Abstract
The FRS2 family of adaptor/scaffold proteins has two members, FRS2α and FRS2β. Both proteins contain N‐terminal myristylation sites for localization on the plasma membrane and a PTB domain for binding to limited species of receptor tyrosine kinases (RTKs), including the FGF receptor, the neurotophin receptor, RET, and ALK. Activation of these RTKs allows FRS2 proteins to become phosphorylated of tyrosine residues and then bind to Grb2 and Shp2, a SH2 domain‐containing adaptor and a tyrosine phosphatase, respectively. Subsequently, Shp2 activates a Ras/ERK pathway and Grb2 activates a Ras/ERK, phosphatidyl inositol (PI)‐3 kinase and ubiquitination/degradation pathways by binding to SOS, Gab1, and Cbl via the SH3 domains of Grb2. FRS2α acts as ‘a conning center’ in FGF signaling mainly because it induces sustained levels of activation of ERK via Shp2‐binding sites and Grb2‐binding sites, though the contribution of the former is greater. Indeed, FRS2α knockout mice and mice with mutated Shp2‐binding sites exhibit a variety of phenotypes due to defects in FGF signaling in vivo. Although FRS2β binds to the EGF receptor, it does not induce tyrosine phosphorylation on the receptor. Instead, it inhibits EGF signaling, resulting in inhibition of EGF‐induced cell proliferation and cell transformation. Based on these findings, the involvement of FRS2 proteins in tumorigenesis should be studied extensively to be validated as candidate biomarkers for the effectiveness of treatments targeting RTKs such as the FGF receptor and EGF receptor. (Cancer Sci 2008; 99: 1319–1325)
Signal transduction pathways through receptor tyrosine kinases (RTKs) play pivotal roles in a number of aspects of physiological and pathological biology including cancer.( 1 ) Ample amounts of evidence indicate that proteins lacking catalytic activity, so called scaffolding adaptor proteins, relay many key events of signal transduction from upstream components such as receptors to downstream elements.( 1 , 2 , 3 ) The scaffold adaptor proteins that are upstream of RTK signal transduction are classified into two groups. One is comprised of docking proteins that have multiple tyrosine phosphorylation sites to dock downstream signaling proteins. They also often have other domains to bind other molecules. This group includes fibroblast growth factor (FGF) receptor substrate 2 (FRS2), a new SH2‐containing sequence (Shc), Grb2‐associated binder (Gab),( 4 , 5 ) insulin receptor substrate (IRS), and downstream of kinase (Dok)‐family proteins.( 6 ) Several proteins in this group are subdivided into a group of membrane‐linked docking proteins (MLDP)( 7 ) (Fig. 1). MLDP is localized in the lipid component of plasma membranes, since the protein contains a membrane‐anchor domain of a stretch of hydrophobic amino acid residues and/or a pleckstrin homology (PH) domain at or close to the N‐terminus. The other is comprised of adaptor proteins in a narrow sense. They have only SH3 or/and SH2 domains to bind signaling proteins.( 7 , 8 ) This group includes Grb2, Crk, and Nck.
Figure 1.

Membrane‐linked docking proteins (MLDPs). Schematic structure of several MLDPs. The phosphotyrosine binding domain (PTB) binds to phosphrylated tyrosine residues on receptors or signaling proteins. The pleckstrin homology (PH) domain binds to phospholipids such as phosphatidyl inositol (PI)‐3 phosphate. The proline‐rich domains are important for binding to SH3 containing proteins such as Src family tyrosine kinases or Grb2. Y designates potential tyrosine (Y) phosphorylation sites. The ERK binding domain in FRS2β, Μet binding domain in Gab1, or insulin receptor binding domain in IRS2 contain a unique sequence for binding to ERK, Met, or insulin receptors.
Some of the scaffold adaptor proteins have a phosphotyrosine binding (PTB) domain or a Src homology (SH)2 domain to bind specific residues containing a tyrosine residue that becomes phosphorylated by activated RTKs or other tyrosine kinases. All the scaffold adaptor proteins act in the specification and/or amplification of the signal transduction pathway. Since these scaffold proteins were first discovered almost two decades ago, signal transduction pathways have emerged as a very complex network. In this review, I would like to summarize what is known about FRS2 proteins, a relatively new family of docking/adaptor proteins.
