inhibits the ability of the Rad9 mediator to promote Rad53 autophosphorylation [372]. Another study suggests that Cdc5- and Cdk1-dependent Rad53 phosphorylation in G2/M may function to lower the threshold required for DNA damage checkpoint activation, contributing to the robustness and maintenance of the checkpoint [371]. Following checkpoint activation, Cdc5 is also phosphorylated in a Rad53-dependent manner [374], leading to Cdc5 inactivation and prevention of mitotic exit [44]. Rad53 also suppress the MEN by preventing the release of the Cdc14 phosphatase from the nucleolus in a Cdc5-independent manner [365]. Taken together, these studies demonstrate that RAD53 and CHK1 function in parallel, partially redundant, pathways that co-operate to ensure efficient G2/M checkpoint arrest.
Conclusions and future perspectives
Our knowledge of the complex regulatory mechanisms governing DNA damage checkpoint activation has advanced significantly over recent years. However, our understanding of these intricate pathways is still far from complete. Recent research has uncovered several elegant regulatory mechanisms that modulate divergent aspects of the DDR at different levels, in particular the early steps of checkpoint activation. CDK has fast emerged as a key post-translational regulator of the DDR [68–71, 118, 191, 375, 376]. Accumulating evidence suggests an expanding network of kinases and substrates being identified as novel players in the DDR. This further adds to the complexity of these signalling networks, especially in vertebrates where DDR signalling and cross-talk between proteins is more complex [46]. Recent phosphoproteomic screens have significantly impacted our understanding of how phosphorylation mediates DDR events, resulting in a major expansion of the kinase network through the identification of novel damage-induced phosphorylation events induced by non DDR protein kinases [377, 378]. Understanding the functional cross-talk between signalling proteins and pathways, also conserved in yeast, is a critical area of research that will yield important insights into how complex mechanisms governing the DDR are regulated.
Additionally to phosphorylation and other PTMs, microRNAs (miRNAs) and RNA-binding proteins (RBPs) represent an exciting post-transcriptional level of regulation for the DDR [379, 380]. miRNAs are often deregulated in cancer [381], and siRNA-mediated knockdown of Dicer (essential component in miRNA biogenesis) increases cellular sensitivity to cisplatin and UV, as well as perturbed cell cycle control following UV-induced DNA damage [382, 383]. It is likely that miRNAs and RBPs orchestrate the expression of several protein clusters involved in cell cycle control and the DDR (recent evidence implicates miR-182 in the regulation of BRCA1 function [384]). Identification of novel players involved in miRNA-mediated gene regulation will be crucial to our understanding of the DDR and the potential role miRNAs play in cancer development and progression. Understanding the context-dependent regulation of DNA damage checkpoint activation at the molecular level will provide further insight into how defects in DNA damage signalling pathways can drive tumourigenesis. Ultimately, this knowledge will facilitate the development of more efficacious and targeted cancer therapies and will improve our knowledge of cancer initiation and progression.
Another emerging direction of research is the identification of novel functions for checkpoint proteins and their involvement in controlling nuclear dynamics in response to DNA damage. It was already shown that 53BP1 is a modulator of chromatin dynamics and plays an important role in uncapped telomere rejoining [385], long-range V(D)J recombination [386] and the repair of lesions induced within regions of heterochromatin [387]. More recently, it has been shown that the E3 ubiquitin ligase activity of the BRCA1 tumour suppressor protein has a novel role, key for genomic stability, in maintaining centromeric sites on chromosomes in a structurally condensed state known as pericentric heterochromatin [388]. The exact mechanism underlying these novel functions represents an important area for future investigation. The molecular mechanisms by which the putative scRad9 vertebrate orthologues mediate DSB signalling in this context are only beginning to emerge, and further studies should lead to a greater understanding of the role of nuclear dynamics in the DDR and the maintenance of genomic integrity.
Interestingly, and more specifically relevant to vertebrate cells, it has recently been discovered that several proteins required for the maintenance of genome stability also play key roles in other cellular contexts. For example, p53 and histone H2AX have been implicated in the regulation of developmental potency [389–393] and embryonic stem (ES) cell proliferation [394]. The observation that GABAA receptor activation can induce γ-H2AX foci in undamaged cells provides a potential paradigm shift across many areas of research: in addition to demonstrating a novel mechanism for regulating ES cell proliferation, the authors have shown that proteins involved in the DDR can be activated in contexts outside the remit of DNA damage [394]. It will be important to elucidate the molecular mechanisms that link GABA signalling to PIKK activation. It is also tempting to speculate that other key players in the DDR may be activated by additional receptor–ligand signalling loops and that checkpoint proteins likely have cellular roles outside the context of DNA damage. However, it will be important to explore these novel activation mechanisms in the context of genome instability. Ultimately, a comprehensive understanding of the cellular and molecular response to DNA damage will greatly impact our knowledge of cell biology in a wide variety of contexts, ranging from development to disease.
Acknowledgments
We apologise to authors whose publications have not been cited due to space limitations. We thank T. Weinert for critical reading of the manuscript. This work was supported by an Irish Research Council for Science, Engineering and Technology (IRCSET) grant to K.F., a Cancer Research Ireland project grant to M.G. (CR105GRE), the European Union FP6 Integrated Project “Radiosensitivity of Individuals and Susceptibility to Cancer induced by Ionising RADiations” contract number F16R-CT-2003-508842 and Science Foundation Ireland Principal Investigator award 07/IN1/B958 to N.F.L.
Conflict of interest
All co-authors have seen and agree with the contents of the manuscript. The authors certify that the submission is original work and is not under review at any other publication. The authors declare no conflict of interest.
Abbreviations
- ATM
Ataxia telangiectasia mutated
- ATR
ATM and Rad3-related
- ATRIP
ATR interacting protein
- BRCT
BRCA1 carboxyl terminal
- CAD
Chk1 activation domain
- CDK
Cyclin-dependent kinase
- DDK
Dbf4-dependent kinase
- DDR
DNA damage response
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- DSB
Double-strand break
- GCRs
Gross chromosomal rearrangements
- G1
Gap phase 1
- G2
Gap phase 2
- HR
Homologous recombination
- IR
Ionising radiation
- M
Mitosis
- MEN
Mitotic exit network
- MMS
Methyl methanesulfonate
- MRX/MRN
Mre11-Rad50-Xrs2/MRE11-RAD50-NBS1
- NHEJ
Non-homologous end joining
- PIKK
Phosphoinositide 3-kinase related kinase
- PTM
Post-translational modification
- RNR
Ribonucleotide reductase
- RPA
Replication protein A
- SCD
SQ/TQ cluster domain
- UV
Ultraviolet
Contributor Information
Noel Francis Lowndes, Phone: +353-91-495848, FAX: +353-91-495504, Email: noel.lowndes@nuigalway.ie.
