Abstract
Survivin has multiple functions including cytoprotection, inhibition of cell death, and cell‐cycle regulation, especially at the mitotic process stage, all of which favor cancer survival. Many studies on clinical specimens have shown that survivin expression is invariably up‐regulated in human cancers and is associated with resistance to chemotherapy or radiation therapy, and linked to poor prognosis, suggesting that cancer cells survive with survivin. It is also reported that survivin inhibition, alone or in combination with the other therapies, induces or enhances apoptosis and mitotic catastrophe in tumor cells. Moreover, certain antitumor agents can reduce survivin expression. These findings suggest that survivin may be a promising molecular target against human malignancies. (Cancer Sci 2008; 99: 1709–1714)
Abbreviations:
- AIF
apoptosis‐inducing factor
- IAP
inhibitor of apoptosis protein
- P13K
phosphatidylinositol 3‐kinase
- siRNA
short interfering RNA
Originally identified as a member of the IAPs, survivin was discovered to play critical roles in cell division and cell survival.( 1 , 2 ) Structurally, survivin contains a single BIR (baculoviral IAP repeats) domain, which is shared by other members of the IAPs, such as XIAP, c‐IAP1, c‐IAP2, and livin (Fig. 1).( 3 ) This domain contributes to its function in apoptosis inhibition. However, instead of a carboxyl terminal RING finger shared by others, survivin contains an extended carboxyl‐terminal alpha‐helical coiled‐coil which is thought to be important for its interaction with microtubules, hence its roles in cell division.( 2 , 4 ) Functioning simultaneously at cell division and apoptosis inhibition, survivin obviously plays a pivotal role in determining cell survival.
Figure 1.

Structure and functions of the survivin protein.
Cells are programmed to die when their existence is no longer required during development or when irreparable damage occurs. Survivin counteracts this process, along with other IAPs. Survivin blocks apoptosis induced by various stimuli including FAS/CD95,( 5 ) irradiation,( 6 ) and chemotherapeutic drugs, such as taxol( 2 ) or staurosporine.( 7 ) Inhibition of apoptosis is believed to be through direct binding to caspase‐3 and caspase‐7, preventing their activation.( 5 , 8 ) Besides being a direct inhibitor for caspases, survivin can interact with Smac/DIABLO physically.( 9 ) Survivin also provides cytoprotection to cells against caspase‐independent cell death through the inhibition of the AIF pathway, which is known to induce caspase‐independent DNA fragmentation.( 10 )
Survivin shows cell‐cycle‐dependent expression during cell division. Together with Aurora‐B kinase, survivin forms as component of the chromosome passenger complex, which plays a role in chromosome segregation and cytokinesis in cell division.( 11 ) Increased survivin expression is observed in the G2/M phase. The increase in survivin expression is believed to protect cells against a possible default induction of apoptosis in the case of aberrant mitosis.( 2 ) Survivin is also required for the maintenance of the spindle assembly checkpoint to allow proper microtubule alignment to ensure cell propagation.( 12 )
Lack of survivin during cell division causes polyploidy as well as apoptosis( 13 ) (Fig. 2). Polyploidy occurs following incomplete segregation or incomplete cytokinesis. The features of polyploidy are similar to that of mitotic catastrophe, which is also known as mitotic death, a form of nonapoptotic cell death, featuring the morphology of multinucleated giant cells.( 14 ) They undergo slow or delayed cell death. Polyploidy cells after genotoxic stress express Rad51, a protein involved in the DNA damage repair mechanism, suggesting a possible selection or repair process in these cells.( 15 ) Other factors, including the genome guardian, p53 protein and cell‐cycle regulatory protein, p21waf1/cip1, also work together with survivin in response to DNA damage caused by anticancer agents.
Figure 2.

Effect of inhibition of survivin.
In this review, we introduce the regulation and expression of survivin in human cancers, and we propose the possibility that survivin is a promising therapeutic target against human malignancies.
Survivin regulation
One of the mechanisms of survivin regulation is at the transcriptional level (Fig. 3). Survivin promoter activity is regulated through the β‐catenin activated T‐cell factor (TCF) transcription factor.( 16 ) The expression of survivin is also Sp1‐dependent, with transcriptional activity requiring two critical Sp1 sites.( 17 ) Another report indicates that Sp1‐mediated survivin expression is further subject to a p53‐mediated transcriptional repression.( 18 ) Other transcriptional regulation is mediated by Stat3.( 19 ) Stat3 inhibitors down‐regulate survivin in human breast and ovarian cancer cells.( 20 ) However, we have reported that the Sp1 transcription factor, rather than Stat3, dominantly regulated promoter activity of survivin in esophageal cancer and gastric cancer; thus the regulation mode appears to be highly cell‐type specific.( 21 ) Hypoxia‐inducible factor‐1 alpha (HIF‐1α) is another transcription factor involved in survivin regulation. Epidermal growth factor (EGF)–stimulated survivin up‐regulation was mediated by HIF‐1α in breast cancer cell lines.( 22 )
Figure 3.

