Abstract
The role of intercellular communication in the regulation of bacterial multicellular behavior has received widespread attention, and a variety of signal molecules involved in bacterial communication have been discovered. In addition to the N-acyl-homoserine lactones, 4-hydroxy-2-alkylquinolines (HAQs), including the Pseudomonas quinolone signal, have been shown to function as signal molecules in Pseudomonas aeruginosa. In this study we unraveled the biosynthetic pathway of HAQs using feeding experiments with isotope-labeled precursors and analysis of extracted HAQs by gas chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy. Our results show that the biosynthesis of various HAQ metabolites is directed via a common metabolic pathway involving a “head-to-head” condensation of anthranilic acid and β-keto fatty acids. Moreover, we provide evidence that the β-keto-(do)decanoic acids, crucial for the biosynthesis of the heptyl and nonyl derivatives of the 4-hydroxyquinolines in P. aeruginosa, are at least in part derived from a common pool of β-hydroxy(do)decanoic acids involved in rhamnolipid biosynthesis.
Pseudomonas aeruginosa is a ubiquitous gram-negative bacterial pathogen that causes infections not only in human hosts but also in animals and even plants. This pathogen has been identified as one of the leading causes of nosocomial infections (4, 16, 23) and is responsible for fatal chronic lung infections in patients with cystic fibrosis (CF). P. aeruginosa coordinates its population behavior, such as biofilm formation (6, 7, 11) and virulence factor production, by means of small extracellular signal molecules, so-called autoinducers, that are released into the environment under appropriate conditions (15, 19, 21). Since intercellular communication leads to cooperative and coordinated bacterial behavior in a cell density-dependent manner, it is referred to as quorum sensing (2, 8, 18). A common feature of intercellular communication is the transcriptional activation of quorum-sensing-controlled genes when the bacterial signal molecules reach a certain threshold. P. aeruginosa produces two major cell-to-cell signals that are members of the acyl-homoserine lactone signal family, N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-homoserine lactone) and N-butyryl-l-homoserine lactone (C4-homoserine lactone). In addition to the two acyl-homoserine lactone-type autoinducers, signal molecules belonging to the family of 4-hydroxy-2-alkylquinolines (HAQs) (9) have been identified, which include in addition to N oxides (exhibiting antimicrobial activities) 3,4-hydroxy-2-heptylquinoline (PQS) (20) and 4-hydroxy-2-heptylquinoline. The latter two molecules have been shown to be involved in intracellular communication.
There is growing global concern that multiresistant pathogenic bacteria are emerging and will gradually render antimicrobial treatment ineffective. Hence, there is an urgent need for the development of novel therapeutic approaches for the treatment of bacterial infections. An alternative to growth inhibition, which is the mode of action of most traditional antibiotics, could be the attenuation of virulence factor production. In this approach, cell-to-cell signals have been recognized as promising targets for alternative therapeutic strategies that decrease bacterial virulence (13, 14, 24) as significant amounts of signal molecules have been detected at sites of infection in vivo (5, 17). Analysis of the underlying metabolic events of intercellular bacterial communication and elucidation of the biosynthesis of the signal molecules might contribute to our understanding and provide an opportunity to interfere with the control of virulence factor production. Previous studies strongly suggested that anthranilic acid is a precursor of PQS, and it was demonstrated that inhibition of anthranilic acid resulted in a loss of PQS production (1). To produce PQS, a condensation of anthranilic acid and a β-ketodecanoic acid in a multistep reaction was proposed. More recently, anthranilic acid has been shown to be a common precursor of HAQs, including PQS, 4-hydroxy-2-heptylquinoline, and N oxides (9). Moreover, a PQS biosynthetic gene cluster has been identified (10). This pqsABCDE operon codes for a putative coenzyme A ligase (pqsA), two β-keto-acyl-acyl carrier protein synthases (pqsB, pqsC), and a FabH1 homologous transacetylase (pqsD), whereas pqsE seems to encode a response effector protein which itself is not involved in the biosynthesis of PQS. Although it has clearly been demonstrated that the pqsABCD genes are essential for PQS biosynthesis, their enzymatic function remains to be elucidated. In this study we used feeding experiments with labeled isotopes, and we confirmed by gas chromatography (GC)-mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy that P. aeruginosa synthesizes HAQs via a common biosynthetic pathway involving the “head-to-head” condensation of anthranilic acid and β-keto fatty acids. Moreover, PQS biosynthesis seems to be dependent not only on an intact pqsABCD operon but also on the availability of β-keto acids. Interestingly, at least some of these acids seem to be derived from a common pool of β-hydroxy-keto acids involved in rhamnolipid biosynthesis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Clinical P. aeruginosa strain SCV 20265, isolated from the respiratory tract of a CF patient who attended the cystic fibrosis clinic at Hanover Medical School, Hanover, Germany (12), was used in this study. This P. aeruginosa strain produced large amounts of HAQs and therefore was especially suitable for the feeding experiments with labeled precursors. P. aeruginosa was routinely cultured at 37°C on Columbia or Luria-Bertani (LB) agar. Transposon mutants of SCV 20265 with an insertion in the carB, pyrB, or pyrD gene that was generated using the transposon construction vector EZ:TN pMOD-2 (Epicentre) were grown in LB medium supplemented with 50 μg/ml gentamicin. The PAO1 wild-type strain and the rhlG:Tc mutant strain used in this study were kindly provided by G. Soberon-Chavez (Departmento de Microbiologia Molecular, Universidad Nacional Autonoma de Mexico) (3).
Broth cultures of P. aeruginosa SCV 20265 and transposon mutants affected in genes of the pyrimidine metabolic pathway were grown in LB medium containing in some cases, as indicated below, either 5 mM orotic acid or 5 mM UMP.
Extraction of extracellular P. aeruginosa HAQ metabolites and thin-layer chromatography (TLC).
To isolate HAQ metabolites, P. aeruginosa cultures grown on agar plates were harvested and suspended in methanol. Following a centrifugation step and evaporation of the methanol to completion, the dried residue was washed several times with distilled water. Alternatively, the secondary metabolites were extracted from P. aeruginosa broth cultures with dichloromethane. Briefly, the bacterial cultures were extracted with 1 volume of dichloromethane by vigorous shaking. After centrifugation at 2,000 × g for 15 min the lower organic layer was evaporated.
TLC was performed using a Silica Gel 60 F254 TLC plate. The extracted P. aeruginosa material was dissolved in methanol and separated by TLC using tert-butyl methyl ether-n-hexane (10:1) as the solvent. Fluorescent spots were visualized under UV light and photographed. 4-Hydroxy-2-heptylquinoline was chemically synthesized as described previously (22). Starting from 4-hydroxy-2-heptylquinoline, PQS was synthesized by the procedures described by Pesci et al. (20). The two HAQs and 4-hydroxy-2-heptylquinoline-N-oxide, the latter purchased from Sigma (St. Louis, MO), were used as standards in TLC.
Isotope labeling.
Isotope-labeled anthranilic acid (15N; 1 mg/ml) or [1,2-13C]acetate (2 mg/ml) was added to a bacterial culture that had been grown for 24 h on 0.2% agarose in modified Vogel-Bonner medium (3.3 mM MgSO4, 10 mM citric acid, 28 mM NaNH4HPO4, 37 mM K2HPO4, 214 mM d-gluconic acid; pH 7.4). The plates were incubated for another 48 h before HAQs were methanol extracted. The anthranilic acid-labeled samples were subjected to GC-MS, whereas the 13C-labeled samples were subjected to one-dimensional (1D) 13C NMR spectroscopy.
To increase the 13C label of extracted HAQs, P. aeruginosa SCV 20265 was cultured by using 0.2% agarose in modified Vogel-Bonner medium without d-gluconic acid supplemented with 2 mg/ml [1,2-13C]acetate, [1-13C]acetate, or [2-13C]acetate with and without 5 mM orotic acid or 10 mg β-ketodecanoic fatty acid. In order to avoid 13C incorporation into the HAQ moiety originating from anthranilic acid, 1 mg/ml of unlabeled anthranilic acid was added to the medium in these experiments. The plates were incubated for 3 days, and the HAQs were methanol extracted and subjected to TLC and GC—MS-MS.
