Abstract
Angiotensin II (Ang II) stimulates hypertrophy of glomerular mesangial cells. The signalling mechanism by which Ang II exerts this effect is not precisely known. Downstream potential targets of Ang II are the extracellular-signal-regulated kinases 1 and 2 (ERK1/ERK2). We demonstrate that Ang II activates ERK1/ERK2 via the AT1 receptor. Arachidonic acid (AA) mimics the action of Ang II on ERK1/ERK2 and phospholipase A2 inhibitors blocked Ang II-induced ERK1/ERK2 activation. The antioxidant N-acetylcysteine as well as the NAD(P)H oxidase inhibitors diphenylene iodonium and phenylarsine oxide abolished both Ang II- and AA-induced ERK1/ERK2 activation. Moreover, dominant-negative Rac1 (N17Rac1) blocks activation of ERK1/ERK2 in response to Ang II and AA, whereas constitutively active Rac1 resulted in an increase in ERK1/ERK2 activity. Antisense oligonucleotides for Nox4 NAD(P)H oxidase significantly reduce activation of ERK1/ERK2 by Ang II and AA. We also show that protein synthesis in response to Ang II and AA is inhibited by N17Rac1 or MEK (mitogen-activated protein kinase/ERK kinase) inhibitor. These results demonstrate that Ang II stimulates ERK1/ERK2 by AA and Nox4-derived reactive oxygen species, suggesting that these molecules act as downstream signal transducers of Ang II in the signalling pathway linking the Ang II receptor AT1 to ERK1/ERK2 activation. This pathway involving AA, Rac1, Nox4, reactive oxygen species and ERK1/ERK2 may play an important role in Ang II-induced mesangial cell hypertrophy.
Keywords: angiotensin II, arachidonic acid, extracellular-signal-regulated kinase 1 and 2 (ERK1/ERK2), hypertrophy, Nox4 NADPH oxidase, reactive oxygen species
Abbreviations: AA, arachidonic acid; Ang II, angiotensin II; DAG, diacylglycerol; DPI, diphenylene iodonium; ERK1/ERK2, extracellular-signal-regulated kinase 1 and 2; HA, haemagglutinin; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MCs, mesangial cells; MEK, MAPK/ERK kinase; NAC, N-acetylcysteine; Nox, NAD(P)H oxidase; PAO, phenylarsine oxide; PD98059, 2-(2′-amino-3′-methoxyphenyl)oxanaphthalen-4-one; PKB, protein kinase B; PLA2, phospholipase A2; ROS, reactive oxygen species; RT, reverse transcriptase; VSMC, vascular smooth-muscle cells
INTRODUCTION
The octapeptide hormone Ang II (angiotensin II) activates MCs (mesangial cells) and stimulates cell growth, hypertrophy and the synthesis of extracellular matrix components [1]. These effects of Ang II contribute to the pathogenesis of fibrosis of the glomerular microvascular bed [1].
Ang II activates PLA2 (phospholipase A2) to generate AA (arachidonic acid) [2,3]. A direct role of AA has been implicated in certain cellular responses via activation of protein kinases and/or phosphatases [2,4–8]. In the kidney, Ang II-induced AA release mediates the activation of ERK (extracellular-signal-regulated kinase) and JNK (c-Jun N-terminal kinase) in epithelial cells [4,5,9]. In MCs, AA has been shown to activate JNK and Akt/PKB (protein kinase B) in response to interleukin-1 and Ang II respectively by a mechanism that does not require prostanoid production [2,6]. ROS (reactive oxygen species), including superoxide radical (O2•−) and H2O2, are also important chemical second messengers generated in response to Ang II [2,10]. ROS are important components of biological responses of vascular cells and myocytes including growth, migration and modification of the extracellular matrix [10]. NAD(P)H oxidase systems of the Nox family are a major source of ROS implicated in Ang II-induced oxidative stress [10–12]. The Nox oxidases are membrane-integrated proteins and their activation requires stimulus-induced membrane translocation of cytosolic proteins including the small GTPase Rac [11,13]. Recently, we have demonstrated that ROS generated by Nox4 play a role in hypertrophic response to Ang II and AA in MCs [12].
The MAPKs (mitogen-activated protein kinases) ERK1 and ERK2 are serine/threonine kinases that transduce signals from the cell membrane to the nucleus in response to classic growth factors and G-protein-coupled receptors, as well as cellular stresses [14]. ERK1/ERK2 are stimulated by growth factors and mitogenic stimuli, and play a pivotal role in cell growth and differentiation [14–18]. In MCs, ROS appear to be critical in mediating the activation of ERK by 5-hydroxytryptamine, which acts on a G-protein-coupled receptor [19]. Nevertheless, the signalling mechanisms involved in G-protein-coupled receptor activation of ERK by ROS have not been fully elucidated. Moreover, it is important to note that the sensitivity of ERK to oxidative signals is controversial and appears to be cell-type specific [18,20].
