Abstract
Mucosal-associated invariant T (MAIT) cells are antimicrobial T cells abundant in the gut, but mechanisms for their migration into tissues during inflammation are poorly understood. Here, we used acute pediatric appendicitis (APA), a model of acute intestinal inflammation, to examine these migration mechanisms. MAIT cells were lower in numbers in circulation of patients with APA but were enriched in the inflamed appendix with increased production of proinflammatory cytokines. Using the patient-derived appendix organoid (PDAO) model, we found that circulating MAIT cells treated with inflammatory cytokines elevated in APA up-regulated chemokine receptors, including CCR1, CCR3, and CCR4. They exhibited enhanced infiltration of Escherichia coli–pulsed PDAO in a CCR1-, CCR2-, and CCR4-dependent manner. Close interactions of MAIT cells with infected organoids led to the PDAO structural destruction and death. These findings reveal a previously unidentified mechanism of MAIT cell tissue homing, their participation in tissue damage in APA, and their intricate relationship with mucosal tissues during acute intestinal inflammation in humans.
Patient-derived organoid model reveals the mechanism of MAIT cell recruitment and pathological roles in acute appendicitis.
INTRODUCTION
Mucosal-associated invariant T (MAIT) cells are an evolutionarily conserved innate-like T cell population abundant in the human blood and barrier tissues, including the gastrointestinal tract [reviewed in (1, 2)]. Human MAIT cells are defined by their semi-invariant T cell receptor (TCR) usage and recognize microbial metabolite antigens presented by the major histocompatibility complex related 1 (MR1) molecule (1, 3, 4). The most potent MR1-binding antigens to date are 5-(2-oxopropylideneamino)-5-d-ribitylaminouracil (5-OP-RU) and 5-(2-oxoethylideneamino)-5-d-ribitylaminouracil (5-OE-RU), both of which are derived from vitamin B2 (riboflavin) biosynthesis (5, 6). Because of the strong reactivity of MAIT cells to these microbial metabolites (5, 7, 8), MR1 tetramer loaded with 5-OP-RU was developed to identify MAIT cells in multiple eutherian mammals, including humans (9–13). Human MAIT cells are also identifiable through their expression of TCR Vα7.2 combined with high levels of the C-type lectin CD161 (14–16). MAIT cells mediate antimicrobial immunity by eliciting type 1, type 17, cytotoxic, and innate-like effector responses within the gastrointestinal tract (1, 2). MAIT cells respond to riboflavin-producing microbes via a combination of MR1/TCR-dependent and cytokine-dependent pathways and release cytolytic proteins and antimicrobial cytokines to kill both intracellular and extracellular bacteria and orchestrate adaptive immune responses (14, 17–21). In addition, MAIT cells respond to riboflavin-auxotrophic intestinal bacteria through MR1/TCR-independent, cytokine-dependent pathways to exert antimicrobial effector responses (22–25). TCR-dependent activation can synergize with cytokine-derived signals to promote MAIT cell activation and further facilitate the recruitment, maturation, and transactivation of other immune cells that lead to the clearance of the pathogens (23, 26).
Circulating MAIT cells express high levels of gut-homing and inflammation-associated chemokine receptors, including CCR2, CCR5, CCR6, CCR9, and CXCR6 (14, 27–30), as well as tissue-homing molecules including α4β1, α4β7, αΕβ7, and αLβ2 integrins (12, 24, 28, 31–36). However, although MAIT cells are implicated in the setting of acute intestinal infections and inflammation, there is no conclusive evidence for their trafficking to the gut or mechanisms by which this may occur (2, 37–47). In addition, many of these studies were conducted using circulating MAIT cell samples [reviewed in (2)]. Given the functional differences between circulating and mucosal MAIT cells (48), there are limitations to the extrapolation of gut MAIT cell function from such studies. Likewise, murine models offer limited translation to human bacterial infection and inflammation due to naturally low numbers of MAIT cells in mice and differences in genetics, gastrointestinal microbiota, and pathogens between species [reviewed in (49)].
In the present study, we investigate acute pediatric appendicitis (APA), a severe inflammation of the appendix as an outcome of an acute bacterial intestinal infection, to study the changes of MAIT cell numbers and their effector responses in human gastrointestinal mucosa during acute inflammation. Moreover, we have established a three-dimensional (3D) patient-derived appendix organoid (PDAO) and MAIT cell coculture system to delineate the cellular interplay in the intestinal mucosa in this setting. Organoids are micro-sized 3D models of organs that are derived from embryonic stem cells, inducible pluripotent stem cells, or adult stem cell–containing tissue-derived primary cells (50, 51). This emerging, state-of-the-art technology is increasingly used to study complex cellular interactions within the tissue microenvironment, thus enabling a more profound understanding of biological processes during homeostasis and in disease settings (2, 52). Our findings indicate that circulating MAIT cells migrate into the appendix following activation by proinflammatory cytokines elevated in appendicitis. Furthermore, our robust PDAO model evaluated MAIT cell migration toward and interaction with intestinal mucosal epithelium and its involvement in tissue damage during APA. Collectively, our comprehensive study using peripheral blood (PB), primary tissue samples, and PDAO during acute appendicitis reveals the complex interactions between MAIT cells and intestinal mucosal epithelium in the setting of acute bacterial gastrointestinal infections and inflammation in humans.
RESULTS
Clinical characteristics of pediatric patients with acute appendicitis and control participants
The pediatric cohorts comprised 48 patients with acute appendicitis and 37 control participants. There was no significant difference in age and sex ratios between the two groups (table S1). The levels of white blood cells, neutrophils, and monocytes were all significantly increased, while the lymphocyte count was significantly decreased in patients with APA (table S1 and fig. S1A). Gangrenous and perforated appendicitis accounted for most acute appendicitis in this study, followed by acute purulent appendicitis, simple acute appendicitis, and peri-appendicular abscess, respectively (fig. S1B). The vast majority of the patients received an appendectomy within 96 hours of the onset of symptoms (fig. S1C). The most common culturable bacteria from the appendix samples in this cohort were the gram-negative bacteria (GNB) family Enterobacteriaceae, particularly Escherichia coli (55%), followed by Klebsiella pneumoniae, and other Enterobacteriaceae. Pseudomonas aeruginosa and other GNB were less commonly cultured, with gram-positive bacteria being the least common (fig. S1D). In almost a quarter of cases, no bacteria were culturable (fig. S1D), and most of such patients appeared to suffer the milder form of APA, with very few gangrenous or perforated appendixes (fig. S1E).
MAIT cells decline in the circulation of patients with acute appendicitis
In light of the decrease of PB lymphocyte counts during acute appendicitis, further investigation was undertaken to determine which lymphocyte populations were affected (fig. S2A). Strikingly, T cell counts were strongly decreased in patients with APA compared with the control group (fig. S2B). In contrast, CD19+ B cell levels were unchanged in children with acute appendicitis (fig. S2C). The Uniform Manifold Approximation and Projection dimensionality reduction analysis indicated that the decrease in T cell populations was broad, with a profound decrease of the antimicrobial innate-like MAIT cells and γδT cells (Fig. 1A). Deeper analyses indicated that the numbers of MAIT cells, γδT cells, CD8+ T cells, and CD4− CD8− double-negative (DN) T cells were all significantly decreased (Fig. 1, B to E), whereas CD4+ T cell numbers were not affected (fig. S2D). However, within the T cell population, only the proportion of MAIT cells and γδT cells decreased in patients with acute appendicitis compared to control participants, whereas the proportion of other T cell subpopulations was unaffected (Fig. 1, F and G, and fig. S2, E to G).
Fig. 1. The decline in circulating MAIT cells in APA and correlation with disease severity.
(A) The Uniform Manifold Approximation and Projection dimensionality reduction analysis of T cell populations in healthy children and patients with APA (n = 6). (B to E) The numbers of circulating MAIT cells [(B) n = 30 to 45], γδT cells [(C) n = 27 to 30], CD8+ T cells [(D) n = 30 to 32], and CD4− CD8− DN T cells [(E) n = 30 to 32] in healthy children and patients with APA. (F and G) The frequency of circulating MAIT cells [(F) n = 30 to 45] and γδT cells [(G) n = 27 to 30] in healthy children and patients with APA. (H) The numbers of MAIT cells in the circulation of patients with APA of varying severity (SA, n = 12; AP, n = 13; GP, n = 19; PA, n = 1). (I to K) The correlation of circulating MAIT cell numbers in APA with neutrophil counts (n = 43) (I) and the levels of CRP (n = 43) (J) and D-dimer (n = 41) (K). (L) MAIT cell numbers in the circulation of patients with APA before and after appendectomy (n = 9). Truncated violin plots show all data points, median, and quartiles. Statistical significance was determined using Mann-Whitney’s test [(B) to (G)], one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test (H) or Wilcoxon’s signed-rank test (L). Correlations were calculated using the Spearman test [(I) to (K)]. ****P < 0.0001, **P < 0.01, *P < 0.05, ns, not significant. HC, healthy children; A(P)A, acute (pediatric) appendicitis; SA, simple acute appendicitis; AP, acute purulent appendicitis; GP, gangrenous/perforative; PA, periappendicular abscess.
MAIT cells and γδT cells belong to the innate-like antimicrobial T cell population and are poised to mediate rapid and robust responses against bacterial infections (16–18, 29, 53, 54). Given that the most common cause of acute appendicitis is bacterial infection leading to severely inflamed appendix and the consistent decrease in the numbers and frequency of MAIT cells and γδT cells, further investigations were performed to understand their potential roles in acute appendicitis. Circulating MAIT cell levels were lower in pediatric patients with the more severe appendicitis (Fig. 1H). In addition, the numbers of PB MAIT cells correlated negatively with neutrophil levels, as well as plasma levels of the inflammatory markers C-reactive protein (CRP) and D-dimer (Fig. 1, I to K). These associations were not observed for γδT cells and other T cell populations, except for CD4+ T cells (fig. S2, H to K, and table S2). The numbers of circulating MAIT cells significantly increased at 5 to 7 days after appendectomy compared to those just before the surgery (Fig. 1L), coinciding with the decline of CRP levels and pan-leukocyte, neutrophil, and monocyte counts (table S3). Of note, total lymphocyte counts had not yet significantly recovered at this early time point after appendectomy (table S3). These findings indicate that the circulating levels of MAIT cells decrease during APA and correlate with disease severity. This suggests a potential role of MAIT cells in the pathogenesis of appendicitis.
MAIT cells are enriched in the inflamed appendix and display altered expression of chemokine and homing receptors
As circulating MAIT cells during acute appendicitis negatively correlated with disease severity, MAIT cells may have redistributed to the appendix. To assess this possibility, MAIT cell levels were determined in the more inflamed portions of the appendix compared to the less or noninflamed parts. Appendiceal tissue inflammation severity was assessed by gross and pathological examinations and the influx of CD14+ monocytes and CD66b+ neutrophils (fig. S3, A to D), as well as by the heat flow produced by the equivalent number of single cells released from each tissue part using an isothermal microcalorimetry methodology (fig. S3E). The number of MAIT cells, identified as MR1-5-OP-RU–reactive TCR Vα7.2+ T cells (fig. S3F), were significantly elevated in more inflamed parts of the appendix tissue compared with the less or noninflamed parts (Fig. 2, A and B). In contrast, total T cells, CD4+ T cells, CD8+ T cells, DN T cells, and γδ T cells were not significantly different between the two parts of the appendix (fig. S3, G to K). Furthermore, the proportion of MAIT cells was substantially higher in the more inflamed part of the appendix than in blood (Fig. 2C). Conversely, the proportions of total T cells, CD8+ T cells, DN T cells, and γδ T cells were significantly lower in both inflamed and less/noninflamed parts of the appendix compared with those of PB (fig. S3, L to O). Although the proportions of CD4+ T cells in both inflamed and less/noninflamed parts of the appendix were higher than those in the circulation, there was no difference between these appendix parts (fig. S3P). Collectively, the MAIT cell population in blood was dramatically reduced during APA but enriched in the inflamed parts of the appendix. No such pattern was observed in other T cell populations, suggesting that MAIT cells may participate in the pathogenesis of acute appendicitis in children.