Muriel Grenon, Email: muriel.grenon@nuigalway.ie.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Elledge SJ, Ciccia A. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 4.Weinert TA, Hartwell LH. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae . Science. 1988;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- 5.Lowndes NF, Murguia JR. Sensing and responding to DNA damage. Curr Opin Genet Dev. 2000;10(1):17–25. doi: 10.1016/S0959-437X(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408(6811):433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 7.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19(2):238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28(5):739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Clemenson C, Marsolier-Kergoat MC. DNA damage checkpoint inactivation: adaptation and recovery. DNA Repair (Amst) 2009;8(9):1101–1109. doi: 10.1016/j.dnarep.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg RA, Hanahan D. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Bartek J, Jackson SP. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartwell LH, Unger MW. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinert T, Hartwell L. Control of G2 delay by the rad9 gene of Saccharomyces cerevisiae . J Cell Sci. 1989;Suppl 12:145–148. doi: 10.1242/jcs.1989.supplement_12.12. [DOI] [PubMed] [Google Scholar]
- 14.Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic control of the cell division cycle in yeast: V. genetic analysis of cdc mutants. Genetics. 1973;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisby M, Rothstein R. Choreography of recombination proteins during the DNA damage response. DNA Repair (Amst) 2009;8(9):1068–1076. doi: 10.1016/j.dnarep.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan DO. The cell cycle: principles of control. Primers in biology. London: New Science; 2007. [Google Scholar]
- 17.Futcher B. Cyclins and the wiring of the yeast cell cycle. Yeast. 1996;12(16):1635–1646. doi: 10.1002/(SICI)1097-0061(199612)12:16<1635::AID-YEA83>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Bahler J. Cell-cycle control of gene expression in budding and fission yeast. Annu Rev Genet. 2005;39:69–94. doi: 10.1146/annurev.genet.39.110304.095808. [DOI] [PubMed] [Google Scholar]
- 19.Forsburg SL, Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- 20.Lim HH, Goh PY, Surana U. Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol Cell Biol. 1996;16(11):6385–6397. doi: 10.1128/mcb.16.11.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto A, Guacci V, Koshland D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133(1):99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425(6960):859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- 23.Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325(5948):1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enserink JM, Kolodner RD. An overview of Cdk1-controlled targets and processes. Cell Div. 2010;5:11. doi: 10.1186/1747-1028-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwell LH, Culotti J, Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci USA. 1970;66(2):352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartwell LH, Culotti J, Pringle JR, Reid BJ. Genetic control of the cell division cycle in yeast. Science. 1974;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- 27.Fitz Gerald JN, Benjamin JM, Kron SJ. Robust G1 checkpoint arrest in budding yeast: dependence on DNA damage signaling and repair. J Cell Sci. 2002;115(Pt 8):1749–1757. doi: 10.1242/jcs.115.8.1749. [DOI] [PubMed] [Google Scholar]
- 28.Siede W, Friedberg AS, Dianova I, Friedberg EC. Characterization of G1 checkpoint control in the yeast Saccharomyces cerevisiae following exposure to DNA-damaging agents. Genetics. 1994;138(2):271–281. doi: 10.1093/genetics/138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siede W, Friedberg AS, Friedberg EC. RAD9-dependent G1 arrest defines a second checkpoint for damaged DNA in the cell cycle of Saccharomyces cerevisiae . Proc Natl Acad Sci USA. 1993;90(17):7985–7989. doi: 10.1073/pnas.90.17.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siede W, Allen JB, Elledge SJ, Friedberg EC. The Saccharomyces cerevisiae MEC1 gene, which encodes a homolog of the human ATM gene product, is required for G1 arrest following radiation treatment. J Bacteriol. 1996;178(19):5841–5843. doi: 10.1128/jb.178.19.5841-5843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longhese MP, Clerici M, Lucchini G. The S-phase checkpoint and its regulation in Saccharomyces cerevisiae . Mutat Res. 2003;532(1–2):41–58. doi: 10.1016/j.mrfmmm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82(5):841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- 33.Lindahl T. Suppression of spontaneous mutagenesis in human cells by DNA base excision-repair. Mutat Res. 2000;462(2-3):129–135. doi: 10.1016/S1383-5742(00)00024-7. [DOI] [PubMed] [Google Scholar]
- 34.Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- 35.Lovejoy CA, Cortez D. Common mechanisms of PIKK regulation. DNA Repair (Amst) 2009;8(9):1004–1008. doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Critchlow SE, Jackson SP. DNA end-joining: from yeast to man. Trends Biochem Sci. 1998;23(10):394–398. doi: 10.1016/S0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K, Kodama S, Watanabe M. Recruitment of ATM protein to double strand DNA irradiated with ionizing radiation. J Biol Chem. 1999;274(36):25571–25575. doi: 10.1074/jbc.274.36.25571. [DOI] [PubMed] [Google Scholar]
- 38.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 39.Dart DA, Adams KE, Akerman I, Lakin ND. Recruitment of the cell cycle checkpoint kinase ATR to chromatin during S-phase. J Biol Chem. 2004;279(16):16433–16440. doi: 10.1074/jbc.M314212200. [DOI] [PubMed] [Google Scholar]
- 40.Cuadrado M, Martinez-Pastor B, Murga M, Toledo LI, Gutierrez-Martinez P, Lopez E, Fernandez-Capetillo O. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J Exp Med. 2006;203(2):297–303. doi: 10.1084/jem.20051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8(1):37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 42.Adams KE, Medhurst AL, Dart D, Lakin ND. Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene. 2006;25(28):3894–3904. doi: 10.1038/sj.onc.1209426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stracker TH, Usui T, Petrini JH. Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (Amst) 2009;8(9):1047–1054. doi: 10.1016/j.dnarep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge SJ. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science. 1999;286(5442):1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- 45.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9(4):297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 46.Bensimon A, Aebersold R, Shiloh Y. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett. 2011;585(11):1625–1639. doi: 10.1016/j.febslet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25(5):409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett CB, Lewis AL, Baldwin KK, Resnick MA. Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc Natl Acad Sci USA. 1993;90(12):5613–5617. doi: 10.1073/pnas.90.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae . Microbiol Mol Biol Rev. 1999;63(2):349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu D, Topper LM, Wilson TE. Recruitment and dissociation of nonhomologous end joining proteins at a DNA double-strand break in Saccharomyces cerevisiae . Genetics. 2008;178(3):1237–1249. doi: 10.1534/genetics.107.083535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97(5):621–633. doi: 10.1016/S0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 52.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118(6):699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Clerici M, Mantiero D, Guerini I, Lucchini G, Longhese MP. The Yku70-Yku80 complex contributes to regulate double-strand break processing and checkpoint activation during the cell cycle. EMBO Rep. 2008;9(8):810–818. doi: 10.1038/embor.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Hefferin ML, Chen L, Shim EY, Tseng HM, Kwon Y, Sung P, Lee SE, Tomkinson AE. Role of Dnl4-Lif1 in nonhomologous end-joining repair complex assembly and suppression of homologous recombination. Nat Struct Mol Biol. 2007;14(7):639–646. doi: 10.1038/nsmb1261. [DOI] [PubMed] [Google Scholar]
- 55.Shim EY, Chung WH, Nicolette ML, Zhang Y, Davis M, Zhu Z, Paull TT, Ira G, Lee SE. Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J. 2010;29(19):3370–3380. doi: 10.1038/emboj.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412(6847):607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 57.Gravel S, Larrivee M, Labrecque P, Wellinger RJ. Yeast Ku as a regulator of chromosomal DNA end structure. Science. 1998;280(5364):741–744. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- 58.Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72(1):131–142. doi: 10.1016/0092-8674(93)90057-W. [DOI] [PubMed] [Google Scholar]
- 59.Spagnolo L, Rivera-Calzada A, Pearl LH, Llorca O. Three-dimensional structure of the human DNA-PKcs/Ku70/Ku80 complex assembled on DNA and its implications for DNA DSB repair. Mol Cell. 2006;22(4):511–519. doi: 10.1016/j.molcel.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Weterings E, Verkaik NS, Bruggenwirth HT, Hoeijmakers JH, van Gent DC. The role of DNA dependent protein kinase in synapsis of DNA ends. Nucleic Acids Res. 2003;31(24):7238–7246. doi: 10.1093/nar/gkg889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiom K. Coping with DNA double strand breaks. DNA Repair (Amst) 2010;9(12):1256–1263. doi: 10.1016/j.dnarep.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Smith GC, Divecha N, Lakin ND, Jackson SP. DNA-dependent protein kinase and related proteins. Biochem Soc Symp. 1999;64:91–104. [PubMed] [Google Scholar]
- 63.Chen L, Trujillo K, Ramos W, Sung P, Tomkinson AE. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol Cell. 2001;8(5):1105–1115. doi: 10.1016/S1097-2765(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 64.Wiltzius JJ, Hohl M, Fleming JC, Petrini JH. The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nat Struct Mol Biol. 2005;12(5):403–407. doi: 10.1038/nsmb928. [DOI] [PubMed] [Google Scholar]