We have reported that AA/redox-dependent activation of Akt/PKB represents a critical signalling pathway, which mediates protein synthesis and MC hypertrophy in response to Ang II [2,12]. However, activation of Akt/PKB is not sufficient to obtain full hypertrophic response. To identify additional pathway(s) contributing to MC hypertrophy, we investigated the effects of Ang II and AA on ERK1/ERK2 activity, redox status and protein synthesis in MCs. We show that Ang II activates ERK1/ERK2 via an AA/PLA2 and Rac1/Nox4-induced ROS generation leading to MC hypertrophy. These observations suggest that ERK1/ERK2 is a critical component of the AA-, Rac1- and Nox4-dependent signalling pathway involved in Ang II-induced hypertrophy in MCs.
EXPERIMENTAL
Materials
Tissue culture materials were obtained from Gibco/BRL (Rockville, MD, U.S.A.). LIPOFECTAMINE™ was obtained from Life Technologies. Nonidet P40, PMSF, aprotinin, leupeptin, Na3VO4, NAC (N-acetylcysteine), DPI (diphenylene iodonium), PAO (phenylarsine oxide), H2O2, mepacrine, myelin basic protein, Ang II and AA were purchased from Sigma (St. Louis, MO, U.S.A.). Aristolochic acid was obtained from Calbiochem (La Jolla, CA, U.S.A.). PD98059 [2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one] was from Alexis Biochemical (San Diego, CA, U.S.A.). Rabbit anti-ERK1/-ERK2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Mouse anti-phospho-specific ERK antibody was from Cell Signaling Technology (Beverly, MA, U.S.A.). 2′,7′-Dichlorodihydrofluorescin diacetate was from Molecular Probes (Eugene, OR, U.S.A.). [3H]Thymidine and [γ-32P]ATP were from DuPont–New England Nuclear (Boston, MA, U.S.A.). [3H]Leucine was from Amersham Biosciences (Piscataway, NJ, U.S.A.). Nox4 cDNA was a gift from Dr K.-H. Krause (Geneva University Hospitals, Geneva, Switzerland). Myc epitope-tagged mammalian expression construct Myc-N17Rac1 and Myc-L61Rac1 were kindly provided by Dr A. Hall (University College London, London, U.K.). HA (haemagglutinin) epitope-tagged expression construct of dominant-negative HA-Akt (K179M) has been described previously [2].
Cell culture and transfections
Rat glomerular MCs were isolated and characterized as described in [21]. These cells were used between 15th and 30th passages. Selected experiments were performed in primary and early-passaged MCs to confirm the results obtained with late passages. Cells were maintained in RPMI 1640 tissue culture medium, supplemented with antibiotic/antifungal solution and 17% (v/v) foetal bovine serum. The rat aortic VSMCs (vascular smooth-muscle cells; a gift from Dr A. Hahn, Basel, Switzerland) were kept in Dulbecco's modified Eagle's medium, supplemented with 8 mM Hepes, 8 mM Tes, antibiotic/antifungal solution and 17% FBS (fetal bovine serum) as described previously [22]. Transient transfection of vector, Myc-N17Rac1, Myc-L61Rac1 or HA-Akt (K179M) plasmids was performed with LIPOFECTAMINE™ with a modification of the manufacturer's procedure as described previously [23].
Antisense oligonucleotides were designed near the ATG start codon of rat Nox4 (5′-AGCTCCTCCAGGACAGCGCC-3′). Antisense and the corresponding sense oligonucleotides were synthesized as phosphorothiolated oligonucleotides and purified by HPLC (Advanced Nucleic Acid Core Facility, The University of Texas Health Science Center, San Antonio, TX, U.S.A.). Antisense and sense oligonucleotides for Nox4 were transfected by electroporation as described previously [12].
Northern blot and reverse transcriptase (RT)–PCR detection of Nox4
Total RNA was isolated from MCs and VSMCs by using RNAzol B method (Cinna Biotex, Houston, TX, U.S.A.). For Northern-blot analysis, total RNA from MCs was separated electrophoretically on formaldehyde–agarose gel, transferred on to Gene Screen membrane and hybridized with full-length mouse Nox4 cDNA as described in [12]. For RT–PCR amplification of Nox4, total RNA from MCs or VSMCs was reverse-transcribed with the SuperScript reverse transcriptase (Life Technologies) and PCR for Nox4 was performed with Opti-prime™ PCR Optimization kit (Stratagene) using specific primers 5′-GATGTTGGGCCTAGGATTGTGT-3′ (forward primer) and 5′-CAGCCAGGAGGGTGAGTGTCTAA-3′ (reverse primer). Primers for β-actin were 5′-AGGCCAACCGCGAGAAGATGA-3′ (forward primer) and 5′-GAAGTCCAGGGCGACGTAGCA-3′ (reverse primer). Amplification was performed for 35 cycles at 93 °C for 1 min, 85 °C for 1 min and 72 °C for 1 min. The expected sizes of the PCR products for Nox4 and β-actin are 534 and 331 bp respectively. PCR-amplified DNA was separated on agarose gel, stained with ethidium bromide and visualized and photographed under UV light.