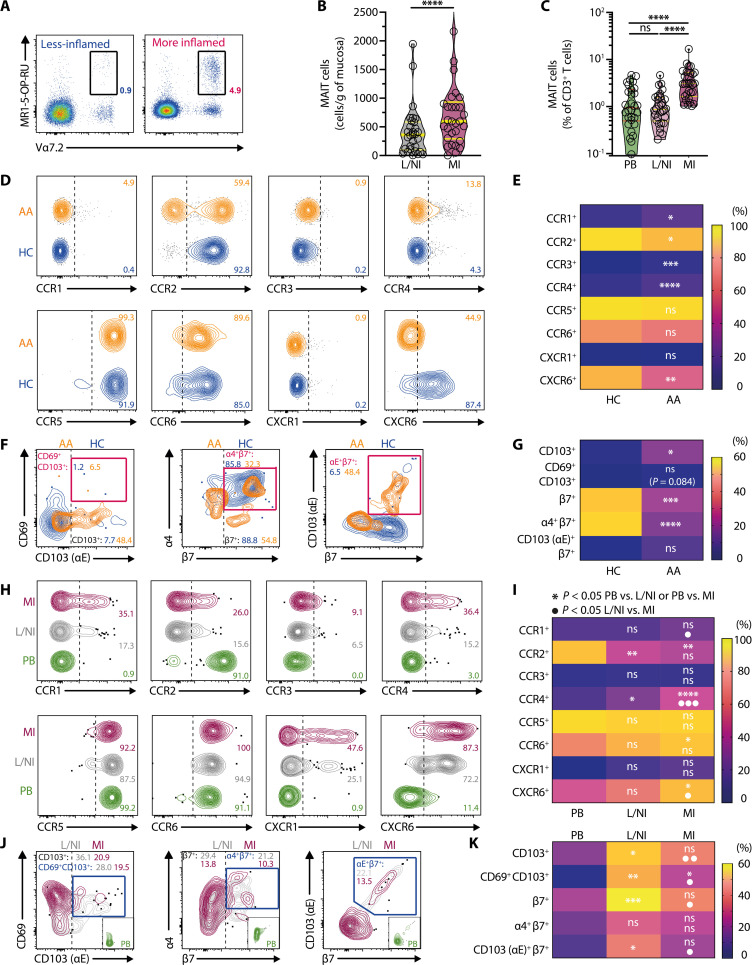
Fig. 2. Enrichment of MAIT cells in inflamed parts of the appendix and altered expression of chemokine receptors and tissue-homing molecules.
(A) Identification of MAIT cells in less/noninflamed and more inflamed appendix tissues. (B and C) The number (B) and proportion (C) of MAIT cells in the blood (n = 30) and in less/noninflamed (n = 30) and more inflamed segments (n = 30) of appendix tissues. (D and E) Flow cytometry plots (D) and percentage (E) of chemokine receptor expression on circulating MAIT cells in healthy children (n = 11 to 13) and patients with APA (n = 7 to 15). (F and G) Flow cytometry plots (F) and percentage (G) of tissue-homing molecule expression on circulating MAIT cells in healthy children (n = 13) and patients with APA (n = 9). (H and I) Flow cytometry plots (H) and percentage (I) of chemokine receptor expression on MAIT cells from the PB (n = 7 to 14) and less/noninflamed (n = 7 to 14) and more inflamed (n = 7 to 14) appendix tissues of patients with APA. (J and K) Flow cytometry plots (J) and percentage (K) of tissue-homing molecule expression on MAIT cells from the PB (n = 7 to 9) and from less/noninflamed (n = 7 to 9) and more inflamed (n = 7 to 9) appendix tissues of patients with APA. Data presented as heatmaps show the mean, whereas truncated violin plots show all data points, median, and quartiles. Statistical significance was determined using Wilcoxon’s signed-rank test (B), Mann-Whitney’s test [(E) and (G)], unpaired t test [(E) α4β7], the Friedman test followed by Dunn’s post hoc test (C), or repeated measure one-way ANOVA followed by Tukey’s post hoc test [(I) and (K)]. Mixed-effects analysis followed by Tukey’s post hoc test was performed for CCR4 (I). ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05; AA, acute appendicitis; PB, peripheral blood; L/NI, less/noninflamed; MI, more inflamed.
Elevated MAIT cell numbers in the inflamed portions of the appendix may reflect enhanced local tissue-resident MAIT cell proliferation from stimulation by riboflavin-autotrophic bacteria infecting the appendix (fig. S1D). However, there was a lack of Ki-67 expression by MAIT cells in blood, as well as both inflamed and noninflamed portions of the appendix (fig. S3, Q and R), suggesting that the increase in appendiceal MAIT cell numbers in the inflamed parts was less likely due to the proliferation of local tissue-resident MAIT cells. To address the possibility that this accumulation was due to PB MAIT cells homing to the inflamed appendix, expression of tissue-homing receptors was examined in the PB MAIT cells of APA and control groups. There was a significant reduction in MAIT cells expressing the chemokine receptors CCR2 and CXCR6, while there was no difference in the proportion of MAIT cells expressing CCR5 and CCR6 (Fig. 2, D and E). Unexpectedly, MAIT cells from children with acute appendicitis expressed significantly more CCR1 and CCR4 than those of control children (Fig. 2, D and E). This was intriguing as CCR1 and CCR4 are not commonly expressed by MAIT cells under a steady state in healthy humans (55). Although there was a significant difference in CCR3-expressing MAIT cells between the cohorts, the absolute levels of expression were very low (<5%) (Fig. 2, D and E). Of note, MAIT cell expression of the gut-homing integrin α4β7 was decreased in the blood of children with acute appendicitis, whereas expression of the tissue residency marker CD103 was elevated (Fig. 2, F and G). The circulating MAIT cells expressing the integrin αEβ7, the receptor for gut epithelial cells adhesion molecule E-Cadherin, were not significantly affected (Fig. 2, F and G). These findings indicate that circulating MAIT cells during APA have an altered chemokine and tissue-homing receptor expression pattern, potentially due to the activation and redistribution of specific MAIT cell subsets into the inflamed appendix.
Subsequent experiments were carried out to explore whether the alteration in chemokine and tissue-homing receptor expression patterns observed in circulating MAIT cells was reflected in their appendiceal counterparts. MAIT cells from the more inflamed appendix expressed more CCR1, CCR4, and CXCR6 than those from the less/noninflamed appendix (Fig. 2, H and I). Compared to those from circulation, MAIT cells from the less/noninflamed part of the appendix expressed significantly more CCR4 (Fig. 2, H and I). In contrast, MAIT cells from the more inflamed part of the appendix expressed more CCR4, CCR6, and CXCR6 (Fig. 2, H and I). CCR2 expression was significantly lower in appendiceal MAIT cells from either part when compared to those in circulation (Fig. 2, H and I). No significant differences were observed in CCR3 and CCR5 expression levels across PB and appendiceal tissues (Fig. 2, H and I).
Expression of integrin β7, CD69, and CD103 in MAIT cells was higher in less/noninflamed parts of appendiceal tissues than in PB (Fig. 2, J and K). However, expression of these gut homing and tissue residency markers were significantly lower in the more inflamed part than in the less/noninflamed part and, in most cases, were comparable to those of circulating MAIT cells (Fig. 2, J and K). These observations suggest a dilution effect of tissue-resident appendiceal MAIT cells by circulating MAIT cells that may have trafficked to the more inflamed parts of the appendix. Together, the altered expression patterns of chemokine receptors, gut-homing integrins, and tissue residency markers in appendiceal MAIT cells support the notion that circulating MAIT cells may have trafficked to the inflamed appendix during APA.
Elevated proinflammatory cytokines in the plasma and appendix tissue are associated with chemokine receptor expression alterations in MAIT cells
Acute bacterial appendicitis often leads to elevated levels of various proinflammatory cytokines (56). MAIT cells are highly responsive to multiple proinflammatory cytokines (25, 26, 57–59). Therefore, we measured the levels of various proinflammatory cytokines in the plasma and appendiceal tissues in patients with APA, with particular attention to cytokines that have substantial effects on MAIT cells, such as interleukin-1β (IL-1β), IL-7, IL-12, IL-18, and IL-23. Several of these MAIT cell–activating cytokines were elevated in the plasma, as well as in the inflamed portions of appendix tissues, including IL-1β, IL-18, and IL-23 (Fig. 3, A to H). Short-term in vitro culture of MAIT cells from healthy donors with IL-18 in the presence of IL-12, a combination commonly used to activate MAIT cells, or with IL-1β and IL-23, led to the alteration of chemokine receptor expression similar to those observed in appendicitis (Fig. 2, D, E, H, and I), including up-regulation of CCR1, CCR3, CCR4, and CXCR1; down-regulation of CCR2; and no apparent effect on CCR5 (Fig. 3, I to L). The chemokine receptor alterations were also seen and enhanced during longer-term in vitro culture of MAIT cells from healthy donors with these cytokines in the presence of the MR1 ligand and MAIT cell antigen 5-OP-RU (Fig. 3, I to L), which is produced by most GNB commonly found infecting the inflamed appendix (fig. S1D). The up-regulation of CCR4 was particularly notable (Fig. 3, I to L) and mimicked the pattern observed in circulating and appendiceal MAIT cells from patients with appendicitis (Fig. 2, D, E, H, and I), suggesting that CCR4 may play a significant role in MAIT cell migration during appendicitis. The in vitro treatments, on the other hand, led to the decrease of CCR6 and CXCR6 expression by MAIT cells from healthy donors (Fig. 3, I to L), which were not observed in appendicitis (Fig. 2, D, E, H, and I). Nevertheless, the overall MAIT cell chemokine receptor expression patterns following in vitro treatments with these proinflammatory cytokines were comparable to those observed in appendicitis (Fig. 2, D, E, H, and I). This suggests that such cytokines may prime MAIT cell tissue–homing capacity toward the infected and inflamed appendix.
Fig. 3. Elevated proinflammatory cytokine levels in APA promote altered chemokine receptor expression on MAIT cells.
(A to D) The plasma levels of proinflammatory cytokines were measured in healthy children (n = 8) and patients with APA (n = 15) (A), with significant elevation in pediatric acute appendicitis including IL-1β (B), IL-18 (C), and IL-23 (D). (E to H) The levels of proinflammatory cytokines measured in appendix tissues (n = 7) (E), with significant elevation in the more inflamed parts of the appendix including IL-1β (F), IL-18 (G), and IL-23 (H). (I and J) Histograms (I) of chemokine receptor expression and their levels (J) on MAIT cells of healthy donors on day 0 (n = 13), day 2 (n = 8), and day 12 (n = 11 to 12) following in vitro treatment with IL-12 and IL-18. (K and L) Histograms (K) of chemokine receptor expression and their levels (L) on MAIT cells of healthy donors on day 0 (n = 12), day 2 (n = 8), and day 12 (n = 6) following in vitro treatment with IL-1β and IL-23. Data presented as heatmaps show the mean, whereas truncated violin plots show all data points, median, and quartiles. Statistical significance was determined using Mann-Whitney’s test [(A) to (D)], Wilcoxon’s signed-rank test [(E) to (H)], or mixed-effects analysis followed by Tukey’s post hoc test [(J) and (L)]. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Inflammatory cytokines promote efficient MAIT cell migration to PDAOs
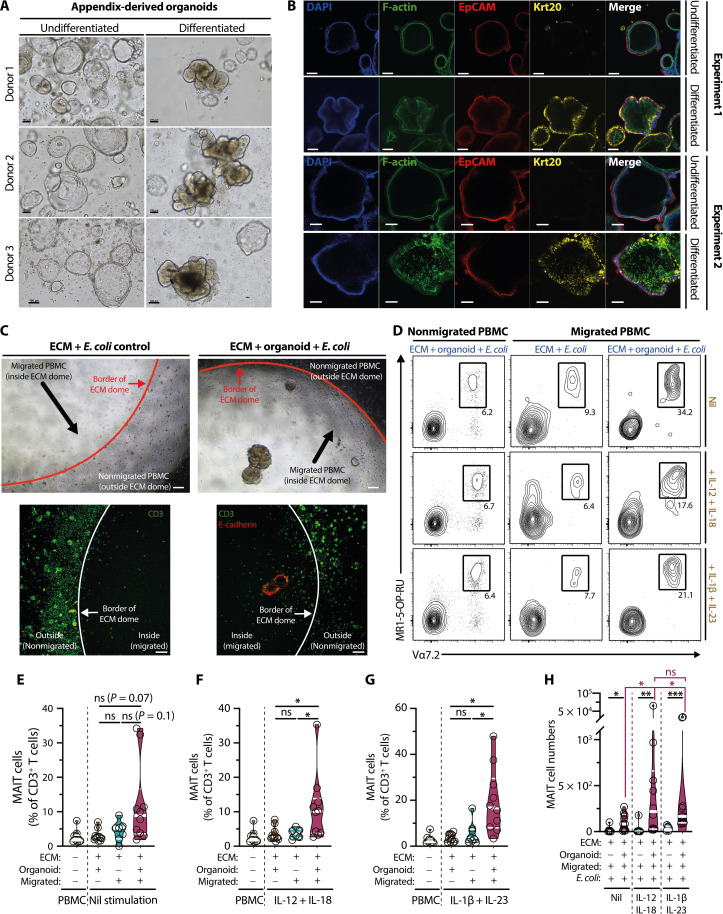
Next, we established the PDAO to test the hypothesis that MAIT cells migrate to the infected and inflamed appendix. Intestinal crypts were isolated according to established protocols and embedded in a dome of Matrigel extracellular matrix (ECM) (60). Such PDAOs were composed of intestinal epithelial cells with “apical-in” and “basal-out” polarity as determined by filamentous (F-) actin and EpCAM stainings (Fig. 4, A and B), thus recapitulating the proper and original polarity of the appendix tissue microenvironment. While undifferentiated, the appendix-derived organoids display the typical cystic appearance (Fig. 4A). However, after applying the differentiation protocol, organoids developed tubular structures resembling the intestine with mature enterocytes as determined by Krt20 staining (Fig. 4, A and B). To test whether there was a preferential migration of PB MAIT cells to PDAO, we cocultured the organoids with peripheral blood mononuclear cells (PBMCs) that were either untreated or prestimulated with IL-12 + IL-18 or IL-1β + IL-23. PBMCs were cultured outside the ECM domes and allowed to migrate into the ECM domes and organoids that were pre-embedded with E. coli (Fig. 4C, fig. S3S), the most common bacteria found in the inflamed appendix (fig. S1D). Using this methodology, there was a clear MAIT cell enrichment within the organoid-containing ECM domes when compared to empty ECM domes, nonmigrated PBMC outside the ECM dome, or the original PBMC population (Fig. 4, D to G). There were significantly more MAIT cells migrated into the ECM domes when E. coli–infected organoids were present, indicating preferential MAIT cell migration to the intestinal organoids (Fig. 4H). The different proinflammatory cytokines did not substantially influence MAIT cell enrichment within the ECM domes containing infected organoids (Fig. 4, D to G). However, there were significantly more migrating MAIT cells into the ECM domes containing E. coli–infected organoids in IL-12 + IL-18– or IL-1β + IL-23–stimulated PBMCs than in unstimulated PBMCs (Fig. 4H).