GC-MS analysis.
4-Hydroxy-2-alkylquinoline derivatives were analyzed after trimethylsilylation (50% pyridine—50% BSTFA [bistrimethylsilyltrifluoroacetamide] containing 1% TMC [trimethylchlorosilane]), (70°C, 1 h) with a Thermo-Finnigan GCQ ion trap mass spectrometer (Finnigan MAT Corp., San Jose, CA) running in the positive-ion electron impact (EI) mode equipped with a 30-m DB5 capillary column. For determination of the exact positions of 13C and 15N atoms after stable isotope labeling studies, MS-MS experiments were performed after isolation of the respective monoisotopic parent ions using excitation energies of ca. 2.0 eV.
NMR spectroscopy.
NMR spectra of the purified selectively 13C-labeled 4-hydroxy-2-alkylquinolines (1D 1H and 13C) and of unlabeled synthetic material (1D 1H and 13C and two-dimensional heteronuclear multiple bond correlation) were recorded at 300 K with Bruker DPX 300 and APX 400 NMR spectrometers locked to the major deuterium resonance of the solvent, CD3OD.
Extraction of P. aeruginosa rhamnolipids.
For isolation of rhamnolipids, P. aeruginosa cultures were grown on soft agar (0.2% agarose [Difco]) in normal Vogel-Bonner medium or Vogel-Bonner medium in which the d-gluconic acid had been replaced by 25 mM sodium acetate. After incubation for 4 days at 37°C, 20-ml portions of the soft agar cultures were extracted with 10 ml dichloromethane by vigorous shaking. After centrifugation at 2,000 × g for 15 min, the lower organic layer was evaporated and subjected to GC-MS.
RESULTS AND DISCUSSION
Analysis of isotope-labeled P. aeruginosa HAQs by GC-MS and NMR.
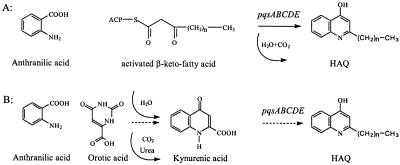
Previous studies on the biosynthesis of HAQs revealed that anthranilic acid is a direct precursor, and it has been speculated that HAQs are produced in a multiple-step reaction by the condensation of anthranilic acid and β-keto fatty acids. However, in a previous screening for HAQ-negative P. aeruginosa transposon mutants, we isolated mutants affected in the carB, pyrB, and pyrD genes. Although these genes are not neighbors on the chromosome, they all encode enzymes successively required for the biosynthesis of orotic acid in the pyrimidine biosynthetic pathway. In these mutants HAQ biosynthesis could be complemented by addition of 5 mM orotic acid to the growth medium, as demonstrated by TLC after 3 days of growth. Thus, it may be argued that orotic acid could be a direct precursor of HAQs. In an initial step anthranilic acid and orotic acid might react to form kynurenic acid, and the pqsABCDE operon could then be responsible for the attachment of an even-number carbon chain (C6 or C8) at the C-2 carbon of the quinolone ring to produce 4-hydroxy-2-heptyl- and 4-hydroxy-2-nonylquinoline, respectively (Fig. 1). To confirm either the previously proposed biosynthetic pathway for HAQs involving the condensation of anthranilic acid and a β-keto-fatty acid or the possibility that anthranilic acid and orotic acid are the precursors of HAQ synthesis via the formation of kynurenic acid, we performed feeding experiments with isotope-labeled substrates. First, P. aeruginosa SCV 20265 was grown in the presence of 15N-labeled anthranilic acid on modified Vogel-Bonner swimming agar plates with d-gluconic acid as the main carbon source. After growth for 3 days the bacterial mass was extracted with methanol, and the isolated secondary metabolites were subjected to GC-MS analysis. The upper panel of Fig. 2 shows a typical gas chromatogram of the trimethylsilylated HAQ derivatives, whereas the lower panel shows the EI mass spectrum of the unlabeled heptyl derivative and the fragmentation pattern assigned in the inserted scheme. Compared to the unlabeled control, all extracted 4-hydroxy-2-alkylquinolines contained approximately 66% 15N, as easily deduced from the corresponding EI mass spectrum of the trimethylsilylated derivatives by the characteristic shift of the molecular ions at m/z 315 (heptyl derivative) and m/z 343 (nonyl derivative) and the intense fragment ion generated by elimination of the alkyl side chain at m/z 231 by 1 Da (Fig. 2). Incorporation of the 15N from labeled anthranilic acid was also observed for all other HAQ metabolites, and this clearly indicated that anthranilic acid served as a common precursor for PQS, 4-hydroxy-2-heptylquinoline, 4-hydroxy-2-nonylquinoline, and 4-hydroxy-2-heptylquinoline-N-oxide. Moreover, since the only nitrogen of anthranilic acid was labeled, this indicates that N-1 of HAQ originates from anthranilic acid.