Survivin regulation at transcriptional and post‐translational levels.
Survivin is a relatively short‐lived protein (t1/2 at 30 min), regulated by proteasome degradation after polyubiquitination.( 23 ) In addition of transcriptional level, the survivin protein becomes stable by phosphorylation of Thr34 by cdc2, which slows down clearance of survivin through the proteasome degradation.( 24 ) Flavopiridol down‐regulates survivin through inhibition of phosphorylation of Thr34.( 25 ) Cyclin‐dependent kinase inhibitor, NU6140, and paclitaxel combination also cause down‐regulation of survivin through the inhibition of phosphorylation of Thr34 and enhanced caspase‐dependent apoptosis in the HeLa cervical carcinoma cell line and an ovarian cell line.( 26 ) Heat shock protein 90 (Hsp90), well known for its chaperone activity, has also been reported to regulate survivin. The disruption of survivin–Hsp90 interaction has been shown to result in the proteasome degradation of survivin, leading to mitotic defects and apoptosis.( 27 )
The Akt signaling pathway is also involved in survivin expression. Geranylgeranyltransferase I inhibitors (GGTIs) induce apoptosis in ovarian cancer cells by inhibition of the PI3K/AKT and survivin pathways.( 28 ) A new class of anticancer drug, Trichostatin A (TCA), inhibitor of mammalian histone deacetylase (HDAC), also transiently induces survivin expression via activation of the EGFR/PI3K/Akt cell survival pathway.( 29 ) Treatment of small cell lung cancer cells with cisplatin leads to up‐regulation of survivin through the Akt survival pathway.( 30 )
Up‐regulation of survivin in human malignancies
Survivin is expressed in embryonic and fetal tissues, but is almost undetectable in adult tissues.( 1 , 3 ) However, overexpression of survivin has been reported in almost all human malignancies including lung cancer, breast cancer; stomach, esophagus, liver, and ovary cancers; and brain and hematological cancers.( 1 , 31 ) Based on detection of protein by immunohistochemistry and mRNA by polymerase chain reaction techniques, overexpression of survivin has been reported in various human malignancies (Table 1 ( 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 )). Similar to its expression, survivin promoter activity is largely silent in normal cell types, but is increased in tumor cell lines.( 46 )
Table 1.
Survivin protein or mRNA expression in human cancers
| Cancer | Expression | Ref No. |
|---|---|---|
| Esophageal cancer | 80% | ( 32 ) |
| Gastric cancer | 34.5~68% | ( 33, 34 ) |
| Colorectal cancer | 63.5% | ( 35 ) |
| Pancreatic cancer | 76.9~88% | ( 36, 37 ) |
| Hepatocellular cancer | 41~87% | ( 38, 39 ) |
| Breast cancer | 70.7~90.2% | ( 40, 41 ) |
| Ovarian cancer | 73.5% | ( 42 ) |
| Lung cancer | 85.5% | ( 43 ) |
| Bladder cancer | 57.8% | ( 44 ) |
| Acute myeloid leukemia | 54.8% | ( 45 ) |
| Acute lymphocytic leukemia | 68.8% | ( 45 ) |
The specific up‐regulation of survivin in cancer cells is further supported by the immunological responses detected against it. Survivin‐specific CD8+ T‐cell‐mediated and CD4+ cell‐mediated immune response have been identified in the peripheral blood of colorectal cancer patients.( 47 ) Cytotoxic T‐lymphocyte response to survivin has also been detected in breast, melanoma, and chronic lymphatic leukemia patients.( 48 ) Induction of survivin‐specific immune response has been confirmed in vitro.( 49 )
Cancer cells thus seem to utilize the cytoprotective character of survivin to ensure its continual progression. During colorectal carcinogenesis, the expression of survivin was found to increase from adenoma with low‐grade dysplasia (2.3%) to high‐grade dysplasia (52.4%) to carcinoma in adenoma (63.3%).( 50 ) Similar results has been found in the tumorigenesis of pancreatic ductal adenocarcinoma. While no expression has been found in normal pancreatic ducts, survivin expression steadily increased from low‐grade pancreatic intraepithelial neoplasia to high‐grade lesions and to the highest in pancreatic ductal adenocarcinoma tissues.( 51 ) A study on ovarian cancer has also reported that survivin expression increased from benign tumors (21.2%) to borderline tumors (47.8%) to ovarian carcinomas (51.1%).( 52 ) Furthermore, most reports have suggested consistencies with a reduced apoptotic index( 53 ) indicating a survival advantage in these tumor cells.