Fig. 4. Enrichment of proinflammatory cytokine-treated PB MAIT cells into PDAOs.
(A and B) Bright-field (A) (n = 2) and confocal microscopy (B) (n = 3) of undifferentiated and differentiated PDAO. Organoid cells of epithelial origin and the basolateral side were identified by positive EpCAM staining (red), whereas the apical side was identified by positive filamentous (F)–actin staining (green). Krt20 staining (yellow) was used to identify differentiated enterocytes. The nuclei were visualized using 4′,6-diamidino-2-phenylindole (DAPI) (blue). (C) Representative bright field (top) and confocal microscopy (bottom) and (D) flow cytometry plots of cytokine-treated or untreated PBMC migration into E. coli– pulsed PDAO or Matrigel ECM controls. In selected experiments (C), PDAOs were identified by E-cadherin staining (red), and T cells were identified by CD3 staining (green). [(D) to G)] Flow cytometry plots of MAIT cells (D) that have not migrated or have migrated to E. coli–ECM controls or to E. coli–pulsed PDAO without cytokine treatment (n = 7 to 10) (E) or treated with IL-12 and IL-18 (n = 6 to 10) (F) or IL-1β and IL-23 (n = 8 to 10) (G). (H) Enumeration of migrated MAIT cells under different stimulation conditions (n = 6 to 10). Statistical significance was determined using Mann-Whitney’s test [(H) E. coli–ECM controls versus E. coli organoids], Friedman’s test followed by Dunn’s post hoc test [(H) ECM+ Org+ Migrated+ E. coli+: nil versus IL-12 + 18 versus IL-1β + 23], or mixed-effects analysis followed by Tukey’s post hoc test [(E) to (G)]. ***P < 0.001, **P < 0.01, *P < 0.05. Scale bars, 100 μm. ECM, extracellular matrix (Matrigel).
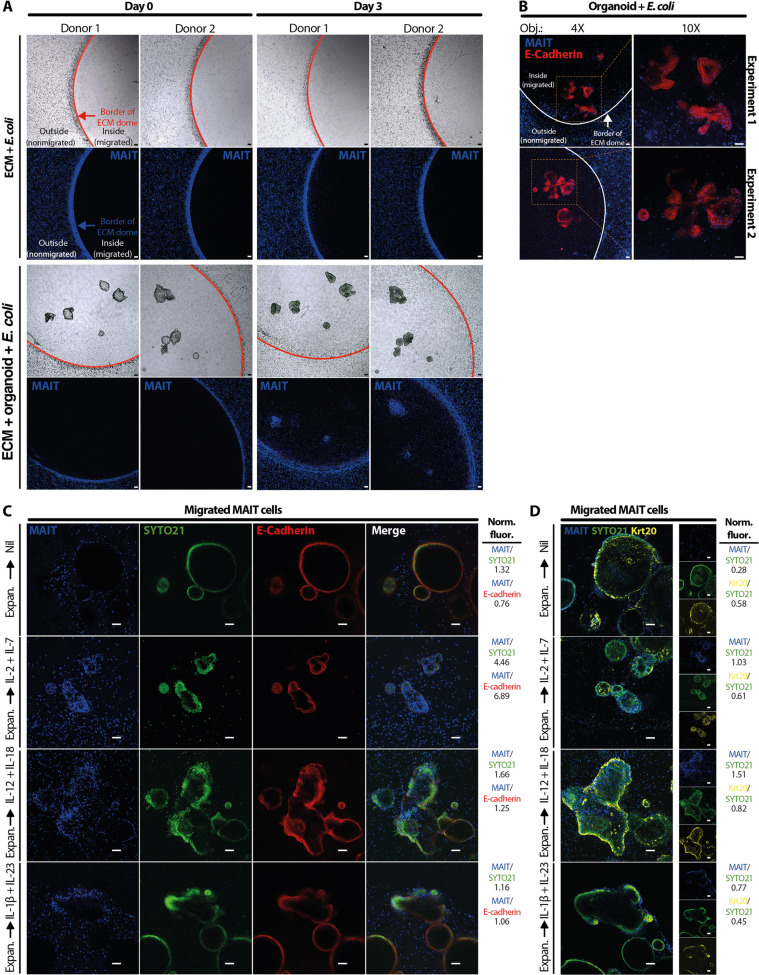
To visualize MAIT cell migration into E. coli–infected appendix organoids by confocal microscopy, the next set of experiments was conducted using expanded MAIT cells with a purity typically >85%, as previously described (17). These IL-2 + IL-7–supplemented expanded MAIT cells had comparable chemokine receptor expression profiles to those of IL-12 + IL-18 and IL-1β + IL-23–stimulated MAIT cells (fig. S4, A and B). Fluorescently labeled MAIT cells migrated into the organoids (fig. S4C), and such migration appeared directed toward the organoids with relatively fewer MAIT cells meandering within the ECM dome distal from the organoids or when the organoids were absent (Fig. 5A and fig. S4D). Further investigation by staining the organoids with an epithelial marker E-Cadherin indicated that these migrated MAIT cells appeared to associate with the organoids (Fig. 5B and fig. S4E). In the next set of experiments, expanded MAIT cells were rested for 2 days in the absence of exogenously added cytokines and then restimulated with IL-12 + IL-18, IL-1β + Il-23, or IL-2 + IL-7. In agreement with previous results (Fig. 4H), expanded MAIT cells restimulated with proinflammatory cytokines seemed to exhibit stronger migration into the appendix organoids than MAIT cells without prestimulation (Fig. 5C and fig. S4F). MAIT cells restimulated with IL-2 + IL-7 appeared to have the greatest capacity to migrate into the appendix organoids (Fig. 5C and fig. S4F), consistent with the ability of IL-7 to enhance MAIT cell functionality (17, 22, 57, 59). To ensure that the differences in migratory capacity of MAIT cells were not due to heterogeneity of the PDAO, the organoids were stained with the intracellular marker of mature enterocytes Krt20 following the live confocal microscopy (Fig. 5D). On the basis of Krt20 expression, the PDAO appeared relatively homogenous (Fig. 5D and fig. S4G), suggesting that the differences in MAIT cell migratory capacity was more likely due to the influence of the cytokines, in line with our previous results shown in Fig. 4H.
Fig. 5. MAIT cells efficiently migrate to E. coli–pulsed PDAOs.
(A) Representative live confocal microscopy of MAIT cells (n = 2) migrating toward E. coli–pulsed PDAOs (n = 5). Expanded and CellTrace Violet–labeled (CTV-) MAIT cells (blue) were cultured outside the Matrigel ECM domes, and confocal microscopy images were acquired 3 days later. E. coli embedded in Matrigel ECM alone served as a control for background MAIT cell migration into the Matrigel ECM domes. (B) In selected experiments (n = 3), E. coli–pulsed appendix-derived organoids were stained using E-cadherin monoclonal antibody (mAb) (red) to examine their interactions with CTV-MAIT cells (blue). Representative live confocal microscopy images from two independent experiments are shown. (C) Expanded MAIT cells were cultured in MAIT cell medium without exogenous cytokines for 48 hours, labeled with CTV, then cultured outside the Matrigel ECM domes of E. coli–pulsed PDAO with or without IL-2 + IL-7, IL-12 + IL-18, or IL-1β + IL-23. Representative live confocal images from seven independent experiments acquired 2 days later show migrating CTV-MAIT cells (blue) infiltrating PDAO visualized by E-cadherin expression (red) and SYTO21-stained nuclei (green). (D) Following live confocal microcopy in (C), the cultures were fixed and stained intracellularly for Krt20 expression (yellow) to identify enterocytes. Normalized fluorescence intensities (Norm. fluor.) of CTV (MAIT cells) and Krt20 to SYTO21 [(C) and (D)] or E-cadherin (C) were shown. Confocal images were acquired with Nikon A1R+ Laser scanning confocal microscope with 4×/0.2 [(A) and (B), left] and 10×/0.45 [(B), right, (C), and (D)] objectives. Imaging was controlled using the Nikon NIS-Elements software and processed with ImageJ 1.53a software. Scale bars, 100 μm.
MAIT cell migration into PDAOs depends on CCR1, CCR2, and CCR4
To investigate the mechanism underlying MAIT cell migration into the infected and inflamed appendix, the plasma of appendicitis patients was first screened for inflammatory mediators, particularly the ligands of the chemokine receptors whose expression by MAIT cells was altered in appendicitis (Fig. 2, D, E, H, and I, and fig. S5A). There was a significant increase in the plasma of appendicitis patients of CCL2, CCL3, CCL8, CCL17, CCL20, CCL22, and CXCL8 (Fig. 6A), while there was no difference in the levels of CCL4, CCL5, CCL7, CCL11, CCL13, CXCL16, and CCL24 when compared to those of healthy controls (fig. S5B). We subsequently measured these inflammatory chemokines in the appendix tissues. Unexpectedly, similar chemokine profiles were observed in more inflamed appendix tissues when compared to less inflamed appendix, i.e., higher levels CCL2, CCL3, CCL8, CCL17, CCL22, CCL20, and CXCL8, but no difference in CCL4, CCL5, CCL7, CCL11, CCL13, CCL24, and CXCL16 levels (Fig. 6B and fig. S5C). These findings suggest that elevated inflammatory chemokines in the plasma were derived from the inflamed appendix tissues, and circulating MAIT cells may be recruited to the inflamed appendix via CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CXCR1, and/or CXCR6-dependent mechanisms.