FIG. 1.
(A) Biosynthetic pathway for P. aeruginosa HAQs involving anthranilic acid and activated β-keto fatty acids as direct precursors. Using labeled precursor substrates, the possibility of an alternative pathway via the formation of kynurenic acid as an intermediate, as shown in panel B, was excluded.
FIG. 2.
(Upper panel) Typical gas chromatogram of total trimethylsilylated quinoline derivatives isolated from P. aeruginosa grown with anthranilic acid and acetic acid as the main carbon source. Peak 1, 4-hydroxy-2-heptylquinoline (whose EI mass spectrum is shown in the lower panel); peak 2, 3,4-dihydroxy-2-heptylquinoline; peak 3, 4-hydroxy-2-nonylquinoline; peak 4, 3,4-dihydroxy-2-nonylquinoline; peak 5, 4-hydroxy-2-hydroxynonylquinoline; peak 6, 4-hydroxy-2-undecenylquinoline (the structures of the peak 5 and 6 compounds were only tentatively assigned using their EI mass spectra alone). (Lower panel) EI mass spectrum of 4-trimethylsilyloxy-2-heptylquinoline and inserted fragmentation scheme.
To shed light on the identity of the carbon substrate, especially at positions 2, 3, and 4 in the quinolone ring system, P. aeruginosa cultures were incubated for 3 days on modified Vogel-Bonner medium containing either [1-13C]acetate, [2-13C]acetate, or [1,2-13C]acetate in addition to unlabeled anthranilic acid as the main carbon source. According to the pattern of label incorporated, one can distinguish whether anthranilic acid and labeled β-keto acid (the pattern is dependent on the [13C]acetate source) condense “head to head” or whether an unusual polyketide synthesis mechanism extending activated anthranilic acid with labeled acetate moieties is responsible for PQS production. Substantial label incorporation into the HAQ metabolites was demonstrated. It is noteworthy that compared to cultures grown in minimal medium with d-gluconic acid as the main carbon source, it was 4-hydroxy-2-nonylquinoline and not 4-hydroxy-2-heptylquinoline that proved to be the main component in the HAQs. A characteristic mass shift of the molecular and fragment ions of the 4-hydroxy-2-alkylquinoline derivatives was observed for all labeling experiments by GC-MS after trimethylsilylation. Whereas the size of the molecular ion of the heptyl derivative increased by 4 Da after labeling with [1-13C]acetate, the size of the molecular ion of the corresponding nonyl derivative increased by 5 Da in accordance with elongation of the alkyl chain by an additional two-carbon unit. The intense fragment comprising the ring system at m/z 231 (Fig. 3, lower panel) incorporated only one 13C atom for both derivatives. Interestingly, the nonyl derivative exhibited in this and all other labeling experiments in which acetate was used as the major carbon source a clearly higher rate of 13C incorporation (ca. 30%) than the heptyl derivative. This behavior can be explained by a later onset of synthesis for the longer-chain derivative than for the shorter-chain derivative. Whereas before addition of acetate predominantly the short-chain variant was synthesized, the availability of large amounts of acetate caused increased synthesis of longer-chain variants. Thus, the chain size seems to depend on both the carbon source and the growth phase.