Immunoprecipitation and ERK1/ERK2 activity assay
MCs grown to near confluency were made quiescent by serum deprivation for 48 h. All incubations were performed in serum-free RPMI 1640 at 37 °C for a specified duration. The cells were lysed in radioimmunoprecipitation buffer (20 mM Tris/HCl, pH 7.5/150 mM NaCl/5 mM EDTA/1 mM Na3VO4/1 mM PMSF/20 μg/ml aprotinin/20 μg/ml leupeptin/1% Nonidet P40) at 4 °C for 30 min. The cell lysates were centrifuged at 10000 g for 30 min at 4 °C. Protein was determined in the cleared supernatant using the Bio-Rad protein assay reagent. For immunoprecipitation, an equal amount of protein (50–100 μg) was incubated with rabbit anti-ERK1/ERK2 for 3 h. Protein A/G–Sepharose beads were added and the resulting mixture was further incubated at 4 °C for 1 h on a rotating device. The beads were washed three times with radioimmunoprecipitation buffer, and twice with PBS. The kinase reaction was performed by incubating the immunobeads in kinase assay buffer (20 mM Hepes, pH 7.4/20 mM MgCl2/25 mM β-glycerophosphate/2 mM dithiothreitol/1 mM Na3VO4) in the presence of 5 μg/ml myelin basic protein and 200 μM unlabelled ATP plus 1 μCi of [γ-32P]ATP for 30 min at 30 °C. This reaction was stopped by the addition of 2× sample buffer, after which the samples were subjected to SDS/PAGE (12.5% gel) and the phosphorylated myelin basic protein was visualized by autoradiography or using a PhosphorImager. The bands were quantified by densitometric analysis using NIH Image and/or PhosphorImager analysis.
Immunoblotting
MC lysates were prepared as described above for ERK1/ERK2 activity assay. For immunoblotting, proteins (25–50 μg) were separated by SDS/PAGE (12.5% gel) and transferred on to PVDF membranes. Blots were incubated with either mouse polyclonal phospho-specific ERK (1:1000) or rabbit anti-ERK1/ERK2 (1:2000) and the primary antibodies were detected using horseradish peroxidase-conjugated IgG (1:2500 or 1:5000). Bands were visualized by enhanced chemiluminescence. Densitometric analysis was performed using NIH Image software.
Measurement of DNA and protein synthesis
[3H]Thymidine and [3H]leucine incorporations into trichloroacetic acid-insoluble material were used to assess DNA and protein synthesis respectively as described in [2].
Statistical analysis
Results are expressed as means±S.E.M. Statistical significance was assessed by Student's unpaired t test. P<0.05 was considered significant.
RESULTS
Effect of Ang II on ERK1/ERK2 activity in MCs
Cultured MCs were exposed to either 1 μM Ang II for different periods of time or to various concentrations of Ang II (0.001–1 μM) for 10 min, and the activation of ERK1/ERK2 was measured. Anti-ERK1/-ERK2 immunoprecipitates were used in an immunocomplex kinase assay using myelin basic protein as a substrate. In addition, since ERK is activated after phosphorylation of a threonine and a tyrosine residue in a TEY motif [14], we also used a specific antibody against phosphorylated ERK to detect the activated form of the kinase by immunoblotting. Ang II caused an increase in ERK1/ERK2 activity in a time-dependent manner, an effect that peaked at 10–15 min and returned to basal levels within 30 min (Figure 1A). Stimulation of MCs with different concentrations of Ang II showed dose-dependent increase in ERK1/ERK2 activity with a threshold at 0.001 μM and a maximal effect occurring at 1 μM (Figure 1B). Ang II had no significant effect on ERK1/ERK2 levels as determined by immunoblotting. To identify the Ang II receptor subtype involved, losartan (an AT1 blocker) and PD123319 (an AT2 blocker) were added at a concentration of 10 μM for 30 min before the addition of 1 μM Ang II. The AT1-selective antagonist losartan, but not the AT2-selective antagonist PD123319, inhibited Ang II-induced activation of ERK1/ERK2. (Figure 1C). These results support a role for the AT1 receptor as a mediator of ERK1/ERK2 activation by Ang II.
Figure 1. Effects of Ang II on ERK1/ERK2 and p38-MAPK activation in MCs.