FRS2 stands for FGF receptor substrate 2.( 9 ) What then is FGF receptor substrate 1? It is actually PLCγ, which was identified as the first substrate of FGF receptor tyrosine kinases.( 10 ) FRS2 is also known as SNT, which stands for suc1‐associated neurotrophic factor target.( 11 ) Historically, SNT was identified as a protein that is tyrosine phosphorylated in response to nerve growth factor (NGF) or FGF in PC12 cells and can bind to p13suc1 a yeast cyclin‐dependent kinase binding protein, immobilized on agarose. It was reported that there is a good correlation between the tyrosine phosphorylation of SNT and differentiation of PC12 cells. Then FRS2 was purified and cloned as a novel protein that is tyrosine phosphorylated in NIH3T3 cells in response to FGF.( 9 ) It turned out to be the same protein as SNT. We identified another protein that has homology with FRS2 in one of the expression sequence tag (EST) fragments. After we cloned the full‐length cDNA of this protein, we named it FRS2β and renamed the original FRS2 FRS2α.( 12 ) Other groups also identified this protein and named it SNT‐2 or FRS3.( 13 , 14 , 15 ) Thus there are now two family members, FRS2α/SNT‐1 and FRS2β/SNT‐2/FRS3, in mammals. There is one homolog in Drosophila, dFRS2, and one homolog in Xenopus, xFRS2.( 16 , 17 , 18 ) In zebrafish, there seems to be two homologs.
Domain structure of FRS2 proteins
FRS2α and FRS2β are similar in structure. Both proteins contain amino acid residues MGSCCS, a consensus myristylation sequence (MGXXXS/T), at the N‐terminus for binding to lipids in the plasma membrane constitutively( 1 , 12 , 19 ) (Fig. 2). Each has a PTB domain and multiple tyrosine phosphorylation sites at the C‐terminus. FRS2α has six tyrosine phosphorylation sites and FRS2β has five. The PTB domains are highly homologous and 72% of amino acids are identical. The residues surrounding each tyrosine phosphorylation site are similar but more than 10 amino acids from the sites there is no similarity. There is also a certain amount of similarity in the C‐terminus.
Figure 2.

Schematic structures of fibroblast growth factor (FGF) receptor substrate 2 (FRS2) family proteins. The phosphotyrosine binding domains (PTBs) of FRS2α and FRS2β have 72% identity and 93% similarity in amino acids. Tyrosine phoshporylated Grb2 binding sites activate Ras/ERK, ubiquination/degradation, and PI‐3 kinase pathways. Tyrosine phoshorylated Shp2 binding sites activate the Ras/ERK pathway stronger than the Grb2 binding site.
Cellular signaling upstream of FRS2 proteins
FRS2 proteins act as docking proteins downstream of limited species of RTKs, including FGF receptors, neurotrophin receptors,( 12 , 20 ) RET,( 21 , 22 , 23 ) and ALK.( 24 ) In particular, emerging evidence indicates that FRS2α is a ‘conning center’ for intracellular signaling in response to FGF as described in detail below. FRS2 proteins bind to these RTKs via the PTB domain and become phosphorylated on tyrosine residues upon activation of these RTKs. In contrast, FRS2 proteins are not good substrates for other RTKs, insulin receptors, platelet‐derived growth factor (PDGF) receptors and so on.( 25 ) FRS2α is phosphorylated on tyrosine residues by epidermal growth factor (EGF) receptor kinases in A431 cells where EGF receptors are overexpressed( 26 ) while EGF‐induced tyrosine phosphorylation of FRS2α seems to be not detectable in other cells that have modest amounts of EGF receptors.( 9 , 25 ) The specificity, in that FRS2 proteins choose only several tyrosine kinases to be phosphorylated, is characteristic of this protein family. The PTB domain is able to bind to specific peptides with or without tyrosine‐phosphorylated residues. The PTB domains of FRS2 proteins bind to unphosphorylated peptides at the juxtamembrane domain of the FGF receptor and the binding is stable with or without activation of the receptors( 13 , 20 ) (Fig. 3). In contrast, binding between the PTB domain of FRS2 and neurotrophin receptor TrkA and TrkB, or RET is mediated by tyrosine phosphorylated peptides in the receptors and is dependent on activation of the RTKs.( 20 , 21 , 22 , 27 , 28 , 29 , 30 ) The t(2;5) chromosomal translocation found in anaplastic large‐cell lymphoma generates the nucleophosmin (NPM)‐ALK chimeric oncoprotein that carries portion of NPM and the tyrosine kinase domain of ALK.( 31 ) NPM‐ALK proteins have two FRS2 binding sites containing phosphorylated tyrosine residues and one FRS2 binding site without tyrosine phosphorylation.( 32 ) The crystallographic structure of the complex between the PTB domain of FRS2α and the peptides containing the binding sites of the FGF receptor or TrkA has revealed that there are two different binding pockets in the PTB domain for the phosphorylated and unphosphorylated peptides on tyrosine residues.( 19 , 27 , 33 ) Due to constitutive binding between the FGF receptor and FRS2 proteins, it is possible that FRS2 is phosphorylated by the FGF receptor tyrosine kinase as efficiently as the receptor itself.
Figure 3.
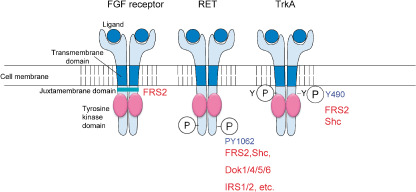
Binding sites of fibroblast growth factor (FGF) receptor substrate 2 (FRS2) proteins in receptor tyrosine kinases (RTKs). FRS2 binds to unphosphorylated peptides in the juxamembrane domain of the FGF receptor. It binds to tyrosine phosphorylated peptides in RET and TrkA, and the binding sites are shared with those of other PTB domain‐containing signaling proteins.