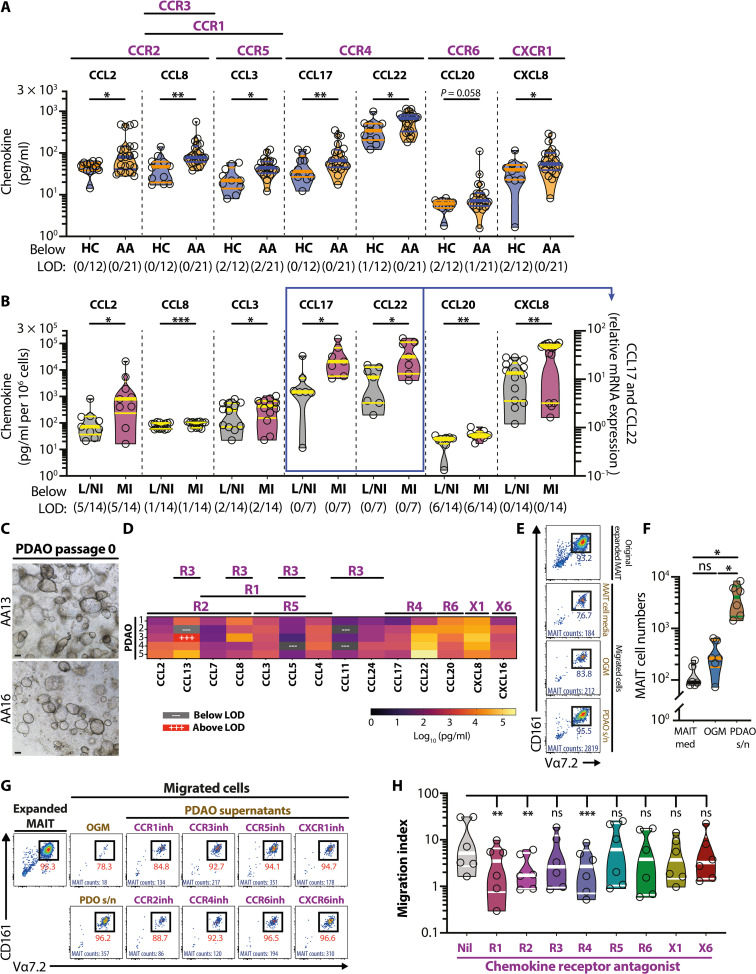
Fig. 6. Elevated appendix-derived chemokines in APA contribute to MAIT cell migration.
(A) The plasma levels of inflammatory chemokines were measured in healthy children (n = 12) and patients with APA (n = 21). (B) The levels of inflammatory chemokines measured in different parts of appendix tissues (n = 7, CCL17 and CCL22; n = 14, all others). (C) Representative bright-field microscopy images of PDAO passage 0 from two patients with APA. (D) Chemokine levels in the supernatants of PDAO passage 0 cultures from five donors. The bead array–based assay was used to determine all chemokine levels, except for CCL17 and CCL22 in (B) where quantitative real-time PCR was used. (E and F) Enumeration of MAIT cell migration toward MAIT cell medium, OGM control, and PDAO culture supernatants. Migrated MAIT cell numbers were enumerated using flow cytometry–based Precision Count Beads. (G and H) Flow cytometry plots (G) and enumeration of MAIT cell transwell migration (H) in the absence or presence of various chemokine receptor antagonists (n = 6). The migration index was defined as the ratio between the number of MAIT cells collected in the bottom chamber in the presence of PDAO supernatants and the number of MAIT cells collected in the bottom chamber in the presence of OGM. Data presented as heatmaps show the mean, whereas truncated violin plots show all data points, median, and quartiles. Statistical significance was determined using Mann-Whitney’s test (A), Wilcoxon’s signed-rank test (B), one-way ANOVA followed by Tukey’s post hoc test (F), or Friedman’s test followed by Dunn’s post hoc test based on the raw, nonnormalized data (H). ***P < 0.001, **P < 0.01, *P < 0.05, L/NI, less/noninflamed; MI, more inflamed; LOD, limit of detection; s/n, supernatant.
Next, PDAOs were cultured to provide inflamed appendiceal secretomes to evaluate the specific chemokine receptors that may mediate MAIT cell migration to the inflamed appendix. To maximize the resemblance of the PDAO with the inflamed primary appendix tissues, only the secretomes from passage 0 PDAOs were used in subsequent experiments (Fig. 6C). Chemokine profiling of the PDAO secretomes revealed comparable profiles when compared to the inflamed primary appendix tissues as well as plasma of patients with appendicitis, with detectable levels of CCL2, CCL3, CCL8, CCL17, CCL22, CCL20, and CXCL8 (Fig. 6D). Of note, CCL20, CCL22, and CXCL8 levels were high in the PDAO secretomes (Fig. 6D). While the levels of CCL5, CCL7, CCL11, and CCL24 were relatively low and comparable to the patterns exhibited by the inflamed primary appendix tissues, PDAO secretomes in contrast had high levels of CCL4, CCL13, and CXCL16 (Fig. 6D). Thus, all of the chemokines that were significantly elevated in primary appendix tissues and plasma were also detected in the PDAO secretomes (fig. S5D). Almost half of the measured chemokines that were not elevated in primary appendix tissues and plasma were also not detected or only present at low levels in the PDAO secretomes (fig. S5D). Taken collectively, the chemokine profiles of the PDAO passage 0 secretomes were similar to those of inflamed primary appendix tissues and plasma of the patients, suggesting a robust PDAO model to evaluate MAIT cell migratory capacity during acute appendicitis. This was next investigated using a transwell-based MAIT cell migration assay and the PDAO secretomes as the chemoattractant (fig. S5E). Because of limited amounts of supernatants, initial experiments were performed to evaluate MAIT cell migratory capacity toward pooled appendix-derived organoids supernatants. MAIT cells rapidly and strongly migrated toward PDAO supernatants but not to the control organoid growth media (OGM) or the MAIT cell expansion media (Fig. 6, E and F), in agreement with the observation that MAIT cells migrated toward the PDAO (Figs. 4, D to H, and 5). Next, to examine which chemokine receptors may contribute to MAIT cell migration during acute appendicitis, MAIT cells expanded in the presence of the MR1 ligand 5-OP-RU and the proinflammatory cytokines IL-12 and IL-18 were pretreated with various chemokine receptor antagonists before the transwell migration assay using PDAO passage 0 supernatants from a single patient with appendicitis. Antagonists for the chemokine receptors CCR1, CCR2, and CCR4 significantly reduced MAIT cell migration to the PDAO supernatants (Fig. 6, G and H). In contrast, antagonists of CCR3, CCR5, CCR6, CXCR1, and CXCR6 had no effects on MAIT cell migration (Fig. 6, G and H). These findings collectively indicate that MAIT cells may migrate to the infected and inflamed appendix via predominantly CCR1-, CCR2-, and CCR4-dependent mechanisms.
MAIT cells from inflamed appendices display activated, inflammatory, and cytotoxic profiles
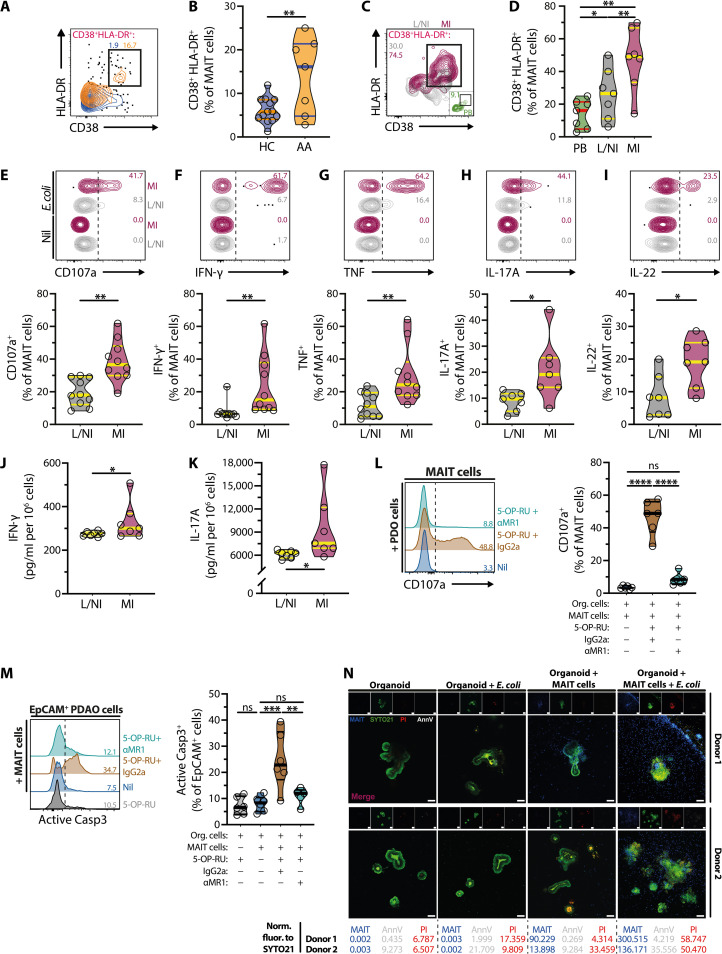
Because MAIT cells release potent antimicrobial, cytotoxic, and proinflammatory effector proteins during gastrointestinal infections caused by many GNB [reviewed in (2)], commonly found during appendicitis (fig. S1D), we next explored their potential inflammatory roles during APA. Circulating MAIT cells from children with acute appendicitis expressed higher levels of the activation markers CD38 and human leukocyte antigen (HLA)–DR (Fig. 7, A and B). Moreover, CD38 and HLA-DR levels were higher in MAIT cells derived from more inflamed parts of the appendix compared with less/noninflamed parts or circulation of the same patients (Fig. 7, C and D). Subsequent evaluation of MAIT cell effector functions following stimulation with fixed E. coli (17, 22, 24, 35, 57, 61) showed higher levels of degranulation (CD107a), interferon-γ (IFN-γ), tumor necrosis factor, IL-17A, and IL-22 by MAIT cells from more inflamed parts of appendix than less/noninflamed parts (Fig. 7, E to I). Of note, there were also significantly higher levels of IFN-γ and IL-17A produced by ex vivo cultures of the more inflamed appendix parts (Fig. 7, J and K). These findings suggest that MAIT cells from patients with appendicitis, especially those from the inflamed appendix, were activated and produced enhanced levels of cytotoxic and proinflammatory mediators following encounters with GNB within the infected appendix.
Fig. 7. MAIT cells display proinflammatory and cytotoxic profiles in acute appendicitis and trigger organoid cell death and structural destruction.
(A and B) CD38 and HLA-DR expression in circulating MAIT cells from HC (n = 13) and patients with APA (n = 7). (C and D) CD38 and HLA-DR expression on MAIT cells from circulation (n = 7) and less/noninflamed (n = 7) and more inflamed (n = 7) appendix tissues. (E to I) Levels of CD107a (n = 10) (E), IFN-γ (n = 10) (F), tumor necrosis factor (n = 10) (G), IL-17A (n = 7) (H), and IL-22 (n = 7) (I) in MAIT cells from less/noninflamed and more inflamed appendix tissues following paraformaldehyde-fixed E. coli stimulation. (J and K) IFN-γ and IL-17A levels in supernatants of ex vivo cultures of less/noninflamed and more inflamed appendix tissues (both n = 7). (L and M) CD107a levels on MAIT cells (L) or activated Caspase (Casp) 3 levels on EpCAM+ organoid cells (M) following 3 hours of 2D coculture with untreated (n = 6) or 5-OP-RU–pulsed organoid cells with anti-MR1 mAb (n = 5) or IgG2a isotype control (n = 6). (N) Representative live confocal images from seven independent experiments (n = 4) of CTV-MAIT cells (blue) infiltrating E. coli–pulsed PDAO visualized by SYTO21 (green). Structural destruction of PDAO was observed visually by confocal microscopy, whereas organoid death was assessed by positive annexin V (AnnV, white) and propidium iodide (PI, red) staining. Normalized fluorescence (Norm. fluor.) intensities of CTV (MAIT cells), AnnV, and PI to those of SYTO21 were shown. Data presented as truncated violin plots show all data points, median, and quartiles. Statistical significance was determined using unpaired t test (B), Wilcoxon’s signed-rank test [(E) to (I)], one-way ANOVA followed by Tukey’s post hoc test (D), or mixed-effects analysis followed by Tukey’s post hoc test [(L) and (M)]. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
MAIT cells migrate to bacteria-infected appendix-derived organoids and mediate MR1-dependent organoid cell death and structural destruction
Having established that MAIT cells migrate to the infected and inflamed appendix by using the PDAO models, as well as their inflammatory and cytotoxic profiles in the appendixes of patients with appendicitis (Fig. 7, A to K), we next explored whether migrated MAIT cells could promote appendiceal tissue damage and may thus participate in the pathogenesis of the disease. Using a 2D cell culture of PDAO that were dissociated into single cells from a 3D culture (fig. S5F), we enumerated MAIT cell cytotoxic capacity and organoid cell death using precise MAIT to organoid cell ratios not possible with the current 3D organoid technology. In addition, we used the MR1 ligand 5-OP-RU to allow rapid measurement of MAIT cell–mediated killing of organoid cells. In such experiments, MAIT cells [typical purity >85% (17, 62)] strongly degranulated (CD107a) following a short-term coculture with 2D organoid cells pulsed with 5-OP-RU but not when cocultured with organoid cells alone (Fig. 7L). Correspondingly, 5-OP-RU–pulsed organoid cells were rapidly and efficiently killed by MAIT cells, as indicated by active Caspase 3 expression (Fig. 7M). Neither 5-OP-RU nor MAIT cell coculture alone triggered organoid cell death (Fig. 7M). MR1-antibody severely inhibited MAIT cell degranulation and killing of 5-OP-RU–pulsed organoid cells (Fig. 7, L and M), indicating that antigen presentation by MR1 was essential. These findings indicate that PDAO could present the bacterial MR1 ligand 5-OP-RU and trigger MR1-dependent MAIT cell cytotoxicity and killing of the organoid cells.