FIG. 3.
Second-order (daughter ion) mass spectra of the monoisotopic molecular ions of the trimethylsilylated derivatives of 4-hydroxy-2-heptylquinoline labeled with four and five 13C atoms by addition of [1-13C]acetic acid and [2-13C]acetic acid, respectively, to the medium in the presence of unlabeled anthranilic acid. The arrows indicate the positions of 13C.
An identical experiment performed with [2-13C]acetate instead of [1-13C]acetate resulted in mass shifts of 5 and 6 Da for the molecular ions of the heptyl and nonyl derivatives, respectively, and a shift of 2 Da for the fragment ion at m/z 231. Therefore, the heterocyclic part of the quinoline ring system must also be synthesized from acetate units, since the labeled acetate could be clearly found not only in the alkyl side chain but also in the ring system. Furthermore, the difference between the two acetate labels can only be explained by the elimination of one CO2 molecule originating from the carboxylate moiety of acetate during biosynthesis. As expected, labeling with [1,2-13C]acetate led to a mass increase of 9 or 11 Da for the molecular ions and a shift of 3 Da of the fragment ion at 231 Da. To confirm these results, MS-MS fragmentation was performed for the molecular ions of the [1-13C]acetate- and [2-13C]acetate-labeled 4-hydroxy-2-heptylquinolines at m/z 319 and 320, respectively, as shown in Fig. 3. The alternating presence of 13C and 12C carbons in the alkyl chain of the molecules can be directly deduced from the corresponding fragments and also the fragments at m/z 232 and 233, respectively, incorporating the quinoline ring system, indirectly supporting the hypothesis that all HAQ derivatives are synthesized by “head-to-head” condensation of anthranilic acid and β-ketodecanoic or β-ketododecanoic acid with elimination of the carboxylate group of the fatty acid as CO2. A similar MS-MS experiment was performed for the molecular ion of the nonyl derivative, and analogous results were obtained (data not shown). The addition of unlabeled orotic acid in addition to anthranilic acid to the medium, which should have drastically reduced the incorporation of 13C into the ring system if the alternative biosynthetic pathway via kynurenic acid is correct, had absolutely no effect on this pattern. Addition of 3-oxo-decanoate, anthranilic acid, and [1,2-13C]acetate to the medium, however, resulted in labeling of the nonyl derivative (3 Da of the ring fragment and 11 Da of the molecular ion; compare with the data described above), whereas the labeling rate of the heptyl derivative was reduced by a factor of approximately 10, demonstrating that predominantly added unlabeled 3-oxo-decanoate is used for the biosynthesis of this derivative.
The biosynthetic pathway via β-keto-(do)decanoic acid could be independently demonstrated by 13C NMR spectroscopy. For this experiment, HAQ produced with relatively small amounts of [1,2-13C]acetate added to the medium was used, resulting in a degree of labeling of ca. 1%. Since the presence of two neighboring 13C atoms can be expected in the side chain and also in the heterocyclic ring system if our proposed biosynthetic model is correct, C-C coupling between intact acetate-derived units should be observed by 1D 13C NMR, whereas the probability of coupling between 13C atoms originating from different acetate units is too low to be observable. In this way, coupling between C-2 of the quinoline ring system and the first carbon of the side chain, C-9, with a characteristic coupling constant was indeed observed (Table 1), unequivocally demonstrating the presence of an intact acetate-derived unit at this position. The observation of doublet signals for the three carbons at the chain end confirmed the regular incorporation of acetate units in the fatty acid section of the molecule. No coupling was detected between C-3 and C-4 of the quinoline ring (Table 1), confirming the elimination of the carboxylate carbon of the final acetate unit as CO2 used to synthesize HAQ derivatives via β-keto fatty acids. Therefore, the C-4 carbons of these molecules must originate from anthranilic acid.
TABLE 1.