Laloo is one of the Src like kinases in Xenopus and functions in FGF signaling. It promotes tyrosine phosphorylation of xFRS2, whose PTB domain seems to bind to Laloo.( 16 , 17 )
We recently found that the PTB domain of FRS2β binds to the EGF receptors. Moreover, FRS2β does not become phosphorylated by EGF receptor tyrosine kinases. Instead, it inhibits the tyrosine kinase activity of the receptors.( 34 , 35 ) Our finding clearly demonstrated that FRS2β is a unique scaffolding adaptor that serves as a negative as well as a positive regulator for RTK signaling dependent on the receptor species.
Cellular signaling downstream of FRS2 proteins
The four tyrosine phosphorylation sites on FRS2α and the three tyrosine phosphorylation sites on FRS2β bind to the Grb2 adaptor( 9 , 12 ) (Fig. 2). All these peptides contain the consensus sequence for the SH2 domain of Grb2, YXNX. The other two tyrosine phosphorylation sites on FRS2α and FRS2β bind to Shp2, the SH2‐containing tyrosine phosphatase.( 12 , 36 , 37 ) Several proteins are constitutively bound via two SH3 domains of Grb2: SOS, Cbl, and Gab1.( 19 , 38 , 39 ) SOS is a guanine nucleotide exchange factor (GEF) for Ras, and FRS2α‐mediated recruitment of Grb2‐SOS induces activation of the Ras/ERK pathway. Cbl functions as an E3 ubiquitin ligase and the ternary complex FRS2α‐Grb2‐Cbl results in ubiquitination and degradation of FRS2α and receptors. The ternary complex FRS2α‐Grb2‐Gab1 enables tyrosine phosphorylation of Gab1 followed by recruitment of PI‐3 kinase and activation of a cell survival pathway. SOS binds to the N‐terminal SH3 domain of Grb2, Gab1 binds to the C‐terminal SH3 domain of Grb2, and Cbl binds to both N‐ and C‐terminal SH3 domains of Grb2. Thus FRS2α assembles both positive and negative signaling proteins to mediate a balanced signal transduction. The FRS2α‐Shp2 complex induces tyrosine phosphorylation of Shp2 followed by strong activation of ERK in response to FGF.( 36 ) Tyrosine phosphorylated Shp2 activates its own tyrosine phosphatase, resulting in strong activation of the Ras/ERK pathway in numerous cell contexts, though the precise mechanisms by which Shp2 activates Ras are still controversial.( 40 )
Growth factor‐induced activation of ERK is subdivided into two phases, a transient phase and a sustained phase.( 41 ) After stimulation with growth factors, the activation peaks within 5 min and returns to basal levels within 1 h in a transient phase, while it lasts for several hours with a gradual reduction in a sustained phase. Several growth factors such as EGF induce prominent levels of ERK activation in a transient manner but not in a sustained manner, while patterns of ERK activation induced by other growth factors such as FGF and NGF fit with a pattern of summation of both transient and sustained phases. In FGF signaling, FRS2α appears to be a critical mediator that is able to induce the sustained phase of ERK activation in cells lacking endogenous FRS2β.( 42 ) When FRS2β is exogenously expressed in FRS2α–null mouse embryonic fibroblasts, it compensates for the loss of FRS2α in FGF‐stimulated activation of ERK.( 12 ) Thus FRS2β is also able to activate ERK in a sustained manner in response to FGF. Compared with the strong activation levels of ERK via the Shp2‐binding sites of FRS2α, the Grb2‐binding sites of FRS2α activate ERK at moderate levels.( 42 ) Nevertheless, the Shp2‐binding sites and Grb2‐binding sites contribute to both phases.
FRS2α is phosphorylated by ERK at multiple threonine residues in response to a variety of ligands, FGF, insulin, EGF, and PDGF( 25 ) (Fig. 4a,b). These include extracellular stimuli that do not induce tyrosine phosphorylation of FRS2α. There are eight canonical ERK phosphorylation sites (PXTP motif) in FRS2α. Expression of a mutant FRS2α deficient in ERK phosphorylation sites resulted in enhanced tyrosine phosphorylation of FRS2α, ERK stimulation, cell migration, and cell proliferation. In addition, activated ERK binds to threonine phosphorylated FRS2α. Thus there is an ERK‐mediated negative feedback mechanism for the control of signaling pathways that are dependent on FRS2α.
Figure 4.