Last, we revisited our 3D PDAO model to evaluate whether MAIT cells can efficiently migrate to and promote structural destruction and death of PDAO cocultured with E. coli. PDAO coculture with E. coli alone (up to 3 days) did not trigger significant organoid structural destruction and death (Fig. 7N, fig. S5G). Observable MAIT cell migration into uninfected 3D organoids was noted within 3 days of coculture; however, this did not promote organoid destruction and death despite the infiltration of MAIT cells into the organoids (Fig. 7N, fig. S5G). In contrast, there appeared to be an increase in MAIT cell migration to PDAO cocultured with E. coli compared to uninfected organoids (Fig. 7N, fig. S5G). MAIT cell migration was accompanied by severe destruction of the PDAO as indicated by the loss of organoid structural integrity as well as organoid cell death as indicated by positive costaining of annexin V and propidium iodide (PI) (n = 5; Fig. 7N, fig. S5G). Furthermore, MAIT cell proximity to, adherence, and infiltration of the 3D organoids were associated with the structural destruction and cell death of the PDAO (Fig. 7N, fig. S5G).
DISCUSSION
Little is known about the roles of MAIT cells in acute intestinal inflammation despite the presence of this MR1-restricted innate-like T cell population in the human gastrointestinal tract. It is also unclear whether circulating MAIT cells may traffic to the inflamed sites and participate in tissue injuries. Here, we use APA and PDAOs as models of acute intestinal inflammation in humans. We report the recruitment of MAIT cells to intestinal tissue and their association with damage and inflammation during APA. Circulating MAIT cell numbers were significantly lower in patients with APA than in healthy children. MAIT cells were more abundant in the inflamed appendix and associated with disease severity. Appendiceal MAIT cells from patients with APA were also highly activated and displayed strong inflammatory T helper 17 (TH17) cell–like profiles following stimulation with E. coli. Both plasma and inflamed appendix parts from children with acute appendicitis had similar profiles of elevated levels of proinflammatory cytokines and chemokines. Moreover, both circulating and appendiceal MAIT cells displayed altered expression of tissue-homing receptors. MAIT cell numbers rapidly recovered following an appendectomy. These results suggest that circulating MAIT cells may traffic to the appendix during APA. Our PDAO model demonstrates that circulating MAIT cells primed with inflammatory cytokines elevated in acute appendicitis rapidly migrated to and infiltrated the PDAO. Such migration was dependent on CCR1-, CCR2-, and CCR4-mediated pathways. The infiltrating MAIT cells further mediated cytotoxicity and structural destruction of the PDAO fed with E. coli, a process dependent on MR1 presentation of bacterial metabolite antigens. Collectively, our findings using PB, primary tissue samples, and PDAO reveal the pathological roles of MAIT cells in APA and the mechanisms of their activation and recruitment through appendix-derived cytokines and chemokines leading to inflammation and tissue damage in acute appendicitis (Fig. 8).
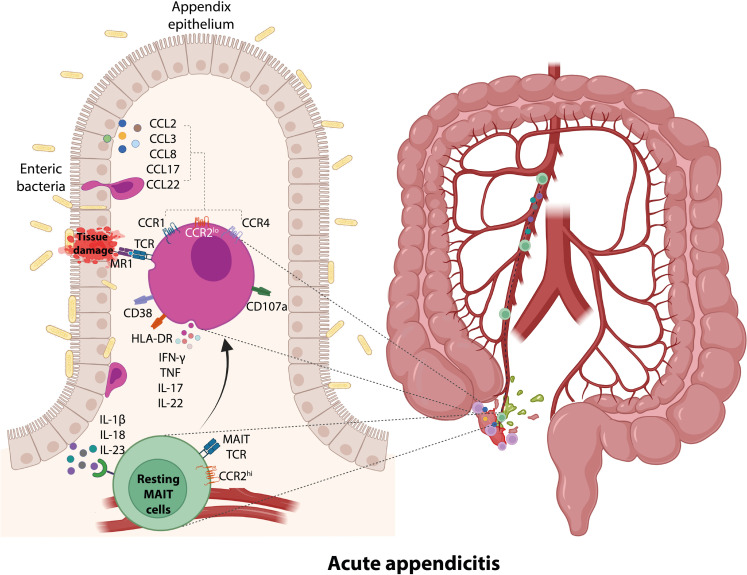
Fig. 8. Proposed model on the potential mechanisms of MAIT cell recruitment to the appendix and their participation in disease pathogenesis during acute appendicitis in children.
During APA, the inflamed appendix secretes inflammatory cytokines and chemokines. These inflammatory cytokines, including IL-1β, IL-18, and IL-23, activate circulating MAIT cells and promote the expression of multiple chemokine receptors not expressed by resting MAIT cells. Inflammatory chemokines secreted by the inflamed appendix, including CCL2, CCL3, CCL8, CCL17, and CCL22, then recruit MAIT cells to the sites of inflammation via CCR1-, CCR2-, and CCR4-dependent fashion. Accumulated MAIT cells recognize the enteric bacteria infection of the appendix and promote MR1-dependent MAIT cell cytotoxic and inflammatory responses, contributing to tissue damage.
The prevailing hypotheses potentially explaining the loss of circulating MAIT cells in infectious and inflammatory diseases include trafficking to and accumulation within infected and inflamed tissues, activation-induced cell death, and pyroptosis (37–43, 45, 47, 61, 63–69). However, MAIT cells were rapidly recovering in numbers in the blood of patients with APA following an appendectomy, and this supports that MAIT cells were only transiently sequestered out of circulation rather than irreversibly lost. MAIT cell numbers were elevated in the inflamed portions of the appendix but not in the noninflamed regions. The lack of Ki-67 expression indicates that such accumulation was not due to the proliferation of local tissue-resident MAIT cells, but rather, it suggests an active recruitment of MAIT cells from the circulation to the inflamed appendix. In line with this notion, there was an alteration in the tissue-homing chemokine receptor expression observed on blood MAIT cells in patients with APA and tissue MAIT cells from the more inflamed parts of the appendix. In addition, levels of inflammatory chemokines were elevated in the plasma and the inflamed parts of the appendix of patients with APA. Intriguingly, our findings from ex vivo–circulating and appendiceal MAIT cells reveal the up-regulation of a set of chemokine receptors that was not previously considered typical for MAIT cells, including CCR1, CCR3, and CCR4. Such changes in MAIT cell chemokine receptor profiles were recapitulated in vitro following treatment of circulating MAIT cells with cytokines found elevated in patients with APA, including IL-1β, IL-18, and IL-23—cytokines strongly associated with MAIT cell effector function. Last, there was a consistent match between the increased chemokine levels in patients with APA and their corresponding chemokine receptors, whose expression was altered on MAIT cells. These include CCL2, CCL3, and CCL8, which are ligands to CCR1, CCR2, and/or CCR3, and CCL17 and CCL22, which are ligands to CCR4. Considering all these observations, our findings suggest that circulating MAIT cells may have trafficked to the inflamed appendix in children with acute appendicitis via these appendix-derived inflammatory chemokines.
The PDAO model provided a valuable tool to evaluate MAIT cell migration and interaction with appendix tissues during APA. Treatments of PB MAIT cells with inflammatory cytokines elevated in patients with APA enhanced MAIT cell migration, enrichment, and infiltration into the PDAO. These treatments also led to apparent interactions with the PDAO and penetration of the enterocyte junctions by MAIT cells, suggesting the intraepithelial nature of human MAIT cells in this setting. These interactions ultimately led to the structural destruction and MR1-dependent killing of organoid cells when the PDAOs were pulsed with E. coli, consistent with the high levels of cytolytic and proinflammatory activity of MAIT cells from the inflamed segments of the appendix. Together, using the PDAO model, our findings indicate that MAIT cells infiltrate the inflamed appendix during APA and participate in appendix tissue injury through cytolytic and proinflammatory cytokine activity. Furthermore, our PDAO model will enable future studies to understand the biology of MAIT cell tissue residency and its role in gut homeostasis and inflammation.
Last, we used our PDAO model to investigate which chemokine receptors exert a major role in MAIT cell recruitment during APA. The chemokine profiles of the original passage PDAO paralleled that the chemokines elevated in the inflamed appendix of patients with APA, thus validating our robust PDAO system to assess circulating MAIT cell migration to inflamed appendix tissues. Blocking experiments indicate that CCR1, CCR2, and CCR4 exert a significant role in MAIT cell recruitment to the inflamed appendix during APA. While CCR2 is constitutively expressed by MAIT cells and mediates extravasation and transendothelial migration (30), its role in recruiting MAIT cells to inflammed gut tissues was previously unknown. In contrast, MAIT cells gain CCR1 and CCR4 expression through activation by proinflammatory cytokines and proliferation induced by MR1 ligand, consistent with the up-regulation of these chemokine receptors following long-term culture of MAIT cells (55). However, CCR1 expression on MAIT cells in patients with APA and its up-regulation following short-term stimulation of PB MAIT cells from healthy adults was relatively modest. There was a notable up-regulation of CCR4 by MAIT cells from patients with APA and from in vitro treatment with proinflammatory cytokines. To our knowledge, there is no previously reported role of CCR4 in driving MAIT cell recruitment in gut infection and inflammation, as CCR4 is mostly expressed by regulatory T cells, TH2 cells, and cutaneous lymphocyte antigen–positive skin-homing T cells (70–73). The strong up-regulation of CCR4 on MAIT cells and high levels of its chemokine ligands CCL17 and CCL22 in patients with APA and PDAO supernatants, as well as a significant contribution of CCR4 to MAIT cell migration toward PDAO, together strongly support the notion that MAIT cells traffic to and infiltrate appendix tissues via the CCL17/CCL22-CCR4 axis. It is currently unclear which of the two CCR4 chemokine ligands plays a dominant role in attracting MAIT cells to the inflamed appendix tissues, although a recent study showed that CCL17 strongly promotes the migration of MAIT cells (55). Future studies are warranted to ascertain the role of the CCL17/CCL22-CCR4 axis and the cellular sources of such chemokines in driving MAIT cell trafficking during various acute intestinal infections and inflammation.
In summary, the current work reveals a previously unidentified mechanism of MAIT cell recruitment to the gut during acute infection and inflammation, furthers our understanding of the pathogenesis of acute appendicitis, and suggests that modulation of MAIT cell homing to the gut, and their proinflammatory activity could be a potential target for intervention in patients with gut inflammation. Specifically, the current work demonstrates that proinflammatory cytokines enhance the capacity of MAIT cells to migrate into the gut tissues by up-regulating specific chemokine receptor pathways that are otherwise not expressed during noninflammatory and steady states. The levels of circulating MAIT cells are considerably lower in children than those in adults (74). Although in our study, we were restricted to using circulating MAIT cells from healthy adults due to sample constraints, MAIT cells are present in low numbers in the intestinal mucosae of the second-trimester fetuses (27) and the appendix of neonates (74), suggesting the tissue-homing capability of MAIT cells to seed the intestinal mucosa even before birth. Whether circulating MAIT cells from children have comparable migratory capacity to that of adults remains to be explored. Last, using the innovative PDAO model, we show that secretion of specific appendix-derived chemokines during appendicitis strongly attracts MAIT cell migration to the appendix, promoting tissue inflammation and damage. The present work also shows the usefulness of the emerging organoid technology to extend our understanding of MAIT cell tissue-homing in a fully human system that is otherwise difficult to achieve using conventional in vitro 2D cell culture and ex vivo experimental methodologies alone. Thus, our findings warrant future studies investigating whether regulating MAIT cell trafficking and down-modulating inflammatory signatures of MAIT cells may promote the resolution of appendiceal inflammation, in addition to the antimicrobial treatment and endoscopic “appendix-sparing” approach demonstrated elsewhere (75, 76).
MATERIALS AND METHODS
Ethics statement
Human samples were collected after informed written consent was obtained from all donors in accordance with study protocols conforming to the provisions of the Declaration of Helsinki. For the pediatric participants, written informed consent was obtained from adult legal guardians of the children before enrolment. The study was approved by the Ethics Committees of Zhangzhou Municipal Hospital of Fujian Province (Ref. 2022KYB277) and Tsinghua Shenzhen International Graduate School (Refs. 202171 and 202295).