- 65.Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BA, Karcher A, Henderson B, Bodmer JL, McMurray CT, Carney JP, Petrini JH, Tainer JA. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418(6897):562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- 66.Lammens K, Bemeleit DJ, Mockel C, Clausing E, Schele A, Hartung S, Schiller CB, Lucas M, Angermuller C, Soding J, Strasser K, Hopfner KP. The Mre11: Rad50 structure shows an ATP-dependent molecular clamp in DNA double-strand break repair. Cell. 2011;145(1):54–66. doi: 10.1016/j.cell.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank-Vaillant M, Marcand S. Transient stability of DNA ends allows nonhomologous end joining to precede homologous recombination. Mol Cell. 2002;10(5):1189–1199. doi: 10.1016/S1097-2765(02)00705-0. [DOI] [PubMed] [Google Scholar]
- 68.Barlow JH, Lisby M, Rothstein R. Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol Cell. 2008;30(1):73–85. doi: 10.1016/j.molcel.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, Haber JE, Foiani M. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431(7011):1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23(24):4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature. 2008;455(7213):689–692. doi: 10.1038/nature07215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kadyk LC, Hartwell LH. Replication-dependent sister chromatid recombination in rad1 mutants of Saccharomyces cerevisiae . Genetics. 1993;133(3):469–487. doi: 10.1093/genetics/133.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44:113–139. doi: 10.1146/annurev-genet-051710-150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rupnik A, Lowndes NF, Grenon M. MRN and the race to the break. Chromosoma. 2010;119(2):115–135. doi: 10.1007/s00412-009-0242-4. [DOI] [PubMed] [Google Scholar]
- 76.Nakada D, Matsumoto K, Sugimoto K. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 2003;17(16):1957–1962. doi: 10.1101/gad.1099003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usui T, Ogawa H, Petrini JH. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol Cell. 2001;7(6):1255–1266. doi: 10.1016/S1097-2765(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 78.D’Amours D, Jackson SP. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev. 2001;15(17):2238–2249. doi: 10.1101/gad.208701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grenon M, Gilbert C, Lowndes NF. Checkpoint activation in response to double-strand breaks requires the Mre11/Rad50/Xrs2 complex. Nat Cell Biol. 2001;3(9):844–847. doi: 10.1038/ncb0901-844. [DOI] [PubMed] [Google Scholar]
- 80.Mantiero D, Clerici M, Lucchini G, Longhese MP. Dual role for Saccharomyces cerevisiae Tel1 in the checkpoint response to double-strand breaks. EMBO Rep. 2007;8(4):380–387. doi: 10.1038/sj.embor.7400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vialard JE, Gilbert CS, Green CM, Lowndes NF. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 1998;17(19):5679–5688. doi: 10.1093/emboj/17.19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrow DM, Tagle DA, Shiloh Y, Collins FS, Hieter P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell. 1995;82(5):831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 83.Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271(5247):357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 84.Fukunaga K, Kwon Y, Sung P, Sugimoto K. Activation of protein kinase Tel1 through recognition of protein-bound DNA ends. Mol Cell Biol. 2011;31(10):1959–1971. doi: 10.1128/MCB.05157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baroni E, Viscardi V, Cartagena-Lirola H, Lucchini G, Longhese MP. The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol Cell Biol. 2004;24(10):4151–4165. doi: 10.1128/MCB.24.10.4151-4165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clerici M, Mantiero D, Lucchini G, Longhese MP. The Saccharomyces cerevisiae Sae2 protein negatively regulates DNA damage checkpoint signalling. EMBO Rep. 2006;7(2):212–218. doi: 10.1038/sj.embor.7400593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 88.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434(7033):605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 89.You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25(13):5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308(5721):551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 91.Jazayeri A, Balestrini A, Garner E, Haber JE, Costanzo V. Mre11-Rad50-Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 2008;27(14):1953–1962. doi: 10.1038/emboj.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33(5):547–558. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Y, Xu Y, Roy K, Price BD. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol Cell Biol. 2007;27(24):8502–8509. doi: 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102(37):13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276(45):42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 96.Downs JA, Lowndes NF, Jackson SP. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature. 2000;408(6815):1001–1004. doi: 10.1038/35050000. [DOI] [PubMed] [Google Scholar]
- 97.Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64(7):2390–2396. doi: 10.1158/0008-5472.CAN-03-3207. [DOI] [PubMed] [Google Scholar]
- 98.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276(51):47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 99.Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, Haber JE, Lichten M. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol. 2004;14(19):1703–1711. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Downs JA, Nussenzweig MC, Nussenzweig A. Chromatin dynamics and the preservation of genetic information. Nature. 2007;447(7147):951–958. doi: 10.1038/nature05980. [DOI] [PubMed] [Google Scholar]
- 101.Costelloe T, Fitzgerald J, Murphy NJ, Flaus A, Lowndes NF. Chromatin modulation and the DNA damage response. Exp Cell Res. 2006;312(14):2677–2686. doi: 10.1016/j.yexcr.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 102.Rossetto D, Truman AW, Kron SJ, Cote J. Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin Cancer Res. 2010;16(18):4543–4552. doi: 10.1158/1078-0432.CCR-10-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y, Price BD. Chromatin dynamics and the repair of DNA double strand breaks. Cell Cycle. 2011;10(2):261–267. doi: 10.4161/cc.10.2.14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 105.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146(5):905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Redon C, Pilch DR, Rogakou EP, Orr AH, Lowndes NF, Bonner WM. Yeast histone 2A serine 129 is essential for the efficient repair of checkpoint-blind DNA damage. EMBO Rep. 2003;4(7):1–7. doi: 10.1038/sj.embor.embor871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toh GW, O’Shaughnessy AM, Jimeno S, Dobbie IM, Grenon M, Maffini S, O’Rorke A, Lowndes NF. Histone H2A phosphorylation and H3 methylation are required for a novel Rad9 DSB repair function following checkpoint activation. DNA Repair (Amst) 2006;5:693–703. doi: 10.1016/j.dnarep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 108.Hammet A, Magill C, Heierhorst J, Jackson SP. Rad9 BRCT domain interaction with phosphorylated H2AX regulates the G1 checkpoint in budding yeast. EMBO Rep. 2007;8(9):851–857. doi: 10.1038/sj.embor.7401036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Javaheri A, Wysocki R, Jobin-Robitaille O, Altaf M, Cote J, Kron SJ. Yeast G1 DNA damage checkpoint regulation by H2A phosphorylation is independent of chromatin remodeling. Proc Natl Acad Sci USA. 2006;103(37):13771–13776. doi: 10.1073/pnas.0511192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114(3):359–370. doi: 10.1016/S0092-8674(03)00566-X. [DOI] [PubMed] [Google Scholar]
- 111.Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, Eckhaus M, Ried T, Bonner WM, Nussenzweig A. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114(3):371–383. doi: 10.1016/S0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296(5569):922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie A, Puget N, Shim I, Odate S, Jarzyna I, Bassing CH, Alt FW, Scully R. Control of sister chromatid recombination by histone H2AX. Mol Cell. 2004;16(6):1017–1025. doi: 10.1016/j.molcel.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, Carpenter PB, Bonner WM, Chen J, Nussenzweig A. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4(12):993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 115.van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19(5):207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Huertas D, Sendra R, Munoz P. Chromatin dynamics coupled to DNA repair. Epigenetics. 2009;4(1):31–42. doi: 10.4161/epi.4.1.7733. [DOI] [PubMed] [Google Scholar]
- 117.Soutoglou E, Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320(5882):1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonilla CY, Melo JA, Toczyski DP. Colocalization of sensors is sufficient to activate the DNA damage checkpoint in the absence of damage. Mol Cell. 2008;30(3):267–276. doi: 10.1016/j.molcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5(7):675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 120.Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, Alt FW, Chen J. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21(2):187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 121.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123(7):1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 122.Al-Hakim A, Escribano-Diaz C, Landry MC, O’Donnell L, Panier S, Szilard RK, Durocher D. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair (Amst) 2010;9(12):1229–1240. doi: 10.1016/j.dnarep.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131(5):887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 124.Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131(5):901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318(5856):1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES, Nakada S, Ylanko J, Olivarius S, Mendez M, Oldreive C, Wildenhain J, Tagliaferro A, Pelletier L, Taubenheim N, Durandy A, Byrd PJ, Stankovic T, Taylor AM, Durocher D. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136(3):420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 127.Doil C, Mailand N, Bekker-Jensen S, Menard P, Larsen DH, Pepperkok R, Ellenberg J, Panier S, Durocher D, Bartek J, Lukas J, Lukas C. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136(3):435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 128.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276(18):14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 129.Bekker-Jensen S, Rendtlew Danielsen J, Fugger K, Gromova I, Nerstedt A, Lukas C, Bartek J, Lukas J, Mailand N. HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat Cell Biol. 2010;12(1):80–86. doi: 10.1038/ncb2008. [DOI] [PubMed] [Google Scholar]
- 130.Watanabe K, Iwabuchi K, Sun J, Tsuji Y, Tani T, Tokunaga K, Date T, Hashimoto M, Yamaizumi M, Tateishi S. RAD18 promotes DNA double-strand break repair during G1 phase through chromatin retention of 53BP1. Nucleic Acids Res. 2009;37(7):2176–2193. doi: 10.1093/nar/gkp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455(7214):770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134(6):981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lobachev KS, Gordenin DA, Resnick MA. The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell. 2002;108(2):183–193. doi: 10.1016/S0092-8674(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 134.Deng C, Brown JA, You D, Brown JM. Multiple endonucleases function to repair covalent topoisomerase I complexes in Saccharomyces cerevisiae . Genetics. 2005;170(2):591–600. doi: 10.1534/genetics.104.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lengsfeld BM, Rattray AJ, Bhaskara V, Ghirlando R, Paull TT. Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol Cell. 2007;28(4):638–651. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clerici M, Mantiero D, Lucchini G, Longhese MP. The Saccharomyces cerevisiae Sae2 protein promotes resection and bridging of double strand break ends. J Biol Chem. 2005;280(46):38631–38638. doi: 10.1074/jbc.M508339200. [DOI] [PubMed] [Google Scholar]
- 137.Dynan WS, Yoo S. Interaction of Ku protein and DNA-dependent protein kinase catalytic subunit with nucleic acids. Nucleic Acids Res. 1998;26(7):1551–1559. doi: 10.1093/nar/26.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mimitou EP, Symington LS (2011) DNA end resection-Unraveling the tail. DNA Repair (Amst). doi:10.1016/j.dnarep.2010.12.004 [DOI] [PMC free article] [PubMed]
- 139.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22(20):2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nicolette ML, Lee K, Guo Z, Rani M, Chow JM, Lee SE, Paull TT. Mre11-Rad50-Xrs2 and Sae2 promote 5′ strand resection of DNA double-strand breaks. Nat Struct Mol Biol. 2010;17(12):1478–1485. doi: 10.1038/nsmb.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mimitou EP, Symington LS. Ku prevents Exo1 and Sgs1-dependent resection of DNA ends in the absence of a functional MRX complex or Sae2. EMBO J. 2010;29(19):3358–3369. doi: 10.1038/emboj.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Engels K, Giannattasio M, Muzi-Falconi M, Lopes M, Ferrari S (2011) 14-3-3 Proteins regulate exonuclease 1-dependent processing of stalled replication forks. PLoS Genet 7(4):e1001367. doi:10.1371/journal.pgen.1001367 [DOI] [PMC free article] [PubMed]
- 143.Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, Ira G, Sung P. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae . Nature. 2010;467(7311):108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature. 2010;467(7311):112–116. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011;25(4):350–362. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci USA. 2008;105(44):16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Niu H, Raynard S, Sung P. Multiplicity of DNA end resection machineries in chromosome break repair. Genes Dev. 2009;23(13):1481–1486. doi: 10.1101/gad.1824209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Longhese MP, Bonetti D, Manfrini N, Clerici M. Mechanisms and regulation of DNA end resection. EMBO J. 2010;29(17):2864–2874. doi: 10.1038/emboj.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27(13):1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283(12):7713–7720. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 151.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450(7169):509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu X, Chen J. DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol Cell Biol. 2004;24(21):9478–9486. doi: 10.1128/MCB.24.21.9478-9486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu X, Fu S, Lai M, Baer R, Chen J. BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev. 2006;20(13):1721–1726. doi: 10.1101/gad.1431006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459(7245):460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huertas P, Jackson SP. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem. 2009;284(14):9558–9565. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen X, Niu H, Chung WH, Zhu Z, Papusha A, Shim EY, Lee SE, Sung P, Ira G. Cell cycle regulation of DNA double-strand break end resection by Cdk1-dependent Dna2 phosphorylation. Nat Struct Mol Biol. 2011;18(9):1015–1019. doi: 10.1038/nsmb.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee SE, Moore JK, Holmes A, Umezu K, Kolodner RD, Haber JE. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94(3):399–409. doi: 10.1016/S0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- 158.Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270(5241):1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- 159.Lazzaro F, Sapountzi V, Granata M, Pellicioli A, Vaze M, Haber JE, Plevani P, Lydall D, Muzi-Falconi M. Histone methyltransferase Dot1 and Rad9 inhibit single-stranded DNA accumulation at DSBs and uncapped telomeres. EMBO J. 2008;27(10):1502–1512. doi: 10.1038/emboj.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Postow L. Destroying the ring: Freeing DNA from Ku with ubiquitin. FEBS Lett. 2011;585(18):2876–2882. doi: 10.1016/j.febslet.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Couto CA, Wang HY, Green JC, Kiely R, Siddaway R, Borer C, Pears CJ, Lakin ND. PARP regulates nonhomologous end joining through retention of Ku at double-strand breaks. J Cell Biol. 2011;194(3):367–375. doi: 10.1083/jcb.201012132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paciotti V, Clerici M, Lucchini G, Longhese MP. The checkpoint protein Ddc2, functionally related to S. pombe Rad26, interacts with Mec1 and is regulated by Mec1-dependent phosphorylation in budding yeast. Genes Dev. 2000;14(16):2046–2059. [PMC free article] [PubMed] [Google Scholar]
- 163.Majka J, Niedziela-Majka A, Burgers PM. The checkpoint clamp activates Mec1 kinase during initiation of the DNA damage checkpoint. Mol Cell. 2006;24(6):891–901. doi: 10.1016/j.molcel.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294(5547):1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 165.Rouse J, Jackson SP. Lcd1p recruits Mec1p to DNA lesions in vitro and in vivo. Mol Cell. 2002;9(4):857–869. doi: 10.1016/S1097-2765(02)00507-5. [DOI] [PubMed] [Google Scholar]
- 166.Kondo T, Wakayama T, Naiki T, Matsumoto K, Sugimoto K. Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science. 2001;294(5543):867–870. doi: 10.1126/science.1063827. [DOI] [PubMed] [Google Scholar]
- 167.Melo JA, Cohen J, Toczyski DP. Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev. 2001;15(21):2809–2821. doi: 10.1101/gad.903501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Itakura E, Takai KK, Umeda K, Kimura M, Ohsumi M, Tamai K, Matsuura A. Amino-terminal domain of ATRIP contributes to intranuclear relocation of the ATR-ATRIP complex following DNA damage. FEBS Lett. 2004;577(1–2):289–293. doi: 10.1016/j.febslet.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 169.Wu X, Shell SM, Zou Y. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene. 2005;24(29):4728–4735. doi: 10.1038/sj.onc.1208674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dore AS, Kilkenny ML, Rzechorzek NJ, Pearl LH. Crystal structure of the rad9-rad1-hus1 DNA damage checkpoint complex–implications for clamp loading and regulation. Mol Cell. 2009;34(6):735–745. doi: 10.1016/j.molcel.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 171.Sohn SY, Cho Y. Crystal structure of the human rad9-hus1-rad1 clamp. J Mol Biol. 2009;390(3):490–502. doi: 10.1016/j.jmb.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 172.Green CM, Erdjument-Bromage H, Tempst P, Lowndes NF. A novel Rad24 checkpoint protein complex closely related to replication factor C. Curr Biol. 2000;10(1):39–42. doi: 10.1016/S0960-9822(99)00263-8. [DOI] [PubMed] [Google Scholar]
- 173.Majka J, Burgers PM. Yeast Rad17/Mec3/Ddc1: a sliding clamp for the DNA damage checkpoint. Proc Natl Acad Sci USA. 2003;100(5):2249–2254. doi: 10.1073/pnas.0437148100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Majka J, Binz SK, Wold MS, Burgers PM. Replication protein A directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J Biol Chem. 2006;281(38):27855–27861. doi: 10.1074/jbc.M605176200. [DOI] [PubMed] [Google Scholar]
- 175.Ellison V, Stillman B. Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol. 2003;1(2):E33. doi: 10.1371/journal.pbio.0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cimprich KA. Probing ATR activation with model DNA templates. Cell Cycle. 2007;6(19):2348–2354. doi: 10.4161/cc.6.19.4755. [DOI] [PubMed] [Google Scholar]
- 177.Navadgi-Patil VM, Burgers PM. The unstructured C-terminal tail of the 9–1-1 clamp subunit Ddc1 activates Mec1/ATR via two distinct mechanisms. Mol Cell. 2009;36(5):743–753. doi: 10.1016/j.molcel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paciotti V, Lucchini G, Plevani P, Longhese MP. Mec1p is essential for phosphorylation of the yeast DNA damage checkpoint protein Ddc1p, which physically interacts with Mec3p. EMBO J. 1998;17(14):4199–4209. doi: 10.1093/emboj/17.14.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de la Torre-Ruiz MA, Green CM, Lowndes NF. RAD9 and RAD24 define two additive, interacting branches of the DNA damage checkpoint pathway in budding yeast normally required for Rad53 modification and activation. EMBO J. 1998;17(9):2687–2698. doi: 10.1093/emboj/17.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brush GS, Kelly TJ. Phosphorylation of the replication protein A large subunit in the Saccharomyces cerevisiae checkpoint response. Nucleic Acids Res. 2000;28(19):3725–3732. doi: 10.1093/nar/28.19.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Navadgi-Patil VM, Burgers PM. Cell-cycle-specific activators of the Mec1/ATR checkpoint kinase. Biochem Soc Trans. 2011;39(2):600–605. doi: 10.1042/BST0390600. [DOI] [PubMed] [Google Scholar]