Negative feedback regulation of fibroblast growth factor (FGF) receptor substrate 2 (FRS2) proteins by activated ERK. (a) Activated FGF receptor phosphorylates tyrosine residues on FRS2α. The resultant activated ERK in turn phoshorylates threonine resiudes on FRS2α and inhibits tyrosine phosphorylation of FRS2α. (b) Activated insulin, epidermal growth factor (EGF), or the platelet‐derived growth factor (PDGF) receptor induce ERK activation without FRS2‐dependent mechanisms. The activated ERK phosphorylates threonine resiudes on FRS2α and inhibits tyrosine phosphorylation of FRS2α.
In contrast, FRS2β may not have many sites of phosphorylation by ERK; however, it still binds to phosphorylated and activated ERK (M Watanabe and N Gotoh, unpublished data). We identified an ERK‐binding site on FRS2β and found that binding between FRS2β and phosphorylated ERK is important for inhibition of EGF receptor tyrosine kinase.( 35 ) The ternary complex, EGF receptor‐FRS2β‐phosphoryalted ERK, may be required for inhibition of EGF signaling. This functions to form a negative feedback loop after activation of ERK downstream of the activated EGF RTK or to maintain the EGF receptor in an inactive state after the activation of ERK by other stimuli.
Several other proteins are reported to bind to FRS2 proteins at sites different from phosphorylated tyrosine residues: SOS, Rnd, Cks1/Cks2, and Sprouty. Cks1 and Cks2 are mammalian homologs of p13suc1. They are cell‐cycle inducers and trigger ubiquitination and degradation of p27kip1. It is reported that Cks1 associates with the unphosphorylated FRS2α via the PTB domain of FRS2α.( 43 ) FGF‐dependent activation of FRS2α causes the release of Cks1 from FRS2α, and promotes degradation of p27kip1. Sprouty is a negative regulator for the Ras/ERK pathway; however, the point at which Sprouty blocks ERK activation depends on the cellular context and/or the identity of the RTK.( 44 , 45 , 46 ) It is reported that tyrosine phosphorylated Sprouty binds to and sequesters Grb2 from the Grb2‐SOS complex on FRS2 in response to FGF.( 47 ) However, another group has reported that there is constitutive interaction between Sprouty and FRS2, and the interaction is increased by FGF resulting in inhibition of ERK signaling.( 48 ) Rnd1 has low intrinsic GTPase activity and is antagonistic of RhoA signaling.( 37 ) It directly interacts with FRS2α and FRS2β. The interaction of FRS2β suppresses the inhibitory effect of Rnd1 on RhoA. Rnd1 binds to the C‐terminus of FRS2β including Shp2‐binding sites. When the FGF receptor is activated, it phosphorylates FRS2β, recruits Shp2, and releases Rnd1 from FRS2β. The liberated Rnd1 then inhibits RhoA activity.
Expression patterns in tissues and physiological functions of FRS2 proteins
In situ hybridization during mouse embryogenesis showed that Frs2α is strongly expressed in the extraembryonic ectoderm where trophoblast stem (TS) cells exist, stem cells for tissues of the placenta, at embryonic day 6 and is ubiquitously expressed during development, though Frs2β is strictly localized in several tissues including the neuroepithelium.( 12 , 49 ) In Xenopus, the transcripts of xFRS2 have an expression pattern that is quite similar to that of FGFR1 during development.( 16 , 18 )
FGF stimulates cell growth and cell migration in fibroblasts and neurite outgrowth in PC12 cells. It seems that FRS2α plays critical roles in all these activities.( 36 , 42 ) FRS2 proteins bind to TrkA and B upon stimulation with NGF or brain derived growth factor (BDNF) and stimulate neurite outgrowth in neuronal cells such as PC12 cells.( 20 , 29 , 30 ) The binding site on TrkA or TrkB is also known as a Shc‐binding site (Fig. 3). There may be competition for which protein binds to this site.( 50 ) Prolonged activation of ERK is associated with differentiation of PC12 cells, while transient activation of ERK is associated with the proliferation of these cells.( 41 ) Since FRS2 but not Shc induces prolonged activation of ERK, the competition may underlie molecular mechanisms to determine whether cells proliferate or differentiate. FRS2α is also involved in the neuronal differentiation of PC12 cells stimulated by activated ALK.( 24 ) The binding site of FRS2α on RET is a common binding site for several proteins carrying SH2 or/and PTB domains, including Shc, Dok1, 4, 5 and 6, and IRS1 and 2( 21 , 22 ) (Fig. 3). Experiments using a genetically engineered RET mutant that is able to bind FRS2α but not Shc and vice versa showed that Shc activates the cell survival pathway of neuroblastoma cells more strongly than FRS2α does.( 51 )
Phenotypes of FRS2α mutant mice generated by gene targeting support the notion that FRS2α is a ‘conning center’ in FGF signaling in vivo (Fig. 5). It is well established that FGF signaling plays a fundamental role in many aspects of animal development. Frs2α‐null mouse embryos showed multiple developmental defects, resulting in early embryonic lethality by E849. This is due to a failure of maintenance of self‐renewing TS cells that are dependent on FGF4. Further, FGF4‐stimulated FRS2α‐mediated ERK activation in TS cells induces expression of Bmp4 that is important for maintenance of primordial germ cells. Using Frs2α‐null embryos we also showed that Bmp4 is important for cell proliferation in the inner cell mass and epiblast (M Murohashi and N Gotoh, unpublished data). In addition, FRS2α plays a role in cell movement through the primitive streak during gastrulation.