Survivin expression has been shown to induce a global transcriptional change in the tumor microenvironment that may promote tumor progression. In addition to the survival of cancer cells, survivin has been implicated in angiogenesis. Molecular targeting of survivin has led to not only inhibition of tumor growth, but also reduction in tumor‐derived blood vessels.( 54 ) Survivin has also showed a cytoprotection effect, possibly mediated by PI3K pathways, on endothelial cells, which is mediated by VEGF.( 55 )
Association between survivin expression and prognosis or therapy resistance
Survivin expression in most cancers is associated with patients’ survival or disease recurrence, and resistance to chemotherapy or radiotherapy. A higher survivin expression has been correlated with an unfavorable survival or disease recurrence in colorectal cancer, particularly in stage II disease( 35 ) in esophageal cancer,( 56 ) hepatocellular carcinoma,( 38 ) lung cancer,( 43 ) glioma,( 53 ) leukemia,( 45 ) and other cancer types. Survivin has been further identified as an independent prognosis factor in multivariate analysis in different patient groups. With regard to chemosensitivity, patients with lower survivin expression were more responsive to preoperative chemotherapy with 5‐flourouracil and cisplatin in esophageal cancer.( 57 ) Overexpression of survivin was also associated with clinical resistance to a taxol‐based regimen for ovarian carcinomas.( 58 ) It is also reported that patients with lower survivin expression in pretreatment biopsies were more responsive to radiotherapies in rectal cancer.( 59 ) Taken together, these findings suggest that diminution of the basal survivin expression in tumor cells would increase sensitivity to cancer therapies.
Modulation of survivin by anticancer drugs
The above evidence in clinics indicates that overexpression of survivin in cancer invariably provides a survival advantage in tumor cells. Therefore, lack of survivin or disruption of the survivin function would cause cell death such as apoptosis and mitotic catastrophe in these cells. In fact, many anticancer drugs currently in use in clinics as well as those still undergoing testing are reportedly shown to have inhibitory effects on survivin. (i) Celecoxib and its derivatives have been shown to down‐regulate survivin in a wide range of tumor cells, including glioblastoma; lymphoma; multiple myeloma; and carcinoma of the breast, colon and prostate, inducing apoptosis.( 60 ) (ii) COX‐2 inhibitor etodolac and oxaliplatin have been shown to significantly down‐regulate survivin in human colon cancer cells, causing growth inhibition and cell death.( 61 ) (iii) Silibilin derivatives, such as Silymarin, which have showed efficacy toward prostate cancer, have also been shown to down‐regulate survivin.( 62 ) (iv) Tetra‐O‐methyl nordihydroguaiaretic acid (a small organic compound with antitumor activity) has been shown to selectively target survivin and cdc2, both Sp1‐regulated proteins that control apoptosis and the cell cycle. Reintroduction of survivin inhibited apoptosis.( 63 ) (v) Proteasome inhibitor (MG132 or MG115) has been shown to down‐regulate survivin in hepatocellular carcinoma cell lines.( 64 ) (vi) Other novel therapeutic approaches using small molecule inhibitors, nucleoside analogs,( 65 ) or natural products or components such as betulinic acid( 66 ) indole‐3‐carbinol, found in vegetables,( 67 ) have demonstrated anticancer effects, and also been shown to down‐regulate survivin after treatment in certain cell lines used in respective studies.
In the meantime, while survivin is down‐regulated by most anticancer drugs, up‐regulations following treatment regimens have been previously reported. Treatment by taxol, adriamycin, or ultraviolet B irradiation in breast cancer MCF‐7 and cervical cancer HeLa cells have resulted in a four‐ to five‐fold increase in survivin expression, possibly by activation of Thr34 phosphorylation as mentioned above.( 68 ) Exposure of cells to radiation or photodynamic therapy similarly caused up‐regulation of survivin.( 69 ) Cisplatin has also led to up‐regulation of survivin through the Akt survival pathway in small cell lung cancer cells.( 30 ) Supposedly these could be responses for tumor cells to survive in resistance to the various toxic stresses.
Therapeutic approaches
Similar to the conserved mechanism in the developmental process, survivin inhibition has been shown to enhance cell death.( 3 ) Various approaches have been attempted to down‐regulate or block survivin in cancer cells to inhibit cell survival and at the same time enhance cell death.
Inhibition of survivin by the molecular approach.
-
1
Molecular antagonist utilizing antisense, siRNA, ribozyme, dominant‐negative mutants have shown satisfactory results. An approach using short hairpin RNA (shRNA) successfully reduced survivin expression, and induced apoptosis and growth inhibition in a lymphoma cell line.( 70 ) Ribozyme‐mediated inhibition of survivin induced polyploidy and caspase‐9‐dependent apoptosis in prostate cancer cells.( 71 ) We have shown that siRNA against survivin induced drastic induction of mitotic catastrophe in esophageal cancer cells (Fig. 4). A therapeutic approach using survivin responsive conditionally replicating adenovirus displayed tumor cell specificity, induced tumor cell death and inhibition of tumor growth,( 72 ) and also sensitized human melanoma cells to gamma irradiation.( 73 ) Replication‐deficient adenovirus encoding a survivin mutant caused massive apoptosis in tumor cells but had no effect on proliferation normal cells.( 74 )
Figure 4.

Survivin inhibition caused mitotic catastrophe. Short interfering RNA (siRNA) against survivin down‐regulated survivin protein expression, inducing mitotic catastrophe in an esophageal squamous cell carcinoma cell line, TE8. The arrows indicate multinucleated giant cells compatible with mitotic catastrophe. Similar phenomena have been observed in other gastrointestinal cancer cell lines.( 21 )
Other treatments plus survivin inhibition. Survivin inhibition by molecular techniques has been shown to increase the sensitivity of cells to chemotherapeutic drugs, melanoma cells to cisplatin treatment,( 75 ) lung cancer cells to adriamycin treatment,( 76 ) and prostate cancer cells to paclitaxel.( 77 ) Inhibition of survivin by siRNA also increased the sensitivity of colorectal and pancreatic cancer cells to radiation therapy.( 59 , 78 ) Combination use of siRNA against survivin and heat shock protein, Hsp90 inhibitor 17‐allylamino‐17‐demethoxygeldamycin (17‐AAG), reduced cell proliferation and enhanced apoptosis.( 79 ) In addition, shepherdin, an antagonist against interaction between Hsp90 and survivin, induced massive death of tumor cells through apoptotic and nonapoptotic mechanisms.( 80 )
Combination therapy with survivin inhibitors and other treatment. Since various anticancer drugs can reduce survivin levels in tumor cells, they may enhance efficacy of the other chemotherapy or radiotherapy treatments. During investigation into why oxaliplatin can enhance the efficacy of other anticancer drugs, we have found that oxaliplatin was a potent inhibitor of survivin in colon cancer cells( 81 ) (Fig. 5a), and also in other gastrointestinal tumor cells.( 21 , 82 ) On the hand, taxol causes cell arrest in mitosis with increased expression of survivin.( 68 ) To our interest, oxaliplatin in combination with taxol displayed a synergic growth inhibition that could be attributable to drastic mitotic catastrophe (Fig. 5b,c). In such a combination treatment, survivin was not enhanced enough compared to inducement by taxol alone (Fig. 5d). Therefore, we postulate that down‐regulation of survivin by oxaliplatin may be one of the events involved in the synergic mechanism. These findings suggest that certain agents (e.g. oxaliplatin) that decrease survivin expression may considerably influence the effectiveness of the other treatment, possibly by breaking the balance for cell survival signaling. This notion should be applicable to radiation therapy in combination with oxaliplatin, as a survivin inhibitor. However, we should emphasize that the combination of survivin inhibitors with mitotic inhibitors, such as taxol, might be more effective than with other antitumor drugs that arrest the cells at G1 phase, taking into account the essential role of survivin in mitosis. This issue should be further addressed in the future.
Figure 5.
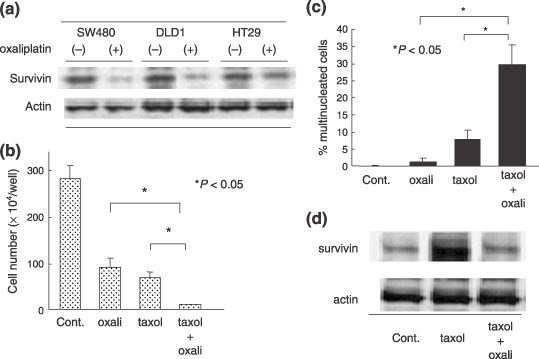
(a) Oxaliplatin induced survivin down‐regulation in colon cancer cells. (b) Combination treatment of oxaliplatin and taxol displayed a synergistic effect on SW480 colon cell growth. (c) Increased cells undergoing mitotic catastrophe were observed in combination treatment group. (d) Oxaliplatin withdrew taxol‐induced survivin up‐regulation.
Also, the mechanisms underlying the down‐regulation of survivin levels by oxaliplatin should be clarified. We suggest two mechanisms, that is, Sp1 down‐regulation (Fig. 6a–c) and proteasome degradation (Fig. 6d), which can mediate survivin inhibition after oxaliplatin treatment, at least in certain gastrointestinal cell lines.
Figure 6.
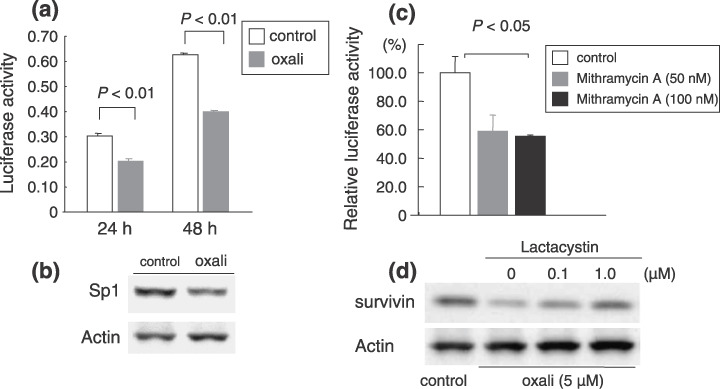
Survivin regulation by oxaliplatin via Sp1‐inactivation and proteasome‐mediated degradation. (a) Survivin promoter activity in esophageal TE7 cells after treatment with oxaliplatin. Oxaliplatin significantly reduced survivin promoter activity. (b) Sp1 expression at 48 h after oxaliplatin treatment. Sp1 expression decreased in oxaliplatin‐treated cells compared to untreated controls. (c) Effect of Sp1 inhibitor (Mithramycin A) on survivin‐promoter activity at 24 h after transfection. Mithramycin A inhibited survivin promoter activity. (d) Proteasome‐mediated degradation. Effects of a proteasome inhibitor (lactacystin) on survivin expression following oxaliplatin treatment. Down‐regulation of survivin expression after oxaliplatin treatment was partly rescued by the addition of the lactacystin. These events (a–d) were observed in several gastrointestinal cancer cells (data not shown).
Targeting overexpression of survivin. Invariably high and specific survivin expression in various human cancers makes it an optimal therapeutic target. Utilizing a fusion of the survivin gene promoter to the coding sequence of active granzyme B has led to increased expression of granzyme B in tumor cells, resulting in a higher rate of apoptotic cell death.( 83 ) Similar approaches utilizing survivin promoter‐driven siRNA targeting human telomerase reverse transcriptase (hTERT) and stathmin were also successful in increasing radiosensitization and growth inhibition, respectively.( 84 , 85 )
Immunological approaches using vaccine have also been developed. Vaccine utilizing survivin antigenic peptide, DNA‐based vaccine, as well as dendritic cells loaded with survivin protein, has demonstrated promising results in inducing cytotoxic T‐lymphocyte (CTL) response, leading to effective eradication of tumor cells in animal models or patients in clinical trials.( 86 , 87 , 88 ) A patient was reported to achieve complete remission of liver metastasis of pancreatic cancer following treatment with survivin vaccine.( 89 ) Further validation of survivin as a target for cancer treatment will be dependent on the outcomes of clinical studies.
Conclusion
Survivin is one of the highly conserved proteins that are indispensable for cell survival in development. Survivin is repressed or down‐regulated in most normal adult tissues; hence its up‐regulation in cancers could make it a highly specific target. The majority of anticancer drugs, although mediated through different regulatory pathways in different types of cancers, lead to down‐regulation of survivin, which causes cell death through apoptosis or mitotic catastrophe. Up‐regulation of survivin by others nevertheless suggests possible mechanisms of compensation to assure cell survival in certain cells. Inhibition of survivin with novel molecular genetic approaches in combination with the use of chemotherapeutic drugs or radiotherapy may improve some of the current regimen. Although survivin is a potential target for therapeutic purposes, the approaches nevertheless should be carefully designed, considering the possibility of hindering its normal functions in limited adult tissues, such as hematopoietic cells. Further investigation is thus necessary to understand the complex cellular circuitry of survivin in cancer as well as in physiological conditions.
References
- 1. Ambrosini G, Adida C, Altieri DC. A novel anti‐apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997; 3: 917–21. [DOI] [PubMed] [Google Scholar]
- 2. Li F, Ambrosini G, Chu EY et al . Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580–4. [DOI] [PubMed] [Google Scholar]
- 3. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003; 3: 46–54. [DOI] [PubMed] [Google Scholar]
- 4. Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 2002; 3: 401–10. [DOI] [PubMed] [Google Scholar]
- 5. Tamm I, Wang Y, Sausville E et al . IAP‐family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, Caspases, and anticancer drugs. Cancer Res 1998; 58: 5315–20. [PubMed] [Google Scholar]
- 6. Lu B, Mu Y, Cao C, Zeng F et al . Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res 2004; 64: 2840–5. [DOI] [PubMed] [Google Scholar]
- 7. Chandele A, Prasad V, Jagtap JC, Shukla R, Shastry PR. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine‐induced apoptosis. Neoplasia 2004; 6: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin S, Sung BJ, Cho YS et al . An anti‐apoptotic protein human survivin is a direct inhibitor of caspase‐3 and ‐7. Biochem 2001; 40: 1117–23. [DOI] [PubMed] [Google Scholar]
- 9. Sun C, Nettesheim D, Liu Z, Olejniczak ET. Solution structure of human survivin and its binding interface with smac/Diablo. Biochem 2005; 44: 11–17. [DOI] [PubMed] [Google Scholar]
- 10. Liu T, Brouha B, Grossman D. Rapid induction of mitochondrial events and caspase‐independent apoptosis in survivin targeted melanoma cells. Oncogene 2004; 23: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skoufias DA, Mollinari C, Lacroix FB, Margolis RL. Human survivin is a kinetochore‐associated passenger protein. J Cell Biol 2000; 151: 1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lens SMA, Wolthuis RMF, Klompmaker R et al . Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J 2003; 22: 2934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang D, Welm A, Bishop M. Cell division and cell survival in the absence of survivin. Proc Natl Acad Sci USA 2004; 101: 15 100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okada H, Mak TW. Pathways of apoptotic and non‐apoptotic death in tumour cells. Nat Rev Cancer 2004; 4: 592–603. [DOI] [PubMed] [Google Scholar]