- 182.Navadgi-Patil VM, Burgers PM. A tale of two tails: activation of DNA damage checkpoint kinase Mec1/ATR by the 9–1-1 clamp and by Dpb11/TopBP1. DNA Repair (Amst) 2009;8(9):996–1003. doi: 10.1016/j.dnarep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee J, Kumagai A, Dunphy WG. The Rad9-Hus1-Rad1 checkpoint clamp regulates interaction of TopBP1 with ATR. J Biol Chem. 2007;282(38):28036–28044. doi: 10.1074/jbc.M704635200. [DOI] [PubMed] [Google Scholar]
- 184.Delacroix S, Wagner JM, Kobayashi M, Yamamoto K, Karnitz LM. The Rad9-Hus1-Rad1 (9–1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 2007;21(12):1472–1477. doi: 10.1101/gad.1547007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Takeishi Y, Ohashi E, Ogawa K, Masai H, Obuse C, Tsurimoto T. Casein kinase 2-dependent phosphorylation of human Rad9 mediates the interaction between human Rad9-Hus1-Rad1 complex and TopBP1. Genes Cells. 2010;15(7):761–771. doi: 10.1111/j.1365-2443.2010.01418.x. [DOI] [PubMed] [Google Scholar]
- 186.Rappas M, Oliver AW, Pearl LH. Structure and function of the Rad9-binding region of the DNA-damage checkpoint adaptor TopBP1. Nucleic Acids Res. 2011;39(1):313–324. doi: 10.1093/nar/gkq743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124(5):943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 188.Mordes DA, Glick GG, Zhao R, Cortez D. TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes Dev. 2008;22(11):1478–1489. doi: 10.1101/gad.1666208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mordes DA, Nam EA, Cortez D. Dpb11 activates the Mec1-Ddc2 complex. Proc Natl Acad Sci USA. 2008;105(48):18730–18734. doi: 10.1073/pnas.0806621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Navadgi-Patil VM, Burgers PM. Yeast DNA replication protein Dpb11 activates the Mec1/ATR checkpoint kinase. J Biol Chem. 2008;283(51):35853–35859. doi: 10.1074/jbc.M807435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pfander B, Diffley JF (2011) Dpb11 coordinates Mec1 kinase activation with cell cycle-regulated Rad9 recruitment. EMBO J. doi:10.1038/emboj.2011.345 [DOI] [PMC free article] [PubMed]
- 192.Schwartz MF, Duong JK, Sun Z, Morrow JS, Pradhan D, Stern DF. Rad9 phosphorylation sites couple Rad53 to the Saccharomyces cerevisiae DNA damage checkpoint. Mol Cell. 2002;9(5):1055–1065. doi: 10.1016/S1097-2765(02)00532-4. [DOI] [PubMed] [Google Scholar]
- 193.Emili A. MEC1-dependent phosphorylation of Rad9p in response to DNA damage. Mol Cell. 1998;2(2):183–189. doi: 10.1016/S1097-2765(00)80128-8. [DOI] [PubMed] [Google Scholar]
- 194.Gilbert CS, van den Bosch M, Green CM, Vialard JE, Grenon M, Erdjument-Bromage H, Tempst P, Lowndes NF. The budding yeast Rad9 checkpoint complex: chaperone proteins are required for its function. EMBO Rep. 2003;4(10):953–958. doi: 10.1038/sj.embor.embor935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gilbert CS, Green CM, Lowndes NF. Budding yeast Rad9 is an ATP-dependent Rad53 activating machine. Mol Cell. 2001;8(1):129–136. doi: 10.1016/S1097-2765(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 196.Granata M, Lazzaro F, Novarina D, Panigada D, Puddu F, Abreu CM, Kumar R, Grenon M, Lowndes NF, Plevani P, Muzi-Falconi M. Dynamics of Rad9 chromatin binding and checkpoint function are mediated by its dimerization and are cell cycle-regulated by CDK1 activity. PLoS Genet. 2010;6(8):e1001047. doi: 10.1371/journal.pgen.1001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grenon M, Costelloe T, Jimeno S, O’Shaughnessy A, Fitzgerald J, Zgheib O, Degerth L, Lowndes NF. Docking onto chromatin via the Saccharomyces cerevisiae Rad9 Tudor domain. Yeast. 2007;24(2):105–119. doi: 10.1002/yea.1441. [DOI] [PubMed] [Google Scholar]
- 198.Huyen Y, Zgheib O, Ditullio RA, Jr, Gorgoulis VG, Zacharatos P, Petty TJ, Sheston EA, Mellert HS, Stavridi ES, Halazonetis TD. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature. 2004;432(7015):406–411. doi: 10.1038/nature03114. [DOI] [PubMed] [Google Scholar]
- 199.Puddu F, Granata M, Di Nola L, Balestrini A, Piergiovanni G, Lazzaro F, Giannattasio M, Plevani P, Muzi-Falconi M. Phosphorylation of the budding yeast 9–1-1 complex is required for Dpb11 function in the full activation of the UV-induced DNA damage checkpoint. Mol Cell Biol. 2008;28(15):4782–4793. doi: 10.1128/MCB.00330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109(6):745–756. doi: 10.1016/S0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 201.Lacoste N, Utley RT, Hunter JM, Poirier GG, Cote J. Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J Biol Chem. 2002;277(34):30421–30424. doi: 10.1074/jbc.C200366200. [DOI] [PubMed] [Google Scholar]
- 202.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 203.Wysocki R, Javaheri A, Allard S, Sha F, Cote J, Kron SJ. Role of dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol. 2005;25(19):8430–8443. doi: 10.1128/MCB.25.19.8430-8443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Giannattasio M, Lazzaro F, Plevani P, Muzi-Falconi M. The DNA damage checkpoint response requires histone H2B ubiquitination by Rad6-Bre1 and H3 methylation by Dot1. J Biol Chem. 2005;280(11):9879–9886. doi: 10.1074/jbc.M414453200. [DOI] [PubMed] [Google Scholar]
- 205.Lancelot N, Charier G, Couprie J, Duband-Goulet I, Alpha-Bazin B, Quemeneur E, Ma E, Marsolier-Kergoat MC, Ropars V, Charbonnier JB, Miron S, Craescu CT, Callebaut I, Gilquin B, Zinn-Justin S. The checkpoint Saccharomyces cerevisiae Rad9 protein contains a tandem tudor domain that recognizes DNA. Nucleic Acids Res. 2007;35(17):5898–5912. doi: 10.1093/nar/gkm607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sweeney FD, Yang F, Chi A, Shabanowitz J, Hunt DF, Durocher D. Saccharomyces cerevisiae Rad9 acts as a Mec1 adaptor to allow Rad53 activation. Curr Biol. 2005;15(15):1364–1375. doi: 10.1016/j.cub.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 207.Du LL, Nakamura TM, Russell P. Histone modification-dependent and -independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev. 2006;20(12):1583–1596. doi: 10.1101/gad.1422606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sanders SL, Portoso M, Mata J, Bahler J, Allshire RC, Kouzarides T. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell. 2004;119(5):603–614. doi: 10.1016/j.cell.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 209.Fang J, Feng Q, Ketel CS, Wang H, Cao R, Xia L, Erdjument-Bromage H, Tempst P, Simon JA, Zhang Y. Purification and functional characterization of SET8, a nucleosomal histone H4-lysine 20-specific methyltransferase. Curr Biol. 2002;12(13):1086–1099. doi: 10.1016/S0960-9822(02)00924-7. [DOI] [PubMed] [Google Scholar]
- 210.Nishioka K, Rice JC, Sarma K, Erdjument-Bromage H, Werner J, Wang Y, Chuikov S, Valenzuela P, Tempst P, Steward R, Lis JT, Allis CD, Reinberg D. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell. 2002;9(6):1201–1213. doi: 10.1016/S1097-2765(02)00548-8. [DOI] [PubMed] [Google Scholar]
- 211.Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G. Structural basis for the methylation state-specific recognition of histone H4–K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127(7):1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Greeson NT, Sengupta R, Arida AR, Jenuwein T, Sanders SL. Di-methyl H4 lysine 20 targets the checkpoint protein Crb2 to sites of DNA damage. J Biol Chem. 2008;283(48):33168–33174. doi: 10.1074/jbc.M806857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kilkenny ML, Dore AS, Roe SM, Nestoras K, Ho JC, Watts FZ, Pearl LH. Structural and functional analysis of the Crb2-BRCT2 domain reveals distinct roles in checkpoint signaling and DNA damage repair. Genes Dev. 2008;22(15):2034–2047. doi: 10.1101/gad.472808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nakamura TM, Du LL, Redon C, Russell P. Histone H2A phosphorylation controls Crb2 recruitment at DNA breaks, maintains checkpoint arrest, and influences DNA repair in fission yeast. Mol Cell Biol. 2004;24(14):6215–6230. doi: 10.1128/MCB.24.14.6215-6230.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nakamura TM, Moser BA, Du LL, Russell P. Cooperative control of Crb2 by ATM family and Cdc2 kinases is essential for the DNA damage checkpoint in fission yeast. Mol Cell Biol. 2005;25(24):10721–10730. doi: 10.1128/MCB.25.24.10721-10730.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Furuya K, Poitelea M, Guo L, Caspari T, Carr AM. Chk1 activation requires Rad9 S/TQ-site phosphorylation to promote association with C-terminal BRCT domains of Rad4TOPBP1. Genes Dev. 2004;18(10):1154–1164. doi: 10.1101/gad.291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes Dev. 1997;11(24):3387–3400. doi: 10.1101/gad.11.24.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mochida S, Esashi F, Aono N, Tamai K, O’Connell MJ, Yanagida M. Regulation of checkpoint kinases through dynamic interaction with Crb2. EMBO J. 2004;23(2):418–428. doi: 10.1038/sj.emboj.7600018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang H, Elledge SJ. Genetic and physical interactions between DPB11 and DDC1 in the yeast DNA damage response pathway. Genetics. 2002;160(4):1295–1304. doi: 10.1093/genetics/160.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Germann SM, Oestergaard VH, Haas C, Salis P, Motegi A, Lisby M. Dpb11/TopBP1 plays distinct roles in DNA replication, checkpoint response and homologous recombination. DNA Repair (Amst) 2011;10(2):210–224. doi: 10.1016/j.dnarep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 221.Lee J, Dunphy WG. Rad17 plays a central role in establishment of the interaction between TopBP1 and the Rad9-Hus1-Rad1 complex at stalled replication forks. Mol Biol Cell. 2010;21(6):926–935. doi: 10.1091/mbc.E09-11-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteomics. 2008;7(7):1389–1396. doi: 10.1074/mcp.M700468-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Smolka MB, Albuquerque CP, Chen SH, Schmidt KH, Wei XX, Kolodner RD, Zhou H. Dynamic changes in protein–protein interaction and protein phosphorylation probed with amine-reactive isotope tag. Mol Cell Proteomics. 2005;4(9):1358–1369. doi: 10.1074/mcp.M500115-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ward IM, Minn K, van Deursen J, Chen J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol. 2003;23(7):2556–2563. doi: 10.1128/MCB.23.7.2556-2563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang B, Matsuoka S, Carpenter PB, Elledge SJ. 53BP1, a mediator of the DNA damage checkpoint. Science. 2002;298(5597):1435–1438. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 226.DiTullio RA, Jr, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J, Halazonetis TD. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol. 2002;4(12):998–1002. doi: 10.1038/ncb892. [DOI] [PubMed] [Google Scholar]
- 227.Wilson KA, Stern DF. NFBD1/MDC1, 53BP1 and BRCA1 have both redundant and unique roles in the ATM pathway. Cell Cycle. 2008;7(22):3584–3594. doi: 10.4161/cc.7.22.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee JH, Goodarzi AA, Jeggo PA, Paull TT. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 2010;29(3):574–585. doi: 10.1038/emboj.2009.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pei H, Zhang L, Luo K, Qin Y, Chesi M, Fei F, Bergsagel PL, Wang L, You Z, Lou Z. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature. 2011;470(7332):124–128. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421(6926):961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 231.Xu X, Stern DF. NFBD1/MDC1 regulates ionizing radiation-induced focus formation by DNA checkpoint signaling and repair factors. FASEB J. 2003;17(13):1842–1848. doi: 10.1096/fj.03-0310com. [DOI] [PubMed] [Google Scholar]
- 232.Cotta-Ramusino C, McDonald ER, 3rd, Hurov K, Sowa ME, Harper JW, Elledge SJ. A DNA damage response screen identifies RHINO, a 9–1-1 and TopBP1 interacting protein required for ATR signaling. Science. 2011;332(6035):1313–1317. doi: 10.1126/science.1203430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yamane K, Wu X, Chen J. A DNA damage-regulated BRCT-containing protein, TopBP1, is required for cell survival. Mol Cell Biol. 2002;22(2):555–566. doi: 10.1128/MCB.22.2.555-566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cescutti R, Negrini S, Kohzaki M, Halazonetis TD. TopBP1 functions with 53BP1 in the G1 DNA damage checkpoint. EMBO J. 2010;29(21):3723–3732. doi: 10.1038/emboj.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105(31):10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jowsey P, Morrice NA, Hastie CJ, McLauchlan H, Toth R, Rouse J. Characterisation of the sites of DNA damage-induced 53BP1 phosphorylation catalysed by ATM and ATR. DNA Repair (Amst) 2007;6(10):1536–1544. doi: 10.1016/j.dnarep.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 237.Van Hoof D, Munoz J, Braam SR, Pinkse MW, Linding R, Heck AJ, Mummery CL, Krijgsveld J. Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell. 2009;5(2):214–226. doi: 10.1016/j.stem.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 238.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127(3):635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 239.Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA, Gygi SP, Livingstone M, Zhang H, Polakiewicz RD, Comb MJ. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci USA. 2007;104(50):19855–19860. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Molina H, Horn DM, Tang N, Mathivanan S, Pandey A. Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci USA. 2007;104(7):2199–2204. doi: 10.1073/pnas.0611217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tao WA, Wollscheid B, O’Brien R, Eng JK, Li XJ, Bodenmiller B, Watts JD, Hood L, Aebersold R. Quantitative phosphoproteome analysis using a dendrimer conjugation chemistry and tandem mass spectrometry. Nat Methods. 2005;2(8):591–598. doi: 10.1038/nmeth776. [DOI] [PubMed] [Google Scholar]
- 242.Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PN, Bohmer FD, Gerke V, Schmidt-Arras DE, Berdel WE, Muller-Tidow C, Mann M, Serve H. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell. 2009;36(2):326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 243.Chen Y, Farmer AA, Chen CF, Jones DC, Chen PL, Lee WH. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996;56(14):3168–3172. [PubMed] [Google Scholar]
- 244.Ruffner H, Verma IM. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci USA. 1997;94(14):7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ruffner H, Jiang W, Craig AG, Hunter T, Verma IM. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol Cell Biol. 1999;19(7):4843–4854. doi: 10.1128/mcb.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chi Y, Welcker M, Hizli AA, Posakony JJ, Aebersold R, Clurman BE. Identification of CDK2 substrates in human cell lysates. Genome Biol. 2008;9(10):R149. doi: 10.1186/gb-2008-9-10-r149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cantin GT, Yi W, Lu B, Park SK, Xu T, Lee JD, Yates JR., 3rd Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J Proteome Res. 2008;7(3):1346–1351. doi: 10.1021/pr0705441. [DOI] [PubMed] [Google Scholar]
- 248.Sun Z, Hsiao J, Fay DS, Stern DF. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science. 1998;281(5374):272–274. doi: 10.1126/science.281.5374.272. [DOI] [PubMed] [Google Scholar]
- 249.Durocher D, Henckel J, Fersht AR, Jackson SP. The FHA domain is a modular phosphopeptide recognition motif. Mol Cell. 1999;4(3):387–394. doi: 10.1016/S1097-2765(00)80340-8. [DOI] [PubMed] [Google Scholar]
- 250.Schwartz MF, Lee S, Duong JK, Eminaga S, Stern DF. FHA domain-mediated DNA checkpoint regulation of Rad53. Cell Cycle. 2003;2(4):384–396. doi: 10.4161/cc.2.4.457. [DOI] [PubMed] [Google Scholar]
- 251.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 252.Usui T, Foster SS, Petrini JH. Maintenance of the DNA-damage checkpoint requires DNA-damage-induced mediator protein oligomerization. Mol Cell. 2009;33(2):147–159. doi: 10.1016/j.molcel.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Blankley RT, Lydall D. A domain of Rad9 specifically required for activation of Chk1 in budding yeast. J Cell Sci. 2004;117(Pt 4):601–608. doi: 10.1242/jcs.00907. [DOI] [PubMed] [Google Scholar]
- 254.Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg JM. A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae . Nature. 2000;403(6770):623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 255.Chen Y, Caldwell JM, Pereira E, Baker RW, Sanchez Y. ATRMec1 phosphorylation-independent activation of Chk1 in vivo. J Biol Chem. 2009;284(1):182–190. doi: 10.1074/jbc.M806530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tapia-Alveal C, Calonge TM, O’Connell MJ. Regulation of chk1. Cell Div. 2009;4:8. doi: 10.1186/1747-1028-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Alcasabas AA, Osborn AJ, Bachant J, Hu F, Werler PJ, Bousset K, Furuya K, Diffley JF, Carr AM, Elledge SJ. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3(11):958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 258.Foss EJ. Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae . Genetics. 2001;157(2):567–577. doi: 10.1093/genetics/157.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Osborn AJ, Elledge SJ. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 2003;17(14):1755–1767. doi: 10.1101/gad.1098303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Smolka MB, Chen SH, Maddox PS, Enserink JM, Albuquerque CP, Wei XX, Desai A, Kolodner RD, Zhou H. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J Cell Biol. 2006;175(5):743–753. doi: 10.1083/jcb.200605081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chen SH, Zhou H. Reconstitution of Rad53 activation by Mec1 through adaptor protein Mrc1. J Biol Chem. 2009;284(28):18593–18604. doi: 10.1074/jbc.M109.018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Katsuragi Y, Sagata N. Regulation of Chk1 kinase by autoinhibition and ATR-mediated phosphorylation. Mol Biol Cell. 2004;15(4):1680–1689. doi: 10.1091/mbc.E03-12-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Oe T, Nakajo N, Katsuragi Y, Okazaki K, Sagata N. Cytoplasmic occurrence of the Chk1/Cdc25 pathway and regulation of Chk1 in Xenopus oocytes. Dev Biol. 2001;229(1):250–261. doi: 10.1006/dbio.2000.9968. [DOI] [PubMed] [Google Scholar]
- 264.Chen P, Luo C, Deng Y, Ryan K, Register J, Margosiak S, Tempczyk-Russell A, Nguyen B, Myers P, Lundgren K, Kan CC, O’Connor PM, O’Connor PM. The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation. Cell. 2000;100(6):681–692. doi: 10.1016/S0092-8674(00)80704-7. [DOI] [PubMed] [Google Scholar]
- 265.Walker M, Black EJ, Oehler V, Gillespie DA, Scott MT. Chk1 C-terminal regulatory phosphorylation mediates checkpoint activation by de-repression of Chk1 catalytic activity. Oncogene. 2009;28(24):2314–2323. doi: 10.1038/onc.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Smits VA, Reaper PM, Jackson SP. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr Biol. 2006;16(2):150–159. doi: 10.1016/j.cub.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 267.Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, Nakanishi M. Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell. 2008;132(2):221–232. doi: 10.1016/j.cell.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 268.Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet. 2002;30(3):290–294. doi: 10.1038/ng845. [DOI] [PubMed] [Google Scholar]
- 269.Lee J, Kumagai A, Dunphy WG. Positive regulation of Wee1 by Chk1 and 14–3-3 proteins. Mol Biol Cell. 2001;12(3):551–563. doi: 10.1091/mbc.12.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Blasina A, de Weyer IV, Laus MC, Luyten WH, Parker AE, McGowan CH. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999;9(1):1–10. doi: 10.1016/S0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 271.Blasius M, Forment JV, Thakkar N, Wagner SA, Choudhary C, Jackson SP. A phospho-proteomic screen identifies substrates of the checkpoint kinase Chk1. Genome Biol. 2011;12(8):R78. doi: 10.1186/gb-2011-12-8-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sidorova JM, Breeden LL. Rad53-dependent phosphorylation of Swi6 and down-regulation of CLN1 and CLN2 transcription occur in response to DNA damage in Saccharomyces cerevisiae . Genes Dev. 1997;11(22):3032–3045. doi: 10.1101/gad.11.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sidorova JM, Breeden LL. Rad53 checkpoint kinase phosphorylation site preference identified in the Swi6 protein of Saccharomyces cerevisiae . Mol Cell Biol. 2003;23(10):3405–3416. doi: 10.1128/MCB.23.10.3405-3416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ghavidel A, Kislinger T, Pogoutse O, Sopko R, Jurisica I, Emili A. Impaired tRNA nuclear export links DNA damage and cell-cycle checkpoint. Cell. 2007;131(5):915–926. doi: 10.1016/j.cell.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 275.Schwob E, Bohm T, Mendenhall MD, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79(2):233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 276.Verma R, Annan RS, Huddleston MJ, Carr SA, Reynard G, Deshaies RJ. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science. 1997;278(5337):455–460. doi: 10.1126/science.278.5337.455. [DOI] [PubMed] [Google Scholar]
- 277.Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 278.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60(21):5934–5936. [PubMed] [Google Scholar]
- 279.Ahn JY, Li X, Davis HL, Canman CE. Phosphorylation of threonine 68 promotes oligomerization and autophosphorylation of the Chk2 protein kinase via the forkhead-associated domain. J Biol Chem. 2002;277(22):19389–19395. doi: 10.1074/jbc.M200822200. [DOI] [PubMed] [Google Scholar]
- 280.Oliver AW, Paul A, Boxall KJ, Barrie SE, Aherne GW, Garrett MD, Mittnacht S, Pearl LH. Trans-activation of the DNA-damage signalling protein kinase Chk2 by T-loop exchange. EMBO J. 2006;25(13):3179–3190. doi: 10.1038/sj.emboj.7601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288(5470):1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 282.Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410(6830):842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- 283.Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994;13(18):4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281(5383):1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 285.Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 1999;18(24):7002–7010. doi: 10.1093/emboj/18.24.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14(3):278–288. [PMC free article] [PubMed] [Google Scholar]
- 287.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 288.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 289.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287(5459):1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 290.Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA. 1999;96(26):14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen L, Gilkes DM, Pan Y, Lane WS, Chen J. ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 2005;24(19):3411–3422. doi: 10.1038/sj.emboj.7600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science. 2001;292(5523):1910–1915. doi: 10.1126/science.1058637. [DOI] [PubMed] [Google Scholar]
- 293.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 294.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12(6):676–684. doi: 10.1016/S0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 295.Segurado M, Tercero JA. The S-phase checkpoint: targeting the replication fork. Biol Cell. 2009;101(11):617–627. doi: 10.1042/BC20090053. [DOI] [PubMed] [Google Scholar]
- 296.Zegerman P, Diffley JF. DNA replication as a target of the DNA damage checkpoint. DNA Repair (Amst) 2009;8(9):1077–1088. doi: 10.1016/j.dnarep.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 297.Myung K, Kolodner RD. Suppression of genome instability by redundant S-phase checkpoint pathways in Saccharomyces cerevisiae . Proc Natl Acad Sci USA. 2002;99(7):4500–4507. doi: 10.1073/pnas.062702199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Puddu F, Piergiovanni G, Plevani P, Muzi-Falconi M (2011) Sensing of replication stress and Mec1 activation act through two independent pathways involving the 9-1-1 complex and DNA polymerase epsilon. PLoS Genet 7(3):e1002022. doi:10.1371/journal.pgen.1002022 [DOI] [PMC free article] [PubMed]
- 299.Paulovich AG, Margulies RU, Garvik BM, Hartwell LH. RAD9, RAD17, and RAD24 are required for S phase regulation in Saccharomyces cerevisiae in response to DNA damage. Genetics. 1997;145(1):45–62. doi: 10.1093/genetics/145.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412(6846):553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- 301.Tercero JA, Longhese MP, Diffley JF. A central role for DNA replication forks in checkpoint activation and response. Mol Cell. 2003;11(5):1323–1336. doi: 10.1016/S1097-2765(03)00169-2. [DOI] [PubMed] [Google Scholar]
- 302.Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412(6846):557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- 303.Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395(6702):615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- 304.Shirahige K, Hori Y, Shiraishi K, Yamashita M, Takahashi K, Obuse C, Tsurimoto T, Yoshikawa H. Regulation of DNA-replication origins during cell-cycle progression. Nature. 1998;395(6702):618–621. doi: 10.1038/27007. [DOI] [PubMed] [Google Scholar]
- 305.Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 1994;8(20):2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- 306.Longhese MP, Neecke H, Paciotti V, Lucchini G, Plevani P. The 70 kDa subunit of replication protein A is required for the G1/S and intra-S DNA damage checkpoints in budding yeast. Nucleic Acids Res. 1996;24(18):3533–3537. doi: 10.1093/nar/24.18.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Brush GS, Morrow DM, Hieter P, Kelly TJ. The ATM homologue MEC1 is required for phosphorylation of replication protein A in yeast. Proc Natl Acad Sci USA. 1996;93(26):15075–15080. doi: 10.1073/pnas.93.26.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Marini F, Pellicioli A, Paciotti V, Lucchini G, Plevani P, Stern DF, Foiani M. A role for DNA primase in coupling DNA replication to DNA damage response. EMBO J. 1997;16(3):639–650. doi: 10.1093/emboj/16.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Longhese MP, Fraschini R, Plevani P, Lucchini G. Yeast pip3/mec3 mutants fail to delay entry into S phase and to slow DNA replication in response to DNA damage, and they define a functional link between Mec3 and DNA primase. Mol Cell Biol. 1996;16(7):3235–3244. doi: 10.1128/mcb.16.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Navas TA, Zhou Z, Elledge SJ. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80(1):29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 311.Lou H, Komata M, Katou Y, Guan Z, Reis CC, Budd M, Shirahige K, Campbell JL. Mrc1 and DNA polymerase epsilon function together in linking DNA replication and the S phase checkpoint. Mol Cell. 2008;32(1):106–117. doi: 10.1016/j.molcel.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010;24(12):1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature. 2010;467(7314):479–483. doi: 10.1038/nature09377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zegerman P, Diffley JF. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature. 2010;467(7314):474–478. doi: 10.1038/nature09373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature. 2007;445(7125):328–332. doi: 10.1038/nature05465. [DOI] [PubMed] [Google Scholar]
- 316.Zegerman P, Diffley JF. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature. 2007;445(7125):281–285. doi: 10.1038/nature05432. [DOI] [PubMed] [Google Scholar]
- 317.Segurado M, Diffley JF. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 2008;22(13):1816–1827. doi: 10.1101/gad.477208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Morin I, Ngo HP, Greenall A, Zubko MK, Morrice N, Lydall D. Checkpoint-dependent phosphorylation of Exo1 modulates the DNA damage response. EMBO J. 2008;27(18):2400–2410. doi: 10.1038/emboj.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Cotta-Ramusino C, Fachinetti D, Lucca C, Doksani Y, Lopes M, Sogo J, Foiani M. Exo1 processes stalled replication forks and counteracts fork reversal in checkpoint-defective cells. Mol Cell. 2005;17(1):153–159. doi: 10.1016/j.molcel.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 320.Smolka MB, Albuquerque CP, Chen SH, Zhou H. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc Natl Acad Sci USA. 2007;104(25):10364–10369. doi: 10.1073/pnas.0701622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.de la Torre Ruiz MA, Lowndes NF. DUN1 defines one branch downstream of RAD53 for transcription and DNA damage repair in Saccharomyces cerevisiae . FEBS Lett. 2000;485(2–3):205–206. doi: 10.1016/S0014-5793(00)02198-0. [DOI] [PubMed] [Google Scholar]
- 322.Zhou Z, Elledge SJ. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75(6):1119–1127. doi: 10.1016/0092-8674(93)90321-G. [DOI] [PubMed] [Google Scholar]
- 323.Chen SH, Smolka MB, Zhou H. Mechanism of Dun1 activation by Rad53 phosphorylation in Saccharomyces cerevisiae . J Biol Chem. 2007;282(2):986–995. doi: 10.1074/jbc.M609322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Huang M, Zhou Z, Elledge SJ. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell. 1998;94(5):595–605. doi: 10.1016/S0092-8674(00)81601-3. [DOI] [PubMed] [Google Scholar]
- 325.Zhao X, Rothstein R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc Natl Acad Sci USA. 2002;99(6):3746–3751. doi: 10.1073/pnas.062502299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Lee YD, Wang J, Stubbe J, Elledge SJ. Dif1 is a DNA-damage-regulated facilitator of nuclear import for ribonucleotide reductase. Mol Cell. 2008;32(1):70–80. doi: 10.1016/j.molcel.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zachos G, Rainey MD, Gillespie DA. Chk1-dependent S-M checkpoint delay in vertebrate cells is linked to maintenance of viable replication structures. Mol Cell Biol. 2005;25(2):563–574. doi: 10.1128/MCB.25.2.563-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zachos G, Rainey MD, Gillespie DA. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 2003;22(3):713–723. doi: 10.1093/emboj/cdg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Trenz K, Smith E, Smith S, Costanzo V. ATM and ATR promote Mre11 dependent restart of collapsed replication forks and prevent accumulation of DNA breaks. EMBO J. 2006;25(8):1764–1774. doi: 10.1038/sj.emboj.7601045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Grallert B, Boye E. The multiple facets of the intra-S checkpoint. Cell Cycle. 2008;7(15):2315–2320. doi: 10.4161/cc.6389. [DOI] [PubMed] [Google Scholar]
- 332.Lee J, Kumagai A, Dunphy WG. Claspin, a Chk1-regulatory protein, monitors DNA replication on chromatin independently of RPA, ATR, Rad17. Mol Cell. 2003;11(2):329–340. doi: 10.1016/S1097-2765(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 333.Guo Z, Kumagai A, Wang SX, Dunphy WG. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14(21):2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6(4):839–849. doi: 10.1016/S1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 335.Chini CC, Chen J. Human claspin is required for replication checkpoint control. J Biol Chem. 2003;278(32):30057–30062. doi: 10.1074/jbc.M301136200. [DOI] [PubMed] [Google Scholar]
- 336.Gotter AL, Suppa C, Emanuel BS. Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork-associated factors. J Mol Biol. 2007;366(1):36–52. doi: 10.1016/j.jmb.2006.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Unsal-Kacmaz K, Chastain PD, Qu PP, Minoo P, Cordeiro-Stone M, Sancar A, Kaufmann WK. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol. 2007;27(8):3131–3142. doi: 10.1128/MCB.02190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol. 2005;25(8):3109–3116. doi: 10.1128/MCB.25.8.3109-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kemp MG, Akan Z, Yilmaz S, Grillo M, Smith-Roe SL, Kang TH, Cordeiro-Stone M, Kaufmann WK, Abraham RT, Sancar A, Unsal-Kacmaz K. Tipin-replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress. J Biol Chem. 2010;285(22):16562–16571. doi: 10.1074/jbc.M110.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277(5331):1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 341.Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277(5331):1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 342.Sorensen CS, Syljuasen RG, FalckSchroeder J, Ronnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover, ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3(3):247–258. doi: 10.1016/S1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 343.Liu P, Barkley LR, Day T, Bi X, Slater DM, Alexandrow MG, Nasheuer HP, Vaziri C. The Chk1-mediated S-phase checkpoint targets initiation factor Cdc45 via a Cdc25A/Cdk2-independent mechanism. J Biol Chem. 2006;281(41):30631–30644. doi: 10.1074/jbc.M602982200. [DOI] [PubMed] [Google Scholar]
- 344.Kim ST, Xu B, Kastan MB. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 2002;16(5):560–570. doi: 10.1101/gad.970602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 2002;16(5):571–582. doi: 10.1101/gad.970702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kitagawa R, Bakkenist CJ, McKinnon PJ, Kastan MB. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 2004;18(12):1423–1438. doi: 10.1101/gad.1200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Luo H, Li Y, Mu JJ, Zhang J, Tonaka T, Hamamori Y, Jung SY, Wang Y, Qin J. Regulation of intra-S phase checkpoint by ionizing radiation (IR)-dependent and IR-independent phosphorylation of SMC3. J Biol Chem. 2008;283(28):19176–19183. doi: 10.1074/jbc.M802299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Krek W, Nigg EA. Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J. 1991;10(2):305–316. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 351.Lee MS, Enoch T, Piwnica-Worms H. mik1 + encodes a tyrosine kinase that phosphorylates p34cdc2 on tyrosine 15. J Biol Chem. 1994;269(48):30530–30537. [PubMed] [Google Scholar]
- 352.Parker LL, Atherton-Fessler S, Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci USA. 1992;89(7):2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Den Haese GJ, Walworth N, Carr AM, Gould KL. The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2. Mol Biol Cell. 1995;6(4):371–385. doi: 10.1091/mbc.6.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 355.Raleigh JM, O’Connell MJ. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J Cell Sci. 2000;113(Pt 10):1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- 356.O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16(3):545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11(4):504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 358.Zeng Y, Piwnica-Worms H. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14–3-3 binding. Mol Cell Biol. 1999;19(11):7410–7419. doi: 10.1128/mcb.19.11.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395(6701):507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- 360.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14–3-3 protein. Nature. 1999;397(6715):172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 361.Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355(6358):368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- 362.Sorger PK, Murray AW. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992;355(6358):365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- 363.Cohen-Fix O, Koshland D. The metaphase-to-anaphase transition: avoiding a mid-life crisis. Curr Opin Cell Biol. 1997;9(6):800–806. doi: 10.1016/S0955-0674(97)80080-4. [DOI] [PubMed] [Google Scholar]
- 364.Gardner R, Putnam CW, Weinert T. RAD53, DUN1 and PDS1 define two parallel G2/M checkpoint pathways in budding yeast. EMBO J. 1999;18(11):3173–3185. doi: 10.1093/emboj/18.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Liang F, Wang Y. DNA damage checkpoints inhibit mitotic exit by two different mechanisms. Mol Cell Biol. 2007;27(14):5067–5078. doi: 10.1128/MCB.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93(6):1067–1076. doi: 10.1016/S0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- 367.Wang H, Liu D, Wang Y, Qin J, Elledge SJ. Pds1 phosphorylation in response to DNA damage is essential for its DNA damage checkpoint function. Genes Dev. 2001;15(11):1361–1372. doi: 10.1101/gad.893201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Agarwal R, Tang Z, Yu H, Cohen-Fix O. Two distinct pathways for inhibiting pds1 ubiquitination in response to DNA damage. J Biol Chem. 2003;278(45):45027–45033. doi: 10.1074/jbc.M306783200. [DOI] [PubMed] [Google Scholar]
- 369.Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8(9):497–507. doi: 10.1016/S0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- 370.Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae . EMBO J. 1998;17(5):1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Schleker T, Shimada K, Sack R, Pike BL, Gasser SM. Cell cycle-dependent phosphorylation of Rad53 kinase by Cdc5 and Cdc28 modulates checkpoint adaptation. Cell Cycle. 2010;9(2):350–363. doi: 10.4161/cc.9.2.10448. [DOI] [PubMed] [Google Scholar]
- 372.Vidanes GM, Sweeney FD, Galicia S, Cheung S, Doyle JP, Durocher D, Toczyski DP. CDC5 inhibits the hyperphosphorylation of the checkpoint kinase Rad53, leading to checkpoint adaptation. PLoS Biol. 2010;8(1):e1000286. doi: 10.1371/journal.pbio.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Donnianni RA, Ferrari M, Lazzaro F, Clerici M, Tamilselvan Nachimuthu B, Plevani P, Muzi-Falconi M, Pellicioli A. Elevated levels of the polo kinase Cdc5 override the Mec1/ATR checkpoint in budding yeast by acting at different steps of the signaling pathway. PLoS Genet. 2010;6(1):e1000763. doi: 10.1371/journal.pgen.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Cheng L, Hunke L, Hardy CF. Cell cycle regulation of the Saccharomyces cerevisiae polo-like kinase cdc5p. Mol Cell Biol. 1998;18(12):7360–7370. doi: 10.1128/mcb.18.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ferreira MG, Cooper JP. Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev. 2004;18(18):2249–2254. doi: 10.1101/gad.315804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhang Y, Shim EY, Davis M, Lee SE. Regulation of repair choice: Cdk1 suppresses recruitment of end joining factors at DNA breaks. DNA Repair (Amst) 2009;8(10):1235–1241. doi: 10.1016/j.dnarep.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Bensimon A, Schmidt A, Ziv Y, Elkon R, Wang SY, Chen DJ, Aebersold R, Shiloh Y (2010) ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci Signal 3(151):rs3. doi:10.1126/scisignal.2001034 [DOI] [PubMed]
- 378.Bennetzen MV, Larsen DH, Bunkenborg J, Bartek J, Lukas J, Andersen JS. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol Cell Proteomics. 2010;9(6):1314–1323. doi: 10.1074/mcp.M900616-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11(9):644–656. doi: 10.1038/nrc3107. [DOI] [PubMed] [Google Scholar]
- 380.Wouters MD, van Gent DC, Hoeijmakers JH, Pothof J (2011) MicroRNAs, the DNA damage response and cancer. Mutat Res. doi:10.1016/j.mrfmmm.2011.03.012 [DOI] [PubMed]
- 381.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39(5):673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 382.Pothof J, Verkaik NS, van IW, Wiemer EA, Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Persengiev SP. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J. 2009;28(14):2090–2099. doi: 10.1038/emboj.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Bu Y, Lu C, Bian C, Wang J, Li J, Zhang B, Li Z, Brewer G, Zhao RC. Knockdown of Dicer in MCF-7 human breast carcinoma cells results in G1 arrest and increased sensitivity to cisplatin. Oncol Rep. 2009;21(1):13–17. [PubMed] [Google Scholar]
- 384.Moskwa P, Buffa FM, Pan Y, Panchakshari R, Gottipati P, Muschel RJ, Beech J, Kulshrestha R, Abdelmohsen K, Weinstock DM, Gorospe M, Harris AL, Helleday T, Chowdhury D. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell. 2011;41(2):210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Dimitrova N, Chen YC, Spector DL, de Lange T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature. 2008;456(7221):524–528. doi: 10.1038/nature07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Difilippantonio S, Gapud E, Wong N, Huang CY, Mahowald G, Chen HT, Kruhlak MJ, Callen E, Livak F, Nussenzweig MC, Sleckman BP, Nussenzweig A. 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature. 2008;456(7221):529–533. doi: 10.1038/nature07476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Noon AT, Shibata A, Rief N, Lobrich M, Stewart GS, Jeggo PA, Goodarzi AA. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol. 2010;12(2):177–184. doi: 10.1038/ncb2017. [DOI] [PubMed] [Google Scholar]
- 388.Zhu Q, Pao GM, Huynh AM, Suh H, Tonnu N, Nederlof PM, Gage FH, Verma IM. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477(7363):179–184. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460(7259):1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El Manira A, Ibanez CF, Ernfors P. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451(7177):460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]