13C NMR data for synthetic 4-hydroxy-2-heptylquinoline and the main components of the biosynthetic product after feeding with doubly labeled [13C]acetatealegend
| Carbon | Shift(s) (ppm)
|
Multiple 13C satellited | Jcc (Hz) (±0.5) | |
|---|---|---|---|---|
| 4-Hydroxy- 2-heptylquinolineb | Main componentsc | |||
| C-2 | 157.1 | 157.1 | d | 44.3 |
| C-3 | 108.8 | 108.8 | s | |
| C-4 | 180.7 | 180.7 | s | |
| C-4a | 125.5 | 125.5 | s | |
| C-5 | 126.0 | 126.0 | s | |
| C-6 | 125.0 | 125.0 | s | |
| C-7 | 133.4 | 133.4 | s | |
| C-8 | 119.0 | 119.1 | s | |
| C-8a | 141.6 | 141.6 | s | |
| C-9 | 35.0 | 35.0 | d | 44.3 |
| C-10 | 30.1 | 30.1-30.8 | ||
| C-11 | 30.2e | 30.1-30.8 | ||
| C-12 | 30.1e | 30.1-30.8 | ||
| C-13 | 32.8 | 32.8 | d | 34.1 |
| C-14 | 23.6 | 23.68, 23.62f | d, d | 34.6, 34.1 |
| C-15 | 14.3 | 14.41, 14.39, 14.35f | d, d | 35.1, 35.6, 35.1 |
As determined by the MS analysis, the main components are a mixture of several compounds, the main components of which are the heptyl and nonyl derivatives. The structure of one heptyl derivative in the tautomeric keto form is
The signals were unambiguously assigned from correlations in the two-dimensional HMBC spectrum.
Overlap of signals prevented assignment of the internal chain carbons in the main components.
Doublet 13C satellite signals centered on the shift of the major signal were observed for the signals indicated by “d” but not for the signals indicated by “s”.
The assignments are interchangeable.
End units from major components of the mixture.
Since we demonstrated that β-keto acids are direct precursors of HAQs, it might be speculated that the probable coenzyme A ligase encoded by pqsA is responsible for the activation of the β-keto acids. pqsD might then be involved in a transacetylation reaction, and finally, β-keto-acyl-acyl carrier protein might be condensed with anthranilic acid to form PQS. These processes are probably also dependent on enzymes of the primary fatty acid metabolism. Future studies will have to elucidate the enzymatic function of the proteins encoded by the pqsABCDE operon and to identify whether and which enzymes of the primary fatty acid metabolism are also required for PQS biosynthesis.
Growth characteristics of P. aeruginosa mutants affected in the pyrimidine metabolic pathway.
To further verify that orotic acid is not a direct precursor of HAQ production, we tested whether HAQ production by mutants with mutations in the pyrimidine metabolic pathway could be restored by not only orotic acid but also UMP. UMP is a direct precursor of DNA biosynthesis, and orotic acid is metabolized into UMP in the pyrimidine metabolic pathway. Indeed, both orotic acid and UMP could restore HAQ production in the carB, pyrB, and pyrD mutant strains. Since the lack of HAQ production in mutants affected in the pyrimidine metabolic pathway cannot be explained by the lack of orotic acid as a direct HAQ precursor, we analyzed the growth characteristics of the carB, pyrB, and pyrD mutant strains compared to those of the wild type. Despite comparable growth behavior in log-phase cultures, not only HAQ production but also growth in stationary-phase cultures was affected in all three mutants. The optical densities approached only about 30% of the optical densities of the wild-type cultures, unless the medium was supplemented with either orotic acid or UMP. These results imply that the absolute bacterial cell density in stationary-phase P. aeruginosa cultures and HAQ production are directly linked and that the mutants affected in the pyrimidine metabolic pathway lack HAQ production due to a general metabolic defect.
Common biosynthetic pathway for the fatty acid moiety of rhamnolipids and P. aeruginosa HAQs.