- 15. Ivanov A, Cragg MS, Erenpreisa J, Emzinsh D, Lukman H, Illidge TM. Endoploidy cells produced after severe genotoxic damage have the potential to repair DNA double strand breaks. J Cell Sci 2003; 116: 4095–106. [DOI] [PubMed] [Google Scholar]
- 16. Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet 2003; 362: 205–9. [DOI] [PubMed] [Google Scholar]
- 17. Li F, Altieri DC. Transcriptional analysis of human survivin gene expression. Biochem J 1999; 344: 305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esteve PO, Chin HG, Pradhan S. Molecular mechanisms of transactivation and doxorubicin‐mediated repression of survivin gene in cancer cells. J Biol Chem 2007; 282: 2615–25. [DOI] [PubMed] [Google Scholar]
- 19. Gritsko T, Williams A, Turkson J et al . Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res 2006; 12: 11–19. [DOI] [PubMed] [Google Scholar]
- 20. Duan Z, Bradner JE. Greenberg et al . SD‐1029 inhibits signal transducer and activator of transcription 3 nuclear translocation. Clin Cancer Res 2006; 12: 6844–52. [DOI] [PubMed] [Google Scholar]
- 21. Ngan CY, Yamamoto H, Takagi A et al . Oxaliplatin induces mitotic catastrophe and apoptosis in esophageal cancer cells. Cancer Sci 2008; 99: 129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng XH, Karna P, Cao Z, Jiang B‐H, Zhou M, Yang L. Cross‐talk between epidermal growth factor receptor and hypoxia‐inducible factor‐1α signal pathways increases resistance to apoptosis by up‐regulating survivin gene expression. J Biol Chem 2006; 281: 25 903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin‐proteasome pathway regulates survivin degradation in a cell cycle‐dependent manner. J Cell Sci 2000; 113: 4363–71. [DOI] [PubMed] [Google Scholar]
- 24. O’Connor DS, Grossman D, Plescia J et al . Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA 2000; 97: 13 103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newcomb EW. Flavopiridol: pleiotropic biological effects enhance its anti‐cancer activity. Anticancer Drugs 2004; 15: 411–19. [DOI] [PubMed] [Google Scholar]
- 26. Pennati M, Campbell AJ, Curto M et al . Potentiaion of paclitaxel‐induced apoptosis by the novel cyclin‐dependent kinase inhibitor NU6140: a possible role for survivin down‐regulation. Mol Cancer Ther 2005; 4: 1328–37. [DOI] [PubMed] [Google Scholar]
- 27. Fortugno P, Beltrami E, Plescia J et al . Regulation of survivin function by Hsp90. Proc Natl Acad Sci USA 2003; 100: 13 791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dan HC, Jiang K, Coppola D et al . Phosphatidylinositol‐3‐OH kinase/AKT and survivin pathways as critical targets fro geranylgeranyltransferase I inhibitor‐induced apoptosis. Oncogene 2004; 23: 706–15. [DOI] [PubMed] [Google Scholar]
- 29. Zhou C, Qiu L, Sun Y et al . Inhibition of EGFR/PI3K/AKT cell survival pathway promotes TSA's effect on cell death and migration in human ovarian cancer cells. Int J Oncol 2006; 29: 269–78. [PubMed] [Google Scholar]
- 30. Belyanskaya LL, Hopkins‐Donaldson S, Kurtz S et al . Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int J Cancer 2005; 117: 755–63. [DOI] [PubMed] [Google Scholar]
- 31. Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adults tissues. Mol Cancer Ther 2006; 5: 1087–98. [DOI] [PubMed] [Google Scholar]
- 32. Grabowski P, Kuhnel T, Muhr‐Wilkenshoff F et al . Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer 2003; 88: 115–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 1998; 58: 1808–12. [PubMed] [Google Scholar]
- 34. Yu J, Leung WK, Ebert MP et al . Increased expression of survivin in gastric cancer patients and in first degree relatives. Br J Cancer 2002; 87: 91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarela AI, Macadam RCA, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut 2000; 46: 645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 2001; 92: 271–8. [DOI] [PubMed] [Google Scholar]
- 37. Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer 2002; 86: 886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikeguchi M, Ueda T, Sakatani T, Hirooka Y, Kaibara N. Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn Mol Pathol 2002; 11: 33–40. [DOI] [PubMed] [Google Scholar]
- 39. Ito T, Shiraki K, Sugimoto K et al . Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology 2000; 31: 1080–5. [DOI] [PubMed] [Google Scholar]
- 40. Nasu S, Yagihashi A, Izawa A et al . Survivin mRNA expression in patients with breast cancer. Anticancer Res 2002; 22: 1839–43. [PubMed] [Google Scholar]
- 41. Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res 2000; 6: 127–34. [PubMed] [Google Scholar]