(A) Time course of ERK1/ERK2 activation by Ang II. Serum-deprived MCs were treated with 1 μM Ang II for the indicated time periods. ERK activation was assessed either using anti-phospho-specific ERK antibodies (top panel) or by immunocomplex kinase assay in ERK1/ERK2 immunoprecipitates with myelin basic protein (MBP) as substrate (second panel from the top). The third panel from the top shows the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. (B) Dose–response of ERK1/ERK2 activation by Ang II. Cells were treated with various concentrations of Ang II (0.001–1 μM) for 5 min. (C) Ang II activates ERK1/ERK2 through the AT1 receptor. Ang II receptor antagonists losartan (10 μM, AT1 receptor specific) and PD123319 (10 μM, AT2 receptor specific) were added to the cultured medium for 30 min before exposure of the cells to 1 μM Ang II for an additional 15 min. In (B, C), ERK1/ERK2 immunoprecipitates were incubated with myelin basic protein and phosphorylation of the substrate was assayed. The top panels are representative images of myelin basic protein phosphorylation by Ang II. The middle panels show the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. In (A–C), each histogram of the bottom panel represents the ratio of the radioactivity incorporated into the phosphorylated myelin basic protein quantified by PhosphorImager analysis divided by the densitometric measurement of ERK1/ERK2 band. Results are expressed as percentage of control where the ratio in the untreated cells was defined as 100%. Values are the means±S.E.M. for three independent experiments. *P<0.05; **P<0.01 versus control.
Role of AA and PLA2 in Ang II-induced ERK1/ERK2 activation in MCs
Although members of MAPK family are known downstream targets of AA [9–14], the role, if any, of AA in the activation of ERK1/ERK2 has not been investigated in MCs. We examined the effect of AA on ERK1/ERK2 activity. As shown in Figure 2(A), exposure of MCs to 30 μM AA caused significant activation of ERK1/ERK2. AA-induced ERK1/ERK2 activation was dose-dependent with a maximal effect occurring at 30 μM (Figure 2B). The time course of ERK1/ERK2 activation demonstrated that the effect of AA on ERK1/ERK2 activity was more rapid (activation first peaking at 5 min after treatment) when compared with the effect of Ang II (activation peaking at 10–15 min), consistent with the contention that AA may mediate ERK1/ERK2 stimulation in response to Ang II (cf. Figures 1A and 2A). One of the mechanisms by which Ang II elicits an increase in AA production is hydrolysis of phospholipids by PLA2 [2,3,9,24]. We examined the effect of PLA2 inhibitors, mepacrine and aristolochic acid. Preincubation of MCs with mepacrine or aristolochic acid abolished ERK1/ERK2 activation induced by Ang II (Figure 2C left panel). On the other hand, RHC-80267, an inhibitor of DAG (diacylglycerol)/DAG lipase, had no effect on Ang II-induced ERK1/ERK2 phosphorylation, suggesting that generation of AA via the DAG/DAG lipase pathway is not involved in ERK1/ERK2 activation (Figure 2C right panel). Collectively, these results indicate that the effect of Ang II on ERK1/ERK2 is most probably mediated by AA via activation of PLA2.
Figure 2. Effects of AA on ERK1/ERK2 activation in MCs.
(A) Time-dependent activation of ERK1/ERK2 by AA. Serum-deprived MCs were treated with 30 μM AA for the time periods indicated. ERK activation was assessed either by using anti-phospho-specific ERK antibodies (top panel) or by immunocomplex kinase assay in ERK1/ERK2 immunoprecipitates with myelin basic protein (MBP) as substrate (second panel from the top). The third panel from the top shows the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. (B) Dose–response of ERK1/ERK2 activation by AA. Cells were treated with various concentrations of AA (5–30 μM) for 15 min. (C) Left panel: effect of PLA2 inhibitors on Ang II-induced ERK1/ERK2 activation. Serum-deprived MCs were preincubated with mepacrine (500 μM, 5 min) or aristolochic acid (50 μM, 30 min) followed by 1 μM Ang II for 10 min. Right panel: effect of DAG lipase inhibitor on Ang II-induced ERK1/ERK2 activation. MCs were preincubated with RHC-80267 (50 μM, 30 min) followed by 1 μM Ang II for 10 min. ERK activation was assessed using anti-phospho-specific ERK antibodies (top panel). In (B, C), the top panels are representative images of myelin basic protein phosphorylation by AA. The middle panel in (B) and bottom panel in (C) respectively show the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. In (A–C), each histogram of the lower panel represents the ratio of the radioactivity incorporated into the phosphorylated myelin basic protein quantified by PhosphorImager analysis divided by the densitometric measurement of ERK1/ERK2 band. Results are expressed as percentage of control, where the ratio in the untreated cells was defined as 100%. Values are the means±S.E.M. for three independent experiments. *P<0.05; **P<0.01 versus control.