Figure 5.

FRS2α is a ‘conning center’ in fibroblast growth factor (FGF) signaling in vivo. Frs2α null mouse embryos showed early embryonic lethality due to a failure of maintenance of trophoblast stem (TS) cells. TS cells are dependent on FGF4 and localized in extraembryonic ectoderm (ExE). The wild‐type ExE is positive in pERK staining but is weak in the mutant. The Frs2α 2F/2F mice have a variety of developmental defects, some of which are reasonably explained by the failure of FGF signaling.
We next generated mutant mice expressing forms of FRS2α that lack either the two Shp2‐binding sites (2F mutant) or the four Grb2‐binding sites (4F mutant) by gene targeting (Fig. 5). Frs2α 2F/2F embryos exhibit a variety of developmental defects and perinatal death, in contrast to the much milder defects in Frs2α 4F/4F mice. All Frs2α 2F/2F embryos were defective in eye development and showed anophthalmia (no eye) or microphthalmia (small eye).( 52 ) Consistent with the critical role of FRS2α in FGF signaling, the level of activated ERK in the Frs2α 2F/2F eye primordium is lower than that of the wild‐type in association with decreased levels of expression of Pax6 and Chx10, molecular markers for lens induction and retinal precursor proliferation, respectively. There are also severe defects in the cerebral cortex's development in Frs2α.2F /2F ( 53 ) The Shp2‐binding sites on FRS2α play critical roles in the maintenance of intermediate progenitor cells committed to a neuronal lineage after asymmetrical cell division of neural stem cells. Moreover, the Shp2‐binding sites on FRS2α play an important role in neural stem/progenitor cells proliferation in response to FGF2. The Frs2α 2F/2F mice have more developmental defects, including lack of carotid body.( 54 )
Fgfr1 mutant mice lacking binding sites for FRS2α and FRS2β die later in embryogenesis than Fgfr1‐null embryos and exhibit defects in neural tube closure and in the development of the tail bud and pharyngeal arches.( 55 ) Although FRS2 proteins play major roles in FGF receptor‐mediated developmental processes, these findings indicate that some essential functions of FGF receptor 1 are mediated by FRS2‐independent signaling.
On the other hand, the functions of FRS2βin vivo are still largely unknown, since there is no report yet about FRS2β knock out mice.
xFRS2 is a critical mediator for FGFR‐mediated induction of mesoderm and the accompanying elongation movements.( 18 ) In addition, xFRS2 cooporates with Laloo for induction of mesoderm.( 17 )
Relevance of FRS2 proteins to cancer
It is known that FGF ligands and their recepors are overexpressed in a variety of cancers, including of the breast, stomach, prostate, pancreas, bladder, and colon.( 19 , 56 , 57 , 58 ) Activating mutations in FGFR3 are observed frequently in bladder and cervical carcinoma and in some cases of multiple myeloma, while activating mutations in FGFR2 are found in gastric carcinoma. Further, several types of fusion proteins involving FGFR1 caused by chromosomal translocation are found in hematologic malignancies. Given that aberrant FGF signaling is important in tumorigenesis based on all these reports, it is reasonable to predict that FRS2 proteins are also involved in tumorigenesis (Fig. 6). Consistent with this idea, expression of the PTB domain of FRS2α inhibits antiestrogen growth of breast carcinoma cells induced by overexpression of FGF1.( 59 )
Figure 6.

Working hypothesis on the role of FRS2α in tumorigenesis. FRS2α appears to be involved in oncogenic addiction to aberrant fibroblast growth factor (FGF) signaling.
Somatic rearrangements of the TrkA gene are detected in a fraction of papillary thyroid carcinomas and produce chimeric proteins. All of them contain a portion of TrkA, including tyrosine kinase domain. The resultant thyroid Trk oncoproteins activate FRS2α and FRS2β.( 60 ) Gene rearrangements leading to fusion of the kinase domain of RET with heterologous proteins containing dimerization motifs result in constitutively activated RET proteins.( 21 , 28 ) Such fusion proteins are expressed in some cases of papillary thyroid carcinoma. Germ line point mutation in RET results in inherited multiple endocrine neoplasm types 2α and 2β and familial medullary thyroid carcinoma. FRS2α is tyrosine phosphorylated by ligand‐stimulated and by constitutively activated oncogenic forms of RET, leading to activation of ERK. The NPM‐ALK oncoprotein activates FRS2α and FRS2β and transforms NIH3T3 cells.( 32 )
Expression of FRS2β suppresses EGF‐induced cell transformation and proliferation.( 35 ) Moreover, the expression of FRS2β is down‐regulated in lung and brain cancer cell lines. These findings suggest that FRS2β is suppressive for tumorigenesis in which aberrant EGF signaling is involved.
Concluding remarks
From extensive studies since FRS2α cDNA was cloned in 1997, it is now clear that FRS2α acts as a ‘conning center’ in signaling via FGF receptors and plays significant roles in the promotion of signaling via several other RTKs. Thus, it is reasonable to predict that FRS2α is involved in the malignancy of tumors, especially when they are addicted to aberrant FGF signaling. On the other hand, FRS2β appears to have inhibitory effects on tumorigenesis when tumors are addicted to aberrant EGF signaling.
Recently, many more small compounds that inhibit RTKs and humanized antibodies against RTKs have been developed and several of them are already in use in the treatment of cancer. FGF receptors are among the targets of such molecular targeted therapies. However, there are few good diagnostic tools with which to decide which patients should be treated with drugs targeting FGF receptors. Therefore, there is an urgent need to develop diagnostic tools such as biomarkers to predict the effectiveness of such drugs. Taking the characteristic that FRS2α promotes signaling via limited species of RTKs into account, it is worth testing if FRS2α can be used as a biomarker. The EGF receptor is one of the most important targets in cancer treatment. We have evidence suggesting that FRS2β is applicable as a biomarker to predict the effectiveness of such drugs.
One direction of research into FRS2 proteins would be the validation of these proteins as candidates for biomarkers using many cancer tissue samples. Another direction would be continuing research aiming at further elucidation of physiological and pathological functions of this protein family and of molecular mechanisms for them.
Acknowledgments
I am grateful to Dr M Shibuya for critical reading of the manuscript. I thank Dr J Schlessinger for supporting my work in his laboratory. Work in my laboratory was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Ministry of Health, Labor, and Welfare of Japan for the 3rd‐term Comprehensive 10‐year Strategy for Cancer Control and for Cancer Research; the Naito Foundation; and the Cell Science Research Foundation.
References
- 1. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103: 211–25. [DOI] [PubMed] [Google Scholar]
- 2. Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science 1997; 278: 2075–80. [DOI] [PubMed] [Google Scholar]
- 3. Pawson T. Dynamic control of signaling by modular adaptor proteins. Curr Opin Cell Biol 2007; 19: 112–16. [DOI] [PubMed] [Google Scholar]
- 4. Nishida K, Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci 2003; 94: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu H, Neel BG. The ‘Gab’ in signal transduction. Trends Cell Biol 2003; 13: 122–30. [DOI] [PubMed] [Google Scholar]
- 6. Yamasaki S, Saito T. Inhibitory adaptors in lymphocytes. Semin Immunol 2004; 16: 421–7. [DOI] [PubMed] [Google Scholar]
- 7. Gotoh N, Tsuchida N. Membrane‐linked docking protein. In: Encyclopedia of Cancer. 2nd Edn. Heidelberg: Springer, in press. [Google Scholar]
- 8. Koch CA, Anderson D, Moran MF, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science 1991; 252: 668–74. [DOI] [PubMed] [Google Scholar]
- 9. Kouhara H, Hadari YR, Spivak‐Kroizman T et al . A lipid‐anchored Grb2‐binding protein that links FGF‐receptor activation to the Ras/MAPK signaling pathway. Cell 1997; 89: 693–702. [DOI] [PubMed] [Google Scholar]
- 10. Mohammadi M, Honegger AM, Rotin D et al . A tyrosine‐phosphorylated carboxy‐terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C‐gamma 1. Mol Cell Biol 1991; 11: 5068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabin SJ, Cleghon V, Kaplan DR. SNT, a differentiation‐specific target of neurotrophic factor‐induced tyrosine kinase activity in neurons and PC12 cells. Mol Cell Biol 1993; 13: 2203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gotoh N, Laks S, Nakashima M, Lax I, Schlessinger J. FRS2 family docking proteins with overlapping roles in activation of MAP kinase have distinct spatial‐temporal patterns of expression of their transcripts. FEBS Lett 2004; 564: 14–18. [DOI] [PubMed] [Google Scholar]
- 13. Xu H, Lee KW, Goldfarb M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J Biol Chem 1998; 273: 17987–90. [DOI] [PubMed] [Google Scholar]
- 14. McDougall K, Kubu C, Verdi JM, Meakin SO. Developmental expression patterns of the signaling adapters FRS‐2 and FRS‐3 during early embryogenesis. Mech Dev 2001; 103: 145–8. [DOI] [PubMed] [Google Scholar]
- 15. Zhou L, McDougall K, Kubu CJ, Verdi JM, Meakin SO. Genomic organization and comparative sequence analysis of the mouse and human FRS2, FRS3 genes. Mol Biol Report 2003; 30: 15–25. [DOI] [PubMed] [Google Scholar]
- 16. Hama J, Xu H, Goldfarb M, Weinstein DC. SNT‐1/FRS2alpha physically interacts with Laloo and mediates mesoderm induction by fibroblast growth factor. Mech Dev 2001; 109: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kusakabe M, Masuyama N, Hanafusa H, Nishida E. Xenopus FRS2 is involved in early embryogenesis in cooperation with the Src family kinase Laloo. EMBO Report 2001; 2: 727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akagi K, Kyun Park E, Mood K, Daar IO. Docking protein SNT1 is a critical mediator of fibroblast growth factor signaling during Xenopus embryonic development. Dev Dyn 2002; 223: 216–28. [DOI] [PubMed] [Google Scholar]
- 19. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005; 16: 139–49. [DOI] [PubMed] [Google Scholar]
- 20. Ong SH, Guy GR, Hadari YR et al . FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol 2000; 20: 979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kodama Y, Asai N, Kawai K et al . The RET proto‐oncogene: a molecular therapeutic target in thyroid cancer. Cancer Sci 2005; 96: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurokawa K, Iwashita T, Murakami H, Hayashi H, Kawai K, Takahashi M. Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction. Oncogene 2001; 20: 1929–38. [DOI] [PubMed] [Google Scholar]
- 23. Melillo RM, Santoro M, Ong SH et al . Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen‐activated protein kinase signaling cascade. Mol Cell Biol 2001; 21: 4177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Degoutin J, Vigny M, Gouzi JY. ALK activation induces Shc and FRS2 recruitment: signaling and phenotypic outcomes in PC12 cells differentiation. FEBS Lett 2007; 581: 727–34. [DOI] [PubMed] [Google Scholar]
- 25. Lax I, Wong A, Lamothe B et al . The docking protein FRS2alpha controls a MAP kinase‐mediated negative feedback mechanism for signaling by FGF receptors. Mol Cell 2002; 10: 709–19. [DOI] [PubMed] [Google Scholar]
- 26. Wu Y, Chen Z, Ullrich A. EGFR and FGFR signaling through FRS2 is subject to negative feedback control by ERK1/2. Biol Chem 2003; 384: 1215–26. [DOI] [PubMed] [Google Scholar]
- 27. Dhalluin C, Yan KS, Plotnikova O et al . Structural basis of SNT PTB domain interactions with distinct neurotrophic receptors. Mol Cell 2000; 6: 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melillo RM, Santoro M, Ong SH et al . Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen‐activated protein kinase signaling cascade. Mol Cell Biol 2001; 21: 4177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Easton JB, Royer AR, Middlemas DS. The protein tyrosine phosphatase, Shp2, is required for the complete activation of the RAS/MAPK pathway by brain‐derived neurotrophic factor. J Neurochem 2006; 97: 834–45. [DOI] [PubMed] [Google Scholar]
- 30. Dixon SJ, MacDonald JI, Robinson KN, Kubu CJ, Meakin SO. Trk receptor binding and neurotrophin/fibroblast growth factor (FGF)‐dependent activation of the FGF receptor substrate (FRS)‐3. Biochim Biophys Acta 2006; 1763: 366–80. [DOI] [PubMed] [Google Scholar]
- 31. Fujimoto J, Shiota M, Iwahara T et al . Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki‐1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci USA 1996; 93: 4181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chikamori M, Fujimoto J, Tokai‐Nishizumi N, Yamamoto T. Identification of multiple SNT‐binding sites on NPM‐ALK oncoprotein and their involvement in cell transformation. Oncogene 2007; 26: 2950–4. [DOI] [PubMed] [Google Scholar]
- 33. Yan KS, Kuti M, Yan S et al . FRS2 PTB domain conformation regulates interactions with divergent neurotrophic receptors. J Biol Chem 2002; 277: 17088–94. [DOI] [PubMed] [Google Scholar]
- 34. Huang L, Gotoh N, Zhang S, Shibuya M, Yamamoto T, Tsuchida N. SNT‐2 interacts with ERK2 and negatively regulates ERK2 signaling in response to EGF stimulation. Biochem Biophys Res Commun 2004; 324: 1011–17. [DOI] [PubMed] [Google Scholar]
- 35. Huang L, Watanabe M, Chikamori M et al . Unique role of SNT‐2/FRS2beta/FRS3 docking/adaptor protein for negative regulation in EGF receptor tyrosine kinase signaling pathways. Oncogene 2006; 25: 6457–66. [DOI] [PubMed] [Google Scholar]
- 36. Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor‐induced PC12 cell differentiation. Mol Cell Biol 1998; 18: 3966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harada A, Katoh H, Negishi M. Direct interaction of Rnd1 with FRS2 beta regulates Rnd1‐induced down‐regulation of RhoA activity and is involved in fibroblast growth factor‐induced neurite outgrowth in PC12 cells. J Biol Chem 2005; 280: 18418–24. [DOI] [PubMed] [Google Scholar]
- 38. Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3‐kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci USA 2001; 98: 6074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong A, Lamothe B, Li A, Schlessinger J, Lax I. FRS2 alpha attenuates FGF receptor signaling by Grb2‐mediated recruitment of the ubiquitin ligase Cbl. Proc Natl Acad Sci USA 2002; 99: 6684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Curr Opin Genet Dev 2007; 17: 23–30. [DOI] [PubMed] [Google Scholar]
- 41. Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal‐regulated kinase activation. Cell 1995; 80: 179–85. [DOI] [PubMed] [Google Scholar]
- 42. Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking‐protein FRS2 alpha in FGF receptor‐mediated signal transduction pathways. Proc Natl Acad Sci USA 2001; 98: 8578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, Lin Y, Bowles C, Wang F. Direct cell cycle regulation by the fibroblast growth factor receptor (FGFR) kinase through phosphorylation‐dependent release of Cks1 from FGFR substrate 2. J Biol Chem 2004; 279: 55348–54. [DOI] [PubMed] [Google Scholar]
- 44. Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative‐feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol 2006; 16: 45–54. [DOI] [PubMed] [Google Scholar]
- 45. Lo TL, Fong CW, Yusoff P et al . Sprouty and cancer: the first terms report. Cancer Lett 2006; 242: 141–50. [DOI] [PubMed] [Google Scholar]
- 46. Li X, Brunton VG, Burgar HR, Wheldon LM, Heath JK. FRS2‐dependent SRC activation is required for fibroblast growth factor receptor‐induced phosphorylation of Sprouty and suppression of ERK activity. J Cell Sci 2004; 117: 6007–17. [DOI] [PubMed] [Google Scholar]
- 47. Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol 2002; 4: 850–8. [DOI] [PubMed] [Google Scholar]
- 48. Tefft D, Lee M, Smith S, Crowe DL, Bellusci S, Warburton D. mSprouty2 inhibits FGF10‐activated MAP kinase by differentially binding to upstream target proteins. Am J Physiol Lung Cell Mol Physiol 2002; 283: L700–6. [DOI] [PubMed] [Google Scholar]
- 49. Gotoh N, Manova K, Tanaka S et al . The docking protein FRS2{alpha} is an essential component of multiple fibroblast growth factor responses during early mouse development. Mol Cell Biol 2005; 25: 4105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meakin SO, MacDonald JI, Gryz EA, Kubu CJ, Verdi JM. The signaling adapter FRS‐2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem 1999; 274: 9861–70. [DOI] [PubMed] [Google Scholar]
- 51. Lundgren TK, Scott RP, Smith M, Pawson T, Ernfors P. Engineering the recruitment of phosphotyrosine binding domain‐containing adaptor proteins reveals distinct roles for RET receptor‐mediated cell survival. J Biol Chem 2006; 281: 29886–96. [DOI] [PubMed] [Google Scholar]
- 52. Gotoh N, Ito M, Yamamoto S et al . Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc Natl Acad Sci USA 2004; 101: 17144–9. Epub 2004 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamamoto S, Yoshino I, Shimazaki T et al . Essential role of Shp2‐binding sites on FRS2alpha for corticogenesis and for FGF2‐dependent proliferation of neural progenitor cells. Proc Natl Acad Sci USA 2005; 102: 15983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kameda Y, Ito M, Nishimaki T, Gotoh N. FRS2 alpha 2F/2F mice lack carotid body and exhibit abnormalities of the superior cervical sympathetic ganglion and carotid sinus nerve. Dev Biol 2008; 314: 236–47. [DOI] [PubMed] [Google Scholar]
- 55. Hoch RV, Soriano P. Context‐specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development 2006; 133: 663–73. [DOI] [PubMed] [Google Scholar]
- 56. Jeffers M, LaRochelle WJ, Lichenstein HS. Fibroblast growth factors in cancer: therapeutic possibilities. Expert Opin Ther Targets 2002; 6: 469–82. [DOI] [PubMed] [Google Scholar]
- 57. Grose R, Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev 2005; 16: 179–86. [DOI] [PubMed] [Google Scholar]
- 58. Desnoyers LR, Pai R, Ferrando RE et al . Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 2008; 27: 85–97. [DOI] [PubMed] [Google Scholar]
- 59. Manuvakhova M, Thottassery JV, Hays S et al . Expression of the SNT‐1/FRS2 phosphotyrosine binding domain inhibits activation of MAP kinase and PI3‐kinase pathways and antiestrogen resistant growth induced by FGF‐1 in human breast carcinoma cells. Oncogene 2006; 25: 6003–14. [DOI] [PubMed] [Google Scholar]
- 60. Ranzi V, Meakin SO, Miranda C, Mondellini P, Pierotti MA, Greco A. The signaling adapters fibroblast growth factor receptor substrate 2 and 3 are activated by the thyroid TRK oncoproteins. Endocrinology 2003; 144: 922–8. [DOI] [PubMed] [Google Scholar]