- 42. Cohen C, Lohmann CM, Cotsonis G, Lawson D, Santoianni R. Survivin expression in ovarian carcinoma correlation with apoptotic markers and prognosis. Mod Pathol 2003; 16: 574–83. [DOI] [PubMed] [Google Scholar]
- 43. Monzo M, Rosell R, Felip E et al . A novel anti‐apoptosis gene: re‐expression of survivin messenger RNA as a prognosis marker in non‐small‐cell lung cancers. J Clin Oncol 1999; 17: 2100–4. [DOI] [PubMed] [Google Scholar]
- 44. Lehner R, Lucia MS, Jarboe EA et al . Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl Immunohistochem Mol Morph 2002; 10: 134–8. [DOI] [PubMed] [Google Scholar]
- 45. Mori A, Wada H, Nishimura Y, Okamoto T, Takemoto Y, Kakishita E. Expression of the antiapoptosis gene survivin in human leukemia. Int J Hematol 2002; 75: 161–5. [DOI] [PubMed] [Google Scholar]
- 46. Bao R, Connoly DC, Murphy M et al . Activation of cancer‐specific gene expression by the survivin promoter. J Natl Cancer Inst 2002; 94: 522–8. [DOI] [PubMed] [Google Scholar]
- 47. Casati C, Dalerba P, Rivoltini L et al . The apoptosis inhibitor protein survivin induces tumor‐specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res 2003; 63: 4507–15. [PubMed] [Google Scholar]
- 48. Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res 2001; 61: 869–72. [PubMed] [Google Scholar]
- 49. Schmidt SM, Schag K, Muller MR et al . Survivin is a shared tumor‐associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood 2003; 102: 571–6. [DOI] [PubMed] [Google Scholar]
- 50. Kawasaki H, Toyoda M, Shinohara H et al . Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 2001; 91: 2026–32. [DOI] [PubMed] [Google Scholar]
- 51. Bhanot U, Heydrich R, Moller P, Hasel C. Survivin expression in pancreatic intraepithelial neoplasia (PanIN): steady increase along the developmental stages of pancreatic ductal adenocarcinoma. Am J Surg Pathol 2006; 30: 754–9. [DOI] [PubMed] [Google Scholar]
- 52. Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol 2002; 21: 315–20. [PubMed] [Google Scholar]
- 53. Chakravarti A, Noll E, Black PM et al . Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 2002; 20: 1063–8. [DOI] [PubMed] [Google Scholar]
- 54. Blanc‐Brude OP, Mesri M et al . Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor‐associated angiogenesis. Clin Cancer Res 2003; 9: 2683–92. [PubMed] [Google Scholar]
- 55. Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA 2002; 99: 4349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ikeguchi M, Kaibara N. Survivin messenger RNA expression is a good prognostic biomarker for oesophageal cancer. Br J Cancer 2002; 87: 883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kato J, Kuwahara Y, Mitani M et al . Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer 2001; 95: 92–5. [DOI] [PubMed] [Google Scholar]
- 58. Zaffaroni N, Pennati M, Colella G et al . Expression of the anti‐apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci 2002; 59: 1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodel F, Hoffman J, Distel L et al . Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res 2005; 65: 4881–7. [DOI] [PubMed] [Google Scholar]
- 60. Pyrko P, Soriano N, Kardosh A et al . Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non‐cyclooxygenase‐2‐inhibitory analog, dimethyl‐celecoxib (DMC), in tumor cells in vitro and in vivo . Mol Cancer 2006; 5: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lin J, Hsiao PW, Chiu TH, Chao JI. Combination of cyclooxygenase‐2 inhibitors and oxaliplatin increases the growth inhibition and death in human colon cancer cells. Biochem Pharmacol 2005; 70: 658–67. [DOI] [PubMed] [Google Scholar]
- 62. Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B and isosilybin A inhibit growth, induce G1 arrest and cause apoptosis in human prostate cancer LNCaP and 22Rv1 cells. Carcinogenesis 2007; 28: 1533–42. [DOI] [PubMed] [Google Scholar]
- 63. Chang CC, Heller JD, Kuo J, Huang RC. Tetra‐O‐methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting cdc2 and survivin expression. Proc Natl Acad Sci USA 2004; 101: 13 239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Inoue T, Shiraki K, Fuke H et al . Proteasome inhibition sensitizes hepatocellular carcinoma cells to TRAIL by suppressing caspase inhibitors and AKT pathway. Anticancer Drugs 2006; 17: 261–8. [DOI] [PubMed] [Google Scholar]
- 65. Radhakrishnan SK, Halasi M, Bhat UG, Kurmasheva RT, Houghton PJ, Gartel AL. Proapoptotic compound ARC targeted Akt and N‐myc in neuroblastoma cells. Oncogene 2008; 27: 694–9. [DOI] [PubMed] [Google Scholar]
- 66. Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res 2007; 67: 2816–23. [DOI] [PubMed] [Google Scholar]
- 67. Rahman KMW, Li Y, Wang Z, Sarkar SH, Sarkar FH. Gene expression profiling revealed survivin as a target of 3,3′‐diinolylmethane‐induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res 2006; 66: 4952–60. [DOI] [PubMed] [Google Scholar]