Participants
Pediatric patients were recruited at the division of pediatric surgery in Zhangzhou Municipal Hospital of Fujian Province, while adult healthy donors were enrolled at Tsinghua Shenzhen International Graduate School. In the acute appendicitis cohort, we included pediatric patients (<14 years old) and admitted for acute appendicitis but excluded those with chronic appendicitis, acute exacerbation of chronic appendicitis, or other comorbidities such as chronic infection and autoimmune diseases. In the healthy children control cohort, we included <14-year-old patients admitted for minor surgical treatments for noninflammatory and noninfectious conditions. Those with acute or chronic infections, autoimmune diseases, elevated CRP levels, and leukocytes were excluded. All participants were screened for and were negative for HBV, HCV, HIV-1/2, and syphilis before surgery.
Blood and tissue processing
The blood was drawn from patients immediately at presentation. Ethylenediaminetetraacetic acid dipotassium (EDTA K2) was used as an anticoagulant. Plasma was separated from the cell pellet after spinning whole blood and stored at −80°C. PBMCs were isolated via Ficoll-Histopaque density gradient separation and cryopreserved in liquid nitrogen. The whole appendix was removed from pediatric patients who underwent surgery for acute appendicitis, dissected into two segments according to the gross severity of inflammation, and immersed in cold RPMI 1640 medium supplemented with gentamicin (50 μg/ml, Gibco) and 1% of penicillin-streptomycin. The samples were sent with ice packs from the hospital to the laboratory and processed within 24 hours.
Generation and maintenance of appendix-derived intestinal organoids
Appendiceal tissues were dissected and opened longitudinally with sterile scalpels, and the mucosae were flushed extensively with cold phosphate-buffered saline (PBS). The mucosae were scraped off and minced into small pieces (<5 mm) using the scalpel. Intestinal crypts were isolated by incubating the tissue fragments in Gentle Cell Dissociation Reagent (GCDR, STEMCELL Technologies) for 30 min on ice with constant agitation and then centrifuged at 500g at 4°C for 5 min. The pellets were dissociated by vigorous pipetting in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEMF12, Gibco) supplemented with 1% bovine serum albumin (Sigma-Aldrich) (DMEMF12-BSA) passed through a 70-μm strainer and centrifuged at 500g at 4°C for 5 min. The crypts were counted using a hemocytometer and resuspended in cold Matrigel ECM solution composed of 50% Matrigel Growth Factor Reduced Basement Membrane Matrix (Corning) and 50% DMEMF12-BSA at a concentration of 20,000 crypts/ml. Fifty microliters of crypt suspensions in Matrigel ECM solution were plated in 24-well plates and incubated for 15 min at 37°C until the Matrigel ECM solution solidified and then cultured with IntestiCult OGM (STEMCELL Technologies) supplemented with Primocin (100 μg/ml, InvivoGen), gentamicin (50 μg/ml, Gibco), and 10 μM Y-27632 (for the first 3 days; STEMCELL Technologies) at 37°C, 5% CO2. Cell media were changed and replenished every 2 to 3 days. In selected experiments, immature organoids (<7 days after passage) were differentiated for a further 5 to 7 days or until tubular structures developed in human IntestiCult Organoid Differentiation Medium (STEMCELL Technologies).
PDAO typically formed large cystic or budded structures in 10 to 14 days when they were ready for passaging. The medium was removed, and PDAO was incubated in GCDR at 37°C for 10 min, followed by manual dissociation of organoids into small fragments through thorough yet gentle pipetting loaded with 200-μl pipette tips. Organoid fragments were pelleted at 500g at 4°C for 5 min and washed once with DMEMF12-BSA. The crypts were then resuspended in cold Matrigel ECM solution at 20,000 crypts/ml and cultured in 24-well plates as described above.
PBMC and MAIT cell migration assays
The PDAOs were typically serially passaged at least five times in a medium containing broad-spectrum antibiotics and antifungals before coculture with the PBMCs to minimize residual bacterial contamination and inflammatory status of the organoids. The intact PDAOs were then carefully harvested from the ECM dome and cocultured with E. coli before being reembedded into the ECM dome. Briefly, mature PDAOs were released from Matrigel via gentle pipetting, resuspended in DMEMF12-BSA, and manually counted by macroscopic visualization of the organoid suspension. Then, five organoids were carefully collected from the organoid suspension for each group using 200-μl pipette tips and were left to settle under 1g for 5 min at room temperature. After removal of supernatants, these mature ogranoids were reembedded in Matrigel ECM solution alone or with formaldehyde-fixed E. coli DH5α (5 × 107 CFU/ml) (77) as indicated, plated, and incubated for 15 min at 37°C until the Matrigel ECM solution became solidified. The ECM alone controls containing the same number of E. coli were included in all experiments as indicated. Organoids were checked and recounted under the microscope to ensure seeding number accuracy. Cell suspensions in MAIT cell medium containing 2 × 106 cells/ml PBMCs or MAIT cells, as indicated, were then added to the organoid cultures. In selected experiments, MAIT cells were labeled with CellTrace Violet (CTV) (Thermo Fisher Scientific) as previously described (17), before being added to the coculture system. The cells were cocultured for 2 to 3 days at 37°C/5% CO2 thereafter harvested for flow cytometric analyses or stained for live confocal microscopy.
For flow cytometric analyses, cells from both the outside and inside of the Matrigel ECM domes were harvested and dissociated into single-cell suspensions. Briefly, supernatants were collected, and the wells were extensively washed with fluorescence-activated cell sorting buffer [Ca2+- and Mg2+-free PBS supplemented with 2% fetal bovine serum (FBS) and 2 mM EDTA] while taking care to preserve the integrity of the domes. The washed fractions were combined with the supernatants and kept on ice. To harvest cells from within the Matrigel ECM domes, the domes were gently aspirated from the wells and then dissociated in prewarmed Trypsin-EDTA (0.25%; Gibco) by extensive pipetting and vortexing for 2 min, followed by addition of FBS at final concentration of 10% (v/v). Cellular suspensions from both the outside and the inside of the Matrigel ECM domes were then pelleted at 500g at 4°C for 5 min, followed by flow cytometric analyses.
In selected experiments, MAIT cell chemotaxis to organoid-derived supernatants was performed. Briefly, 10% (v/v) of pooled supernatants of mature PDAO cultures (>day 7 after passage) from multiple donors, or 5% (v/v) of PDAO passage 0 supernatants from a single patient with appendicitis in OGM were transferred to the bottom chambers of 6.5-mm Transwell plates with 5-μm pore polycarbonate membrane inserts (Corning). 5-OP-RU and IL-12 + IL-18–expanded MAIT cell suspensions were loaded to the insert with the density of 106/ml in MAIT cell medium and incubated at 37°C/5% CO2 for 3 hours and harvested for flow cytometric analyses. In selected experiments, MAIT cells were pretreated with 100 nM of various chemokine receptor antagonists (table S4) for 2 hours before the chemotaxis assay.
MAIT cell cytotoxicity assay in 2D and 3D cell cultures
The MAIT cell cytotoxicity protocols in 2D cell cultures were adapted from our previously described methodology (62). Briefly, mature cultures of PDAOs were harvested as described above and dissociated into single-cell suspension by incubating in GCDR at room temperature for 10 min followed by 0.05% Trypsin-EDTA digestion at 37°C for 5 min. Unviable organoid cells were removed using human dead cell removal kit according to the manufacturer’s instruction (MojoSort, BioLegend). Viable organoid cells were resuspended in MAIT cell medium, plated, and rested at 37°C/5% CO2 for 1 hour, followed by addition of 5-OP-RU at a final concentration of 2 nM and incubated for 2 hours. Anti-MR1 monoclonal antibody (mAb, 26.5; BioLegend) or IgG2a isotype control (MOPC-173; BioLegend) was added and incubated for 1 hour, followed by the addition of CTV-labeled MAIT cells at an effector to target cell (E:T) ratio of 5:1. Anti–CD107a-PE (1:200) was added into the culture medium at the start of the assay to assess MAIT cell degranulation. After 3 hours of culture at 37°C/5% CO2, cells were harvested and stained for flow cytometry as indicated. In the 3D experiment, mature PDAOs were retrieved from the Matrigel ECM domes, incubated with or without formaldehyde-fixed E. coli, reembedded in Matrigel, and cocultured with CTV-labeled MAIT cells for 2 to 3 days, as described above. The cytotoxicity of MAIT cells toward PDAO was visualized using confocal microscopy after staining with annexin V and PI.
Preparation of appendix tissue and appendix-derived organoid supernatants
The supernatants of mature culture of PDAO or from passage 0 of PDAOs were collected every 2 to 3 days during half-media changes. Supernatants were then pooled from multiple donors from different time points or the same PDAO donor from different time points as indicated, aliquoted, snap-frozen in liquid nitrogen, and stored in −80°C until further use. In selected experiments, 106 cells derived from inflamed and less/noninflamed portions of appendix tissues were cultured in MAIT cell medium for 24 hours at 37°C 5% CO2. Supernatants were collected, clarified, and stored in −80°C until further use.
Preparation of 5-OP-RU
The MAIT cell antigen 5-OP-RU was synthesized as a solution in dimethyl sulfoxide (DMSO) (and its concentration quantified by nuclear magnetic resonance spectroscopy) as previously reported (6, 78, 79). It is stable in DMSO solutions but converts rapidly to much less active lumazines in aqueous media, and the exposure time should be minimized as much as possible to maximize activity. All 5-OP-RU working solutions were diluted from the DMSO stocks with the appropriate culture medium immediately before any functional assay.
Flow cytometry
Cells were stained for surface and intracellular molecules in accordance with a protocol described previously (77). The antibodies were listed in table S4. The flow cytometry was performed in CytoFLEX (Beckman Coulter) equipped with 405-, 488-, and 638-nm lasers. The data were analyzed with Flowjo v.10 (BD Biosciences). MAIT cells were identified as Vα7.2+ CD161hi CD3+ T cells or Vα7.2+ MR1–5-OP-RU+ CD3+ T cells as indicated. Cell numbers were enumerated using flow cytometry–based Precision Count Beads (BioLegend) according to the manufacturer’s instructions.
Immunofluorescence confocal microscopy
These protocols used the whole-mount staining of PDAO plated on 96-well or 24-well polystyrene tissue culture-treated plates (Corning). In brief, the PDAOs were first fixed with TF Fix/Perm (BD Biosciences) for 15 min at 37°C and washed carefully with TF Perm/Wash (BD Biosciences) to maintain the integrity of the Matrigel ECM domes. The PDAOs were subsequently stained for 30 min at 37°C in TF Perm/Wash buffer with fluorochrome-conjugated mAbs against EpCAM-1 and Krt20 as listed in table S4. The nuclei and actin filaments of organoids were concurrently counterstained with 4′,6-diamidino-2-phenylindole and phalloidin, respectively, in accordance with the manufactures’ instructions (table S4). In selected experiments, the PDAOs were live-stained with fluorochrome-conjugated mAb against E-cadherin, and the nuclei were counterstained using cell permeant SYTO21 nucleic acid stain for 30 min at 37°C in binding buffer (MAIT cell medium supplemented with 1 mM CaCl2 and 1 mM MgCl2). In the PDAO and CTV-labeled MAIT cells coculture experiments, the cocultures were carefully live-stained with annexin V and PI for 30 min at 37°C to assess MAIT cell–mediated killing of organoids. Nuclei were concurrently stained by using SYTO21. In selected experiments where PBMCs were used, the cocultures were carefully stained with mAbs against E-cadherin and CD3 for 30 min at 37°C in binding buffer. The images were acquired with a Nikon A1R+ laser scanning confocal microscopy equipped with 4×/0.2, 10×/0.45, and 20×/0.45 objectives (Nikon). Imaging was controlled using NIS-Elements software (Nikon) and processed with ImageJ 1.53a software (National Institutes of Health) (80). Integrated fluorescence density was measured in ImageJ by using the Threshold function to separate regions of interest from the background. Lines were drawn using the line tool in the ImageJ software to estimate potential areas of colocalization and define the region of interest for subsequent analysis for overlapping fluorescence intensities.
Statistical analysis
Statistical analyses were performed using Prism software version 10.0.2 (GraphPad). Datasets were first assessed for data normality distribution. Data presented as a heatmap show the mean, whereas data presented as line graphs with shaded error bars represent the mean and SE. Truncated violin plots represent median, the interquartile range, the minimum and maximum observation for each group, and the approximate frequency of data points. Statistically significant differences between samples were determined as appropriate using the unpaired t test or Mann-Whitney’s test for unpaired samples and the paired t test or Wilcoxon’s signed-rank test for paired samples. The Kruskal-Wallis one-way analysis of variance (ANOVA), the Friedman test, ordinary ANOVA, the repeated-measures one-way ANOVA, or mixed-effects analysis followed by the appropriate post hoc test as indicated was used to detect differences across multiple samples. Correlations were assessed using the Spearman rank correlation. Two-sided P < 0.05 was considered significant.
Acknowledgments
We thank all donors, healthcare personnel, study coordinators, administrators, and laboratory managers involved in this work. The MR1 tetramer technology was developed jointly by J. McCluskey, J. Rossjohn, and D. Fairlie, and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne. We thank T. Tsuboi (Tsinghua Shenzhen International Graduate School) for insights into confocal microscopy analyses.
Funding: This work was funded by the following: Tsinghua Shenzhen International Graduate School grant QD2022018C (E.L.), Tsinghua Shenzhen International Graduate School grant JC2022007 (E.L.), Science, Technology and Innovation Commission of Shenzhen Municipality grant WDZC20220819153248002 (E.L.), Shenzhen Municipality Pengcheng Peacock Program (E.L.), National Natural Science Foundation of China grant 61971255 (S.M.), National Natural Science Foundation of China grant 82111530212 (S.M.), Natural Science Foundation of Guangdong Province grant 2021B1515020092 (S.M.), Fujian Medical University Startup Fund for Scientific Research grant 2020QH1293 (B.W.), National Institutes of Health RO1 grant AI148407-01A1 (D.P.F.), NHMRC Investigator grant 2009551 (D.P.F.), and Australian Research Council grant CE200100012 (D.P.F.).
Author contributions: Conceptualization: Y.Z., S.M., and E.L. Methodology: Y.Z., F.H., Z.W., X.C., and E.L. Investigation: Y.Z., F.H., Z.W., X.C., Y.J., A.H., D.H., and E.L. Data analysis: Y.Z., Z.W., C.B., and E.L. Provision of critical materials: B.W., W.R.S., J.Y.W.M., D.P.F., L.-F.W., J.K.S., and P.E.L. Supervision: S.M. and E.L. Writing—original draft: Y.Z. and E.L. Writing—review and editing: J.Y.W.M., D.P.F., J.K.S., S.M., and E.L. All authors reviewed and approved the final manuscript.
Competing interests: E.L. and F.H. are named inventors on a patent application (no. 202211393019.8, China National Intellectual Property Administration) owned by Tsinghua Shenzhen International Graduate School. J.Y.W.M. and D.P.F. are named inventors on a granted patent (no. WO2015/149130, World Intellectual Property Organization) owned by University of Queensland, Monash University, and University of Melbourne. The rest of the authors declare that they have no competing interests associated with this study.
Data and materials availability: All data required to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Materials and Methods
Figs. S1 to S5
Tables S1 to S4
Legend for data file S1
Other Supplementary Material for this manuscript includes the following:
Data file S1
REFERENCES AND NOTES
- 1.Provine N. M., Klenerman P., MAIT cells in health and disease. Annu. Rev. Immunol. 38, 203–228 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y., Han F., Ho A., Xue Y., Wu Z., Chen X., Sandberg J. K., Ma S., Leeansyah E., Role of MAIT cells in gastrointestinal tract bacterial infections in humans: More than a gut feeling. Mucosal Immunol. 16, 740–752 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Kjer-Nielsen L., Patel O., Corbett A. J., le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., Williamson N. A., Purcell A. W., Dudek N. L., McConville M. J., O’Hair R. A. J., Khairallah G. N., Godfrey D. I., Fairlie D. P., Rossjohn J., McCluskey J., MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O., Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Corbett A. J., Eckle S. B. G., Birkinshaw R. W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H., Williamson N. A., Strugnell R. A., van Sinderen D., Mak J. Y. W., Fairlie D. P., Kjer-Nielsen L., Rossjohn J., McCluskey J., T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Mak J. Y. W., Liu L., Fairlie D. P., Chemical modulators of mucosal associated invariant T cells. Acc. Chem. Res. 54, 3462–3475 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souter M. N. T., Awad W., Li S., Pediongco T. J., Meehan B. S., Meehan L. J., Tian Z., Zhao Z., Wang H., Nelson A., le Nours J., Khandokar Y., Praveena T., Wubben J., Lin J., Sullivan L. C., Lovrecz G. O., Mak J. Y. W., Liu L., Kostenko L., Kedzierska K., Corbett A. J., Fairlie D. P., Brooks A. G., Gherardin N. A., Uldrich A. P., Chen Z., Rossjohn J., Godfrey D. I., McCluskey J., Pellicci D. G., Eckle S. B. G., CD8 coreceptor engagement of MR1 enhances antigen responsiveness by human MAIT and other MR1-reactive T cells. J. Exp. Med. 219, e20210828 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassidy F. C., Kedia-Mehta N., Bergin R., Woodcock A., Berisha A., Bradley B., Booth E., Jenkins B. J., Ryan O. K., Jones N., Sinclair L. V., O’Shea D., Hogan A. E., Glycogen-fuelled metabolism supports rapid mucosal-associated invariant T cell responses. Proc. Natl. Acad. Sci. U.S.A. 120, e2300566120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reantragoon R., Corbett A. J., Sakala I. G., Gherardin N. A., Furness J. B., Chen Z., Eckle S. B. G., Uldrich A. P., Birkinshaw R. W., Patel O., Kostenko L., Meehan B., Kedzierska K., Liu L., Fairlie D. P., Hansen T. H., Godfrey D. I., Rossjohn J., McCluskey J., Kjer-Nielsen L., Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 210, 2305–2320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sia W. R., Hey Y. Y., Foo R., Wang L.-F., Leeansyah E., Culture, expansion, and flow-cytometry-based functional analysis of pteropid bat MR1-restricted unconventional T cells. STAR Protoc. 2, 100487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeansyah E., Hey Y. Y., Sia W. R., Ng J. H. J., Gulam M. Y., Boulouis C., Zhu F., Ahn M., Mak J. Y. W., Fairlie D. P., Kwa A. L. H., Sandberg J. K., Wang L. F., MR1-restricted T cells with MAIT-like characteristics are functionally conserved in the pteropid bat Pteropus alecto. iScience 23, 101876 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juno J. A., Wragg K. M., Amarasena T., Meehan B. S., Mak J. Y. W., Liu L., Fairlie D. P., McCluskey J., Eckle S. B. G., Kent S. J., MAIT cells upregulate α4β7 in response to acute simian immunodeficiency virus/simian HIV infection but are resistant to peripheral depletion in pigtail macaques. J. Immunol. 202, 2105–2120 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Greene J. M., Dash P., Roy S., McMurtrey C., Awad W., Reed J. S., Hammond K. B., Abdulhaqq S., Wu H. L., Burwitz B. J., Roth B. F., Morrow D. W., Ford J. C., Xu G., Bae J. Y., Crank H., Legasse A. W., Dang T. H., Greenaway H. Y., Kurniawan M., Gold M. C., Harriff M. J., Lewinsohn D. A., Park B. S., Axthelm M. K., Stanton J. J., Hansen S. G., Picker L. J., Venturi V., Hildebrand W., Thomas P. G., Lewinsohn D. M., Adams E. J., Sacha J. B., MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 10, 802–813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., le Bourhis L., Soudais C., Treiner E., Lantz O., Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Martin E., Stepwise development of MAIT cells in mouse and human. PLOS Biol. 7, e54 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bourhis L., Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Boulouis C., Sia W. R., Gulam M. Y., Teo J. Q. M., Png Y. T., Phan T. K., Mak J. Y. W., Fairlie D. P., Poon I. K. H., Koh T. H., Bergman P., Lim C. M., Wang L. F., Kwa A. L. H., Sandberg J. K., Leeansyah E., Human MAIT cell cytolytic effector proteins synergize to overcome carbapenem resistance in Escherichia coli. PLOS Biol. 18, e3000644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurioka A., Ussher J. E., Cosgrove C., Clough C., Fergusson J. R., Smith K., Kang Y. H., Walker L. J., Hansen T. H., Willberg C. B., Klenerman P., MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 8, 429–440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meierovics A. I., Cowley S. C., MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J. Exp. Med. 213, 2793–2809 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salio M., Gasser O., Gonzalez-Lopez C., Martens A., Veerapen N., Gileadi U., Verter J. G., Napolitani G., Anderson R., Painter G., Besra G. S., Hermans I. F., Cerundolo V., Activation of human mucosal-associated invariant T cells induces CD40L-dependent maturation of monocyte-derived and primary dendritic cells. J. Immunol. 199, 2631–2638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey M. S., Morgan M. P., Liuzzi A. R., Tyler C. J., Khan M. W. A., Szakmany T., Hall J. E., Moser B., Eberl M., Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J. Immunol. 193, 3704–3716 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias J., Sobkowiak M. J., Sandberg J. K., Leeansyah E., Human MAIT-cell responses to Escherichia coli: Activation, cytokine production, proliferation, and cytotoxicity. J. Leukoc. Biol. 100, 233–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamichhane R., Schneider M., de la Harpe S. M., Harrop T. W. R., Hannaway R. F., Dearden P. K., Kirman J. R., Tyndall J. D. A., Vernall A. J., Ussher J. E., TCR- or cytokine-activated CD8+ mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep. 28, 3061–3076.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Dias J., Boulouis C., Gorin J. B., van den Biggelaar R., Lal K. G., Gibbs A., Loh L., Gulam M. Y., Sia W. R., Bari S., Hwang W. Y. K., Nixon D. F., Nguyen S., Betts M. R., Buggert M., Eller M. A., Broliden K., Tjernlund A., Sandberg J. K., Leeansyah E., The CD4−CD8− MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc. Natl. Acad. Sci. U.S.A. 115, E11513–E11522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ussher J. E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T. H., Klenerman P., Willberg C. B., CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng T., Akther H. D., Hackstein C. P., Powell K., King T., Friedrich M., Christoforidou Z., McCuaig S., Neyazi M., Arancibia-Cárcamo C. V., Hagel J., Powrie F.; Oxford IBD Investigators, Peres R. S., Millar V., Ebner D., Lamichhane R., Ussher J., Hinks T. S. C., Marchi E., Willberg C., Klenerman P., TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 28, 3077–3091.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leeansyah E., Loh L., Nixon D. F., Sandberg J. K., Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat. Commun. 5, 3143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffery H. C., van Wilgenburg B., Kurioka A., Parekh K., Stirling K., Roberts S., Dutton E. E., Hunter S., Geh D., Braitch M. K., Rajanayagam J., Iqbal T., Pinkney T., Brown R., Withers D. R., Adams D. H., Klenerman P., Oo Y. H., Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol. 64, 1118–1127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gherardin N. A., Souter M. N. T., Koay H. F., Mangas K. M., Seemann T., Stinear T. P., Eckle S. B. G., Berzins S. P., d’Udekem Y., Konstantinov I. E., Fairlie D. P., Ritchie D. S., Neeson P. J., Pellicci D. G., Uldrich A. P., McCluskey J., Godfrey D. I., Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 96, 507–525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C. H., C/EBPδ drives interactions between human MAIT cells and endothelial cells that are important for extravasation. eLife 7, 32532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakala I. G., Kjer-Nielsen L., Eickhoff C. S., Wang X., Blazevic A., Liu L., Fairlie D. P., Rossjohn J., McCluskey J., Fremont D. H., Hansen T. H., Hoft D. F., Functional heterogeneity and antimycobacterial effects of mouse mucosal-associated invariant T cells specific for riboflavin metabolites. J. Immunol. 195, 587–601 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A., Lawry S. M., Klein B. S., Wang X., Sherer N. M., Zumwalde N. A., Gumperz J. E., LFA-1 ligation by high-density ICAM-1 is sufficient to activate IFN-γ release by innate T lymphocytes. J. Immunol. 201, 2452–2461 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meierovics A., Yankelevich W. J., Cowley S. C., MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. U.S.A. 110, E3119–E3128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z., Wang H., Shi M., Zhu T., Pediongco T., Lim X. Y., Meehan B. S., Nelson A. G., Fairlie D. P., Mak J. Y. W., Eckle S. B. G., de Lima Moreira M., Tumpach C., Bramhall M., Williams C. G., Lee H. J., Haque A., Evrard M., Rossjohn J., McCluskey J., Corbett A. J., Chen Z., Francisella tularensis induces Th1 like MAIT cells conferring protection against systemic and local infection. Nat. Commun. 12, 4355 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias J., Leeansyah E., Sandberg J. K., Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl. Acad. Sci. U.S.A. 114, E5434–E5443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs A., Leeansyah E., Introini A., Paquin-Proulx D., Hasselrot K., Andersson E., Broliden K., Sandberg J. K., Tjernlund A., MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 10, 35–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haga K., Chiba A., Shibuya T., Osada T., Ishikawa D., Kodani T., Nomura O., Watanabe S., Miyake S., MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J. Gastroenterol. Hepatol. 31, 965–972 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Lal K. G., Kim D., Costanzo M. C., Creegan M., Leeansyah E., Dias J., Paquin-Proulx D., Eller L. A., Schuetz A., Phuang-ngern Y., Krebs S. J., Slike B. M., Kibuuka H., Maganga L., Nitayaphan S., Kosgei J., Sacdalan C., Ananworanich J., Bolton D. L., Michael N. L., Shacklett B. L., Robb M. L., Eller M. A., Sandberg J. K., Dynamic MAIT cell response with progressively enhanced innateness during acute HIV-1 infection. Nat. Commun. 11, 272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Bourhis L., Dusseaux M., Bohineust A., Bessoles S., Martin E., Premel V., Coré M., Sleurs D., Serriari N.-E., Treiner E., Hivroz C., Sansonetti P., Gougeon M.-L., Soudais C., Lantz O., MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLOS Pathog. 9, e1003681 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serriari N. E., Eoche M., Lamotte L., Lion J., Fumery M., Marcelo P., Chatelain D., Barre A., Nguyen-Khac E., Lantz O., Dupas J. L., Treiner E., Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 176, 266–274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung D. T., Bhuiyan T. R., Nishat N. S., Hoq M. R., Aktar A., Rahman M. A., Uddin T., Khan A. I., Chowdhury F., Charles R. C., Harris J. B., Calderwood S. B., Qadri F., Ryan E. T., Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLOS Negl. Trop. Dis. 8, e3076 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salerno-Goncalves R., Luo D., Fresnay S., Magder L., Darton T. C., Jones C., Waddington C. S., Blohmke C. J., Angus B., Levine M. M., Pollard A. J., Sztein M. B., Challenge of humans with wild-type Salmonella enterica Serovar Typhi elicits changes in the activation and homing characteristics of mucosal-associated invariant T cells. Front. Immunol. 8, 398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howson L. J., MAIT cell clonal expansion and TCR repertoire shaping in human volunteers challenged with Salmonella Paratyphi A. Nat. Immunol. 9, 253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Souza C., Chen Z., Corbett A. J., Revealing the protective and pathogenic potential of MAIT cells. Mol. Immunol. 103, 46–54 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Bhuiyan T. R., Rahman M. A., Trivedi S., Afroz T., al Banna H., Hoq M. R., Pop I., Jensen O., Rashu R., Uddin M. I., Hossain M., Khan A. I., Chowdhury F., Harris J. B., Calderwood S. B., Ryan E. T., Qadri F., Leung D. T., Mucosal-Associated Invariant T (MAIT) cells are highly activated in duodenal tissue of humans with Vibrio cholerae O1 infection: A preliminary report. PLOS Negl. Trop. Dis. 16, e0010411 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.S. Trivedi, O. Cheng, B. J. Brintz, R. C. Charles, D. T. Leung, Mucosal-associated invariant T (MAIT) cell responses in Salmonella enterica serovar Typhi strain Ty21a oral vaccine recipients. medRxiv 10.1101/2022.10.04.22280651 [Preprint] (2022). 10.1101/2022.10.04.22280651. [DOI]
- 47.Rim S., Dynamics of circulating lymphocytes responding to human experimental enterotoxigenic Escherichia coli infection. Eur. J. Immunol. 53, e2250254 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Sobkowiak M. J., Davanian H., Heymann R., Gibbs A., Emgård J., Dias J., Aleman S., Krüger-Weiner C., Moll M., Tjernlund A., Leeansyah E., Sällberg Chen M., Sandberg J. K., Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 49, 133–143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godfrey D. I., Koay H. F., McCluskey J., Gherardin N. A., The biology and functional importance of MAIT cells. Nat. Immunol. 20, 1110–1128 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Gunther C., Winner B., Neurath M. F., Stappenbeck T. S., Organoids in gastrointestinal diseases: From experimental models to clinical translation. Gut 71, 1892–1908 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lancaster M. A., Knoblich J. A., Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 345, 1247125 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Clevers H., Modeling development and disease with organoids. Cell 165, 1586–1597 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Wang L., Kamath A., Das H., Li L., Bukowski J. F., Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J. Clin. Invest. 108, 1349–1357 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xi X., Han X., Li L., Zhao Z., Identification of a new tuberculosis antigen recognized by γδ T cell receptor. Clin. Vaccine Immunol. 20, 530–539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parrot T., Healy K., Boulouis C., Sobkowiak M. J., Leeansyah E., Aleman S., Bertoletti A., Chen M. S., Sandberg J. K., Expansion of donor-unrestricted MAIT cells with enhanced cytolytic function suitable for TCR redirection. JCI Insight 6, e140074 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stankovic N., Surbatovic M., Stanojevic I., Simić R., Djuricic S., Milickovic M., Grujic B., Savic D., Marinovic V. M., Stankovic M., Vojvodic D., Possible cytokine biomarkers in pediatric acute appendicitis. Ital. J. Pediatr. 45, 125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leeansyah E., Svärd J., Dias J., Buggert M., Nyström J., Quigley M. F., Moll M., Sönnerborg A., Nowak P., Sandberg J. K., Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 infection. PLOS Pathog. 11, e1005072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu B., Liu M., Wang J., Fan H., Yang D., Zhang L., Gu X., Nie J., Chen Z., Corbett A. J., Zhan M. J., Zhang S., Bryant V. L., Lew A. M., McCluskey J., Luo H. B., Cui J., Zhang Y., Zhan Y., Lu G., IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 13, 824–835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallington J. C., Williams A. P., Staples K. J., Wilkinson T. M. A., IL-12 and IL-7 synergize to control mucosal-associated invariant T-cell cytotoxic responses to bacterial infection. J. Allergy Clin. Immunol. 141, 2182–2195.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Grabinger T., Luks L., Kostadinova F., Zimberlin C., Medema J. P., Leist M., Brunner T., Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 5, e1228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leeansyah E., Ganesh A., Quigley M. F., Sönnerborg A., Andersson J., Hunt P. W., Somsouk M., Deeks S. G., Martin J. N., Moll M., Shacklett B. L., Sandberg J. K., Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121, 1124–1135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sia W. R., Boulouis C., Gulam M. Y., Kwa A. L. H., Sandberg J. K., Leeansyah E., Quantification of human MAIT cell-mediated cellular cytotoxicity and antimicrobial activity. Methods Mol. Biol. 2098, 149–165 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Gold M. C., Cerri S., Smyk-Pearson S., Cansler M. E., Vogt T. M., Delepine J., Winata E., Swarbrick G. M., Chua W. J., Yu Y. Y. L., Lantz O., Cook M. S., Null M. D., Jacoby D. B., Harriff M. J., Lewinsohn D. A., Hansen T. H., Lewinsohn D. M., Human mucosal associated invariant T cells detect bacterially infected cells. PLOS Biol. 8, e1000407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cosgrove C., Ussher J. E., Rauch A., Gärtner K., Kurioka A., Hühn M. H., Adelmann K., Kang Y. H., Fergusson J. R., Simmonds P., Goulder P., Hansen T. H., Fox J., Günthard H. F., Khanna N., Powrie F., Steel A., Gazzard B., Phillips R. E., Frater J., Uhlig H., Klenerman P., Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood 121, 951–961 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gérart S., Sibéril S., Martin E., Lenoir C., Aguilar C., Picard C., Lantz O., Fischer A., Latour S., Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood 121, 614–623 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia P., Xing X. D., Yang C. X., Liao X. J., Liu F. H., Huang H. H., Zhang C., Song J. W., Jiao Y. M., Shi M., Jiang T. J., Zhou C. B., Wang X. C., He Q., Zeng Q. L., Wang F. S., Zhang J. Y., Activation-induced pyroptosis contributes to the loss of MAIT cells in chronic HIV-1 infected patients. Mil. Med. Res. 9, 24 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi J., Schmerk C. L., Mele T. S., Rudak P. T., Wardell C. M., Deng G., Pavri F. R., Kim K., Cepinskas G., He W., Haeryfar S. M. M., Longitudinal analysis of mucosa-associated invariant T cells in sepsis reveals their early numerical decline with prognostic implications and a progressive loss of antimicrobial functions. Immunol. Cell Biol. 101, 249–261 (2023). [DOI] [PubMed] [Google Scholar]
- 68.Parrot T., Gorin J.-B., Ponzetta A., Maleki K. T., Kammann T., Emgård J., Perez-Potti A., Sekine T., Rivera-Ballesteros O.; the Karolinska COVID-19 Study Group, Gredmark-Russ S., Rooyackers O., Folkesson E., Eriksson L. I., Norrby-Teglund A., Ljunggren H.-G., Björkström N. K., Aleman S., Buggert M., Klingström J., Strålin K., Sandberg J. K., MAIT cell activation and dynamics associated with COVID-19 disease severity. Sci. Immunol. 5, eabe1670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flament H., Rouland M., Beaudoin L., Toubal A., Bertrand L., Lebourgeois S., Rousseau C., Soulard P., Gouda Z., Cagninacci L., Monteiro A. C., Hurtado-Nedelec M., Luce S., Bailly K., Andrieu M., Saintpierre B., Letourneur F., Jouan Y., Si-Tahar M., Baranek T., Paget C., Boitard C., Vallet-Pichard A., Gautier J. F., Ajzenberg N., Terrier B., Pène F., Ghosn J., Lescure X., Yazdanpanah Y., Visseaux B., Descamps D., Timsit J. F., Monteiro R. C., Lehuen A., Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol. 22, 322–335 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Yoshie O., Matsushima K., CCR4 and its ligands: From bench to bedside. Int. Immunol. 27, 11–20 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Bertolini T. B., CCR4-dependent reduction in the number and suppressor function of CD4+Foxp3+ cells augments IFN-γ-mediated pulmonary inflammation and aggravates tuberculosis pathogenesis. Cell Death Dis. 10, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y., Wu Y., Qi H., Xiao J., Gong H., Zhang Y., Xu E., Li S., Ma D., Wang Y., Li W., Shen H., A new antagonist for CCR4 attenuates allergic lung inflammation in a mouse model of asthma. Sci. Rep. 7, 15038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugiyama D., Nishikawa H., Maeda Y., Nishioka M., Tanemura A., Katayama I., Ezoe S., Kanakura Y., Sato E., Fukumori Y., Karbach J., Jäger E., Sakaguchi S., Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. U.S.A. 110, 17945–17950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ben Youssef G., Tourret M., Salou M., Ghazarian L., Houdouin V., Mondot S., Mburu Y., Lambert M., Azarnoush S., Diana J. S., Virlouvet A. L., Peuchmaur M., Schmitz T., Dalle J. H., Lantz O., Biran V., Caillat-Zucman S., Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J. Exp. Med. 215, 459–479 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costamagna G., Acute appendicitis: Will a novel endoscopic "organ-sparing" approach change the treatment paradigm? Gastrointest. Endosc. 92, 190–191 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Liu B. R., Ma X., Feng J., Yang Z., Qu B., Feng Z. T., Ma S. R., Yin J. B., Sun R., Guo L. L., Liu W. G., Endoscopic retrograde appendicitis therapy (ERAT): A multicenter retrospective study in China. Surg. Endosc. 29, 905–909 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Dias J., Sandberg J. K., Leeansyah E., Extensive phenotypic analysis, transcription factor profiling, and effector cytokine production of human MAIT cells by flow cytometry. Methods Mol. Biol. 1514, 241–256 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Mak J. Y. W., Xu W., Reid R. C., Corbett A. J., Meehan B. S., Wang H., Chen Z., Rossjohn J., McCluskey J., Liu L., Fairlie D. P., Stabilizing short-lived Schiff base derivatives of 5-aminouracils that activate mucosal-associated invariant T cells. Nat. Commun. 8, 14599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mak J. Y. W., Determination of sample concentrations by PULCON NMR spectroscopy. Aust. J. Chem. 75, 160–164 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schneider C. A., Rasband W. S., Eliceiri K. W., NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Figs. S1 to S5
Tables S1 to S4
Legend for data file S1
Data file S1