Role of ERK1/ERK2 in Ang II- and AA-induced MC hypertrophy
Recently, we have shown that Ang II induces protein synthesis and hypertrophy of MCs through an AA-dependent pathway [2]. To study MC hypertrophy in response to Ang II and AA, the incorporation of [3H]leucine, a measure of protein synthesis, was compared with the incorporation of [3H]thymidine, a measure of DNA synthesis. Exposure of quiescent confluent MCs to 1 μM Ang II or 30 μM AA stimulated [3H]leucine incorporation (Figures 3A and 3B), whereas Ang II or AA had no significant effect on [3H]thymidine incorporation (Figure 3D), suggesting that Ang II induces MC hypertrophy. Foetal calf serum (10%, v/v), which served as a positive control, stimulated MC DNA synthesis by 6-fold over control. Since the above studies indicate that Ang II and AA are strong stimulants for ERK1/ERK2, we postulated that this kinase might be involved in Ang II- and AA-induced hypertrophy. Inhibition of the ERK1/ERK2 pathway by treatment of the cells with MEK (MAPK/ERK kinase) inhibitor PD98059 significantly reduced Ang II- and AA-induced [3H]leucine incorporation (Figures 3A and 3B). Importantly, pretreatment of MCs with mepacrine or aristolochic acid abrogated the hypertrophic effect of Ang II, confirming the importance of AA and PLA2 in the hypertrophic response to Ang II (Figure 3A). Taken together, these findings are consistent with a critical role for ERK1/ERK2 in the AA-mediated effect of Ang II on MC hypertrophy.
Figure 3. Roles of ERK1/ERK2 and PLA2 in Ang II-induced hypertrophy.
Serum-deprived MCs were treated with (filled bars) and without (open bars) 1 μM Ang II (A) or 30 μM AA (B) for 48 h in the presence or absence of the indicated inhibitors (mepacrine, 500 μM for 5 min; Aris (aristolochic acid), 50 μM for 30 min; PD98059, 50 μM for 1 h). Protein synthesis was measured by [3H]leucine incorporation as described in the Experimental section. Values are the means±S.E.M. for three independent experiments. **P<0.01 compared with control; ##P<0.01 compared with treatment with Ang II or AA alone. (C) MCs were transfected with HA-tagged inactive Akt/PKB mutant [HA-Akt (K179M)] or vector as control in the presence or absence of PD98059 (50 μM, 1h) and treated with (filled bars) or without (open bars) 1 μM Ang II for 48 h. Protein synthesis was measured by [3H]leucine incorporation as described in the Experimental section. The lower panel shows immunoblot of cells transfected with vector or the dominant negative form of Akt/PKB using anti-HA antibody. Values are the means±S.E.M. for three independent experiments. **P<0.01 compared with control; ##P<0.01 compared with treatment with Ang II+vector; @@P<0.01 compared with Ang II+HA-Akt (K179M). (D) Serum-deprived MCs were treated with 1 μM Ang II, 30 μM AA or 10% FCS for 48 h and DNA synthesis was measured by [3H]thymidine incorporation as described in the Experimental section. Values are the means±S.E.M. for three independent experiments. **P<0.01 compared with control.
The incomplete inhibition of [3H]leucine incorporation by PD98059 suggests that ERK1/ERK2 activation is necessary, but not sufficient, for protein synthesis. We have shown previously that Akt/PKB also participates in Ang II-induced protein synthesis in MCs [2]. Therefore we hypothesized that activation of both ERK1/ERK2 and Akt/PKB pathways may be necessary for a full response. Inhibition of the Akt/PKB pathway by the transfection of MCs with a dominant-negative mutant of Akt/PKB, HA-Akt (K179M), also partially attenuated the Ang II-induced protein synthesis (Figure 3C). Simultaneous inhibition of ERK1/ERK2 and Akt/PKB resulted in additive effect (Figure 3C). These results indicate that both ERK1/ERK2 and Akt/PKB pathways independently contribute to Ang II-induced protein synthesis.