- 68. Wall NR, O’Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res 2003; 63: 230–5. [PubMed] [Google Scholar]
- 69. Asanuma K, Moriai R, Yajima T et al . Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res 2000; 91: 1204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gu CM, Zhu YK, Ma YH et al . Knockdown of survivin gene by vector‐based short hairpin RNA technique induces apoptosis and growth inhibition in Burkitt's lymphoma Raji cell line. Neoplasma 2006; 53: 206–12. [PubMed] [Google Scholar]
- 71. Pennati M, Binda M, Colella G et al . Ribozyme‐mediated inhibition of survivin expression increases spontaneous and drug‐induced apoptosis and decreases the tumorigenic potential of human prostate cancer cells. Oncogene 2004; 23: 386–94. [DOI] [PubMed] [Google Scholar]
- 72. Kamizono J, Nagano S, Murofushi Y et al . Survivin‐responsive conditionally replicating adenovirus exhibits cancer‐specific and efficient viral replication. Cancer Res 2005; 65: 5284–91. [DOI] [PubMed] [Google Scholar]
- 73. Pennati M, Binda M, Colella G et al . Radiosensitization of human melanoma cells by ribozyme‐mediated inhibition of survivin expression. J Invest Dermatol 2003; 120: 648–54. [DOI] [PubMed] [Google Scholar]
- 74. Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest 2001; 108: 981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pennati M, Colella G, Folini M, Citti I, Daidone MG, Zaffaroni N. Ribozyme‐mediated attenuation of survivin expression sensitizes human melanoma cells to cisplatin‐induced apoptosis. J Clin Invest 2002; 109: 285–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yonesaka K, Tamura K, Kurata T et al . Small interfering RNA targeting survivin sensitizes lung cancer cell with mutant p53 to adriamycin. Int J Cancer 2006; 118: 812–20. [DOI] [PubMed] [Google Scholar]
- 77. Zhang M, Mukherjee N, Bermudez RS et al . Adenovirus‐mediated inhibition of survivin expression sensitizes human prostate cancer cells to paclitaxel in vitro and in vivo . Prostate 2005; 64: 293–302. [DOI] [PubMed] [Google Scholar]
- 78. Kami K, Doi R, Koizumi M et al . Downregulation of survivin by siRNA diminishes radioresistance of pancreatic cancer cells. Surgery 2005; 138: 299–305. [DOI] [PubMed] [Google Scholar]
- 79. Paduano F, Villa R, Pennati M et al . Silencing of survivin gene by small interfering RNAs produces supra‐additive growth suppression in combination with 17‐allylamino‐17‐demethoxygeldanamycin in human prostate cancer cells. Mol Cancer Ther 2006; 5: 179–86. [DOI] [PubMed] [Google Scholar]
- 80. Plescia J, Salz W, Xia F et al . Rational design of shepherdin, a novel anticancer agent. Cancer Cell 2005; 7: 457–68. [DOI] [PubMed] [Google Scholar]
- 81. Fujie Y, Yamamoto H, Ngan CY et al . Oxaliplatin, a potent inhibitor of survivin, enhances paclitaxel‐induced apoptosis and mitotic catastrophe in colon cancer cells. Jpn J Clin Oncol 2005; 35: 453–63. [DOI] [PubMed] [Google Scholar]
- 82. Gu J, Yamamoto H, Lu X et al . Low dose oxaliplatin enhances the antitumor efficacy of paclitaxel in human gastric cancer cell lines. Digestion 2006; 74: 19–27. [DOI] [PubMed] [Google Scholar]
- 83. Caldas H, Jaynes FO, Boyer MW, Hammond S, Altura RA. Survivin and granzyme B‐induced apoptosis, a novel anticancer therapy. Mol Cancer Ther 2006; 5: 693–703. [DOI] [PubMed] [Google Scholar]
- 84. Wang R, Lin F, Wang X et al . The therapeutic potential of survivin promoter‐driven siRNA on suppressing tumor growth and enhancing radiosensitivity of human cervical carcinoma cells via downregulating hTERT gene expression. Cancer Biol Ther 2007; 6: 1295–301. [DOI] [PubMed] [Google Scholar]
- 85. Zhang HZ, Wang Y, Gao P et al . Silencing stathmin gene expression by survivin promoter‐driven siRNA vector to reverse malignant phenotype of tumor cells. Cancer Biol Ther 2006; 5: 1457–61. [DOI] [PubMed] [Google Scholar]
- 86. Cho HI, Kim EK, Park SY, Lee SK, Hong YK, Kim TG. Enhanced induction of anti‐tumor immunity in human and mouse by dendritic cells pulsed with recombinant TAT fused human survivin protein. Cancer Lett 2007; 258: 189–98. [DOI] [PubMed] [Google Scholar]
- 87. Idenoue A, Hirohashi Y, Torigoe T et al . A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 2005; 11: 1474–82. [DOI] [PubMed] [Google Scholar]
- 88. Xiang R, Mizutani N, Luo Y et al . A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res 2005; 65: 553–61. [PubMed] [Google Scholar]
- 89. Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA‐A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 2006; 55: 1294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