It is obvious that the majority of the HAQs of P. aeruginosa are either 4-hydroxy-2-heptylquinolines or 4-hydroxy-2-nonylquinolines. Accordingly, either β-ketodecanoic or β-ketododecanoic fatty acids are essential precursors of HAQ biosynthesis. In this context it is intriguing that rhamnolipids of P. aeruginosa are composed of rhamnose and β-hydroxy fatty acids that are the same chain length (namely, β-hydroxydecanoic and β-hydroxydodecanoic fatty acids). Recently, evidence has been provided that the rhlG gene of P. aeruginosa codes for a FabG homolog specifically involved in the synthesis of the β-hydroxy acid moiety required for rhamnolipid biosynthesis (3). While a mutation of the rhlG gene had no apparent effect on the growth rate and total lipid content of P. aeruginosa cells, the production of rhamnolipid was abrogated and the synthesis of poly-β-hydroxyalkanoate was delayed. This suggests that the biosynthetic pathway for the fatty acid moiety of rhamnolipids is separate from the general fatty acid pathway starting with a specific keto-acyl reductase step catalyzed by RhlG. In addition, the rhlG mutant strain was shown to produce significantly less pyocyanin, whose production is strongly dependent on PQS, a defect that could be complemented in the mutant strain after the parental gene was provided on a plasmid in trans (3). Since it was tempting to speculate that RhlG is required to provide a pool of β-hydroxy C10 to C12 fatty acids in P. aeruginosa that are essential precursors of not only rhamnolipid biosynthesis but also of the biosynthesis of HAQs, we tested whether the PAO1 rhlG:Tc mutant (provided by G. Soberon-Chavez) was deficient in HAQ biosynthesis. Indeed, total HAQ production was reduced in the PAO1 rhlG:Tc mutant strain after 4 h, 9 h, and 30 h of growth, as determined by TLC (data not shown). These findings suggest that RhlG plays a role not only in the biosynthesis of fatty acids used as substrates for rhamnolipid and poly-β-hydroxyalkanoate but also in HAQ production, albeit for the latter process an intact rhlG gene is not an absolute requirement. Moreover, assuming a common pathway for the fatty acid moiety of rhamnolipids and HAQs, one would expect that the addition of acetate to the growth medium would lead not only to a shift from 4-hydroxy-2-heptylquinoline to 4-hydroxy-2-nonylquinoline (as demonstrated in this study) but also to a shift in the C side chain length of P. aeruginosa rhamnolipids from C10 to C12. Indeed, acetate supplementation led to a shift in the biosynthesis of rhamnolipids with the longer C12 side chain, as demonstrated by GC-MS. Whereas before addition of acetate the short-chain variants of the rhamnolipids were synthesized predominately, the availability of large amounts of acetate caused increased synthesis of 4-hydroxy-2-nonylquinoline and rhamnolipids with the longer C12 side chain (Fig. 4). These results further strengthen the presumption that the fatty acid moiety of rhamnolipids and HAQs are derived at least in part from the same pool.
FIG. 4.
Comparison of the rhamnolipid constituents and the HAQs present in P. aeruginosa grown on modified Vogel-Bonner medium with d-gluconic acid (upper panel) or acetate (lower panel) as the major carbon source. GC-MS of dichloromethane-extracted and methanolysed samples was performed in triplicate, and representative chromatograms of trimethlysilylated derivatives are shown. Peak 1, β-hydroxydecanoic acid methyl ester; peak 2, rhamnose methylglycoside; peak 3, β-hydroxydodecanoic acid methyl ester; peak 4, 4-hydroxy-2-heptylquinoline; peak 5, 4-hydroxy-2-nonylquinoline. The chromatograms were generated by summation of the most intense fragment ions of the compounds indicated (m/z 204, 231, 259, and 287).
Conclusions.
In this study we elucidated the biosynthetic pathway of HAQs that are signal molecules involved in the regulation of virulence factor production in P. aeruginosa. Using labeled precursors and analysis of extracted HAQs by GC-MS and NMR spectroscopy, we confirmed the previously proposed biosynthetic pathway involving a “head-to-head” condensation of anthranilic acid and β-keto fatty acids. Moreover, the results of this study imply that the P. aeruginosa rhlG gene coding for an NADPH-dependent β-ketoacyl reductase provides a pool of C10 and C12 β-hydroxy fatty acids which not only are essential for rhamnolipid biosynthesis but also serve as substrates for HAQ production.
HAQ signal molecules are produced in the stationary phase of growth, and they seem to be important when the bacterial population is under stress. Under these conditions (for instance, during a chronic infection of a CF lung) maintenance of viability is of major importance for the bacterial population rather than cell division. The elucidation of the biosynthetic pathway and future research on the molecular mechanisms of HAQ signal transduction should provide valuable clues regarding the process of chronic infection and the pathogenesis of persistent disease. More insight could make the HAQ signal molecules a unique drug target for new therapies for treating P. aeruginosa infections.
Acknowledgments
We thank G. Soberon-Chavez for providing the P. aeruginosa PAO1 rhlG transposon mutant, C. Kakoschke, B. Jaschok-Kentner (NMR), and C. Hanko (MS) for excellent technical assistance, and R. Huppmann for helpful discussions. We are especially grateful to Dieter Bitter-Suermann and Jürgen Wehland for their continuous support.
REFERENCES
- 1.Calfee, M. W., J. P. Coleman, and E. C. Pesci. 2001. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:11633-11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camara, M., P. Williams, and A. Hardman. 2002. Controlling infection by tuning in and turning down the volume of bacterial small-talk. Lancet Infect. Dis. 2:667-676. [DOI] [PubMed] [Google Scholar]
- 3.Campos-Garcia, J., A. D. Caro, R. Najera, R. M. Miller-Maier, R. A. Al Tahhan, and G. Soberon-Chavez. 1998. The Pseudomonas aeruginosa rhlG gene encodes an NADPH-dependent beta-ketoacyl reductase which is specifically involved in rhamnolipid synthesis. J. Bacteriol. 180:4442-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chastre, J., and J. Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 5.Collier, D. N., L. Anderson, S. L. McKnight, T. L. Noah, M. Knowles, R. Boucher, U. Schwab, P. Gilligan, and E. C. Pesci. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41-46. [DOI] [PubMed] [Google Scholar]
- 6.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 7.de Kievit, T. R., R. Gillis, S. Marx, C. Brown, and B. H. Iglewski. 2001. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 67:1865-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deziel, E., F. Lepine, S. Milot, J. He, M. N. Mindrinos, R. G. Tompkins, and L. G. Rahme. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 101:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher, L. A., S. L. McKnight, M. S. Kuznetsova, E. C. Pesci, and C. Manoil. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 184:6472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassett, D. J., J. Cuppoletti, B. Trapnell, S. V. Lymar, J. J. Rowe, S. S. Yoon, G. M. Hilliard, K. Parvatiyar, M. C. Kamani, D. J. Wozniak, S. H. Hwang, T. R. McDermott, and U. A. Ochsner. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 54:1425-1443. [DOI] [PubMed] [Google Scholar]
- 12.Haussler, S., I. Ziegler, A. Lottel, F. von Gotz, M. Rohde, D. Wehmhohner, S. Saravanamuthu, B. Tummler, and I. Steinmetz. 2003. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 52:295-301. [DOI] [PubMed] [Google Scholar]
- 13.Hentzer, M., and M. Givskov. 2003. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 112:1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 16.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 17.Middleton, B., H. C. Rodgers, M. Camara, A. J. Knox, P. Williams, and A. Hardman. 2002. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol. Lett. 207:1-7. [DOI] [PubMed] [Google Scholar]
- 18.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 20.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 22.Somanathan, R., and K. M. Smith. 1981. Synthesis of some 2-alkyl-4-quinolone and alkyl-4-methoxyquinoline alkaloids. J. Heterocycl. Chem. 18:1077-1079. [Google Scholar]
- 23.Vasil, M. L. 1986. Pseudomonas aeruginosa: biology, mechanisms of virulence, epidemiology. J. Pediatr. 108:800-805. [DOI] [PubMed] [Google Scholar]
- 24.Williams, P. 2002. Quorum sensing: an emerging target for antibacterial chemotherapy? Expert Opin. Ther. Targets 6:257-274. [DOI] [PubMed] [Google Scholar]