Role of ROS in Ang II-induced ERK1/ERK2 activation
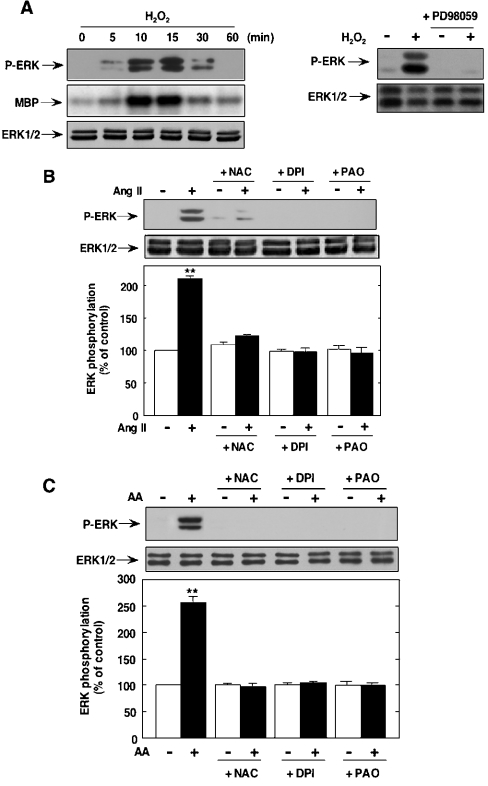
We have recently reported that Ang II and AA rapidly stimulate intracellular ROS generation in MCs [2]. Therefore we studied the effect of H2O2 on ERK1/ERK2 activation. As shown in Figure 4(A) left panel), 200 μM H2O2 induced a robust activation of ERK1/ERK2 with a peak effect occurring 10–15 min after the addition of H2O2. This is consistent with the time course of ERK1/ERK2 activation by Ang II or AA described previously. Incubation of MCs with PD98059 blocked H2O2-induced ERK1/ERK2 phosphorylation, demonstrating that redox-dependent activation of ERK1/ERK2 is mediated by MEK (Figure 4A right panel). These results confirm the previous observations showing that ERK1/ERK2 is redox sensitive and that H2O2 acts via MEK activation [10,20,25]. These results also indicate that ROS may play a role in the effects of Ang II and AA on ERK1/ERK2. To test this hypothesis, we first examined the effect of NAC, an antioxidant, on Ang II- and AA-induced ERK1/ERK2 phosphorylation. As shown in Figures 4(B) and 4(C), NAC blocked ERK1/ERK2 activity stimulated by Ang II or AA. We have previously shown that Ang II- and AA-induced increase in intracellular ROS is inhibitable by DPI, a potent inhibitor of NAD(P)H oxidase [2]. This indicates that Nox activity may be a major source of Ang II- and AA-induced ROS generation in MCs. Therefore we hypothesized that the ROS involved in Ang II- and AA-induced ERK1/ERK2 could be derived from the NAD(P)H oxidase. To assess the role of this enzyme in the activation of ERK1/ERK2 by Ang II and AA, we used the Nox inhibitors, DPI and PAO. DPI, as well as PAO, abrogated Ang II- and AA-stimulated ERK1/ERK2 activation (Figures 4B and 4C respectively). These findings are consistent with a critical role for NAD(P)H oxidase-derived ROS in Ang II- and AA-induced ERK1/ERK2 activation.
Figure 4. Role of ROS in ERK1/ERK2 activation by Ang II and AA.
(A) Left panel: time course of ERK1/ERK2 activation by H2O2. Serum-deprived MCs were treated with 200 μM H2O2 for the time periods indicated. ERK activation was measured either by assessing ERK phosphorylation using phospho-specific anti-ERK antibody (top panel) or by immunocomplex kinase assay in ERK1/ERK2 immunoprecipitates using myelin basic protein as substrate (middle panel). The bottom panel shows the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. Right panel: effect of MEK inhibitor on H2O2-induced ERK1/ERK2 activation. MCs were preincubated with PD98059 (50 μM, 1 h) followed by Ang II for 10 min. ERK activation was measured by assessing ERK phosphorylation using phospho-specific anti-ERK antibody (top panel). (B, C) Role of ROS in ERK1/ERK2 activation by Ang II and AA. Serum-deprived MCs were preincubated with or without 20 mM NAC, 10 μM DPI or 100 μM PAO for 30 min before treatment with 1 μM Ang II (B) or 30 μM AA (C) for 10 min. ERK activity was assessed using anti-phospho-specific ERK antibodies (top panels). The middle panels show the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. In the lower panels, each histogram represents the ratio of the densitometric measurement of phosphorylated ERK band divided by the densitometric measurement of total ERK1/ERK2 band. Results are expressed as percentage of control where the ratio in the untreated cells was defined as 100%. Values are the means±S.E.M. for three independent experiments. **P<0.01 versus control.
Role of Rac1 and Nox4 in Ang II-induced ERK1/ERK2 activation
We have described previously that the release of ROS elicited by Ang II and AA is mediated by Rac1 activation of a Nox4-containing NAD(P)H oxidase in MCs [12]. This observation prompted us to examine the role of the small GTPase Rac1 and Nox4 in Ang II- and AA-induced ERK1/ERK2 activation. As shown in Figure 5(A), expression of dominant-negative mutant of Rac1 (N17) totally inhibited Ang II- and AA-induced ERK1/ERK2 activation. The role of Rac1 in ERK1/ERK2 activation is supported by the observation that constitutively active Rac1 (L61) is sufficient to activate fully ERK1/ERK2 (Figure 5B). Moreover, treatment of MCs with 20 mM NAC inhibits L61Rac1-induced ERK1/ERK2 activity (Figure 5B). Western blot of the cell lysates using anti-Myc antibody confirms successful expression of the mutant protein. Next, we assessed the effect of phosphorothioate-modified antisense (AS) oligonucleotides for Nox4 on the redox-dependent activation of ERK1/ERK2 triggered by Ang II and AA. First, we confirmed by RT–PCR analysis the previous finding that Nox4 is highly expressed in rat MCs (Figure 6A upper panel) and that transfection of the cells with AS Nox4 but not sense (S) Nox4 significantly decreased Nox4 mRNA expression as measured by Northern-blot analysis (Figure 6A lower panel). VSMCs were used as positive controls for Nox4 expression. Importantly, transfection of MCs with AS Nox4, but not S Nox4, significantly reduced activation of ERK1/ERK2 in response to Ang II or AA (Figure 6B). Collectively, these results demonstrate that, in MCs, Rac1 and Nox4 protein function as transducers in the redox-dependent signal transduction pathway leading to ERK1/ERK2 activation in response to Ang II and AA.
Figure 5. Role of Rac1 in ERK1/ERK2 activation by Ang II and AA.
(A) MCs were transiently transfected with Myc-tagged dominant-negative mutant Myc-N17Rac1 (N17) or vector as control and treated with or without 1 μM Ang II or 30 μM AA for 10 min. (B) MCs untreated or treated with NAC (20 mM) for 1h were transfected with constitutively active mutant Myc-L61Rac1 (L61) or vector as control without the addition of Ang II or AA. In (A, B), ERK1/ERK2 immunoprecipitates were incubated with myelin basic protein (MBP) and phosphorylation of the substrate was assayed. The second panel from the top is a representative image of myelin basic protein phosphorylation. The third panel from the top shows the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. The bottom panel shows the immunoblot analysis of cell lysates using anti-Myc antibody to demonstrate mutant Rac1 expression. Each histogram represents the ratio of the radioactivity incorporated into the phosphorylated myelin basic protein quantified by PhosphorImager analysis divided by the densitometric measurement of ERK1/ERK2 band. Results are expressed as percentage of control where the ratio in the untreated cells was defined as 100%. Values are the means±S.E.M. for three independent experiments. **P<0.01 versus control.
Figure 6. Role of Nox4 in ERK1/ERK2 activation by Ang II and AA.
(A) Top panel: RT–PCR analysis showing that Nox4 mRNA is highly expressed in MCs. VSMCs were used as a positive control. Bottom panel, Northern-blot analysis showing that transfection by electroporation of AS Nox4 (1 μM), but not S Nox4 (1 μM), decreased mRNA expression of Nox4. Total RNA (10 μg) from rat MCs was hybridized with Nox4 cDNA. The lower panel shows the ethidium bromide staining of the same blot. (B) MCs were transfected with S Nox4 (1 μM) or AS Nox4 (1 μM) and treated with 1 μM Ang II or 30 μM AA for 5 min. ERK activation was assessed by using anti-phospho-specific ERK antibodies (top panel). The middle panel from the top shows the immunoblot analysis of cell lysates with ERK1/ERK2 antibody. Each histogram of the bottom panel represents the ratio of the densitometric analysis of ERK1/ERK2 phosphorylation divided by the densitometric measurement of ERK1/ERK2 band. Values are the means±S.E.M. for three independent experiments. **P<0.01 versus control; ##P<0.01 versus Ang II or AA.
Role of Rac1 in Ang II-induced hypertrophy
We have previously shown that Nox4-derived ROS mediates MC hypertrophy in response to Ang II and AA [2,12]. Since Rac1 and Nox4 act as upstream regulators of ERK1/ERK2 activation, suggesting that Rac1 is a part of the signalling cascade engaged by Ang II to regulate hypertrophy. However, no direct evidence of Rac1 involvement has been provided. To assess the role of Rac1 in MC protein synthesis, we tested the effect of dominant-negative mutant of Rac1, N17Rac1, on Ang II- and AA-stimulated [3H]leucine incorporation. As shown in Figures 7(A) and 7(B), inhibition of Rac1 by transient transfection of the cells with N17Rac1 nearly abolished Ang II- and AA-induced [3H]leucine incorporation. In contrast, expression of a constitutively active mutant of Rac1, L61Rac1, caused an increase in [3H]leucine incorporation. To assess the role of ROS in Rac1 stimulation of protein synthesis, we treated L61Rac1-transfected cells with the antioxidant NAC. As seen in Figure 7(A), the ability of L61Rac1 to stimulate [3H]leucine incorporation was inhibited by NAC. Western blot of the cell lysates was performed using anti-Myc antibody as a control for protein expression (Figure 7 lower panels). These results suggest that Rac1 plays a critical role in Ang II- and AA-induced hypertrophy. Moreover, these results support the concept that AA acts as a mediator of the signalling pathway activated by Ang II leading to MC hypertrophy via a Rac1-dependent pathway that results in the production of ROS.
Figure 7. Role of Rac1 in Ang II- and AA-induced protein synthesis in MCs.
MCs were transfected with Myc-tagged dominant-negative mutant of Rac1, Myc-N17Rac1 (N17) or vector as control and treated with (filled bars) or without (open bars) 1 μM Ang II (A) or 30 μM AA (B) for 48 h. MCs were also transfected by a Myc-tagged constitutively active form of Rac1, Myc-L61Rac1 (L61), without the addition of Ang II or AA in the presence or absence of 20 mM NAC (A). [3H]Leucine incorporation was then assayed as described in the Experimental section. The bottom panels show immunoblots of cells transfected with empty vector, N17Rac1 or L61Rac1 using anti-Myc antibody to demonstrate mutant Rac1 expression. Values are the means±S.E.M. for three independent experiments. **P<0.01 compared with control; ##P<0.01 compared with treatment with Ang II or AA alone.
DISCUSSION
The present study shows that in MCs Ang II, acting through the AT1 receptor, activates ERK1/ERK2. These results support a critical role for AA, Rac1 and Nox-type NAD(P)H oxidase-derived ROS generation in Ang II-induced activation of ERK1/ERK2. Thus, in MCs, ERK1/ERK2 activation is part of a redox-sensitive signalling pathway. Moreover, we provide the first evidence that the AA-, Rac1- and redox-dependent ERK1/ERK2 pathway contributes to Ang II-induced protein synthesis in MCs.
Ang II stimulates the release of AA after PLA2 activation in a variety of cell types including MCs [2,3,9,24]. Many protein kinases, such as Akt/PKB, ERK, JNK and p38-MAPK, have been identified as intracellular targets for AA [2,4–6,9]. In rabbit renal proximal tubular epithelial cells, AA induces JNK activation and mediates the action of Ang II on ERK [5,9]. In MCs, we have shown previously that Ang II activates Akt/PKB by an AA-dependent pathway [2]. The results presented in this study demonstrate that AA mimics the effect of Ang II on ERK1/ERK2 with a striking parallel in time course, suggesting that ERK1/ERK2 activation by Ang II is also mediated via AA release. Of note is that the concentrations of AA used in this study are within the range of concentrations that can be achieved in cultured cells or in vivo in cells and tissues [26]. Use of PLA2 inhibitors, mepacrine and aristolochic acid, provides further evidence that AA acts as a second messenger in mediating the stimulatory action of Ang II on ERK1/ERK2 activity. The present study is somewhat at variance with the observations of Huang et al. [6], which demonstrated that AA only weakly activates ERK1/ERK2 in the same cell type. MCs express the three known isoforms of PLA2, which include secretory, cytosolic and Ca2+-independent PLA2 [27]. Additional evidence implicates a membrane-associated and G-protein-linked PLA2 as a prime second messenger of Ang II in renal proximal tubule epithelium [28]. Also, the existence of other G-protein-coupled PLA2 in many cell types, including MCs, has been reported [29]. The specific isoform(s) of PLA2 involved in Ang II-induced ERK1/ERK2 activation in MCs remains to be determined. In most mammalian cells, AA is oxidized through cyclo-oxygenases, lipoxygenases and/or cytochrome P450 pathways to yield eicosanoids that mediate most of its biological effects. For instance, metabolites of 12/15-lipoxygenases contribute to Ang II-induced p38-MAPK activation and cell growth in MCs [30]. Nevertheless, it has been demonstrated that AA can directly stimulate members of the MAPK family, such as JNK, p38-MAPK, ERK as well as the MAPK phosphatase-1 [4–7]. In particular, in MCs, AA release in response to interleukin-1 has been shown to activate JNK by a mechanism that does not require enzymic oxygenation [6]. Hence, an important issue that requires further study in our model is the mechanism by which AA itself can activate ERK1/ERK2, i.e. direct activation or indirectly through eicosanoid biosynthesis.
There is now considerable evidence that ROS can function as classic second-messenger molecules [10]. It has been reported that ERK1/ERK2 can be activated by oxidative stress in a variety of cell types including MCs [10,18–20,25]. By using H2O2 as a model oxidant, we confirm that H2O2 leads to the activation of ERK1/ERK2 in MCs. We also demonstrate that the effect of Ang II and AA on ERK1/ERK2 is abrogated by the antioxidant NAC, indicating that ROS act as potential signalling molecules in the regulation of ERK1/ERK2 by Ang II and AA. Moreover, DPI, the inhibitor of flavin-containing oxidases, such as Nox, and PAO a specific reagent of vicinal thiol groups, which prevent phagocyte Nox activation, abolish Ang II- and AA-induced ERK1/ERK2 activation. In phagocytic cells, Rac proteins are involved in the assembly of the neutrophil Nox system, responsible for the subsequent activation of the enzyme [31]. We have previously demonstrated that the small GTPase Rac1 acts as an upstream activator of ROS generation by a NAD(P)H oxidase of the Nox family called Nox4. The Nox oxidases are isoforms of the phagocyte NADPH oxidase. In this family, Nox2 denominates gp91phox, the main subunit of the phagocyte NADPH oxidase and Nox4, which appears to share the same overall structure with Nox2/gp91phox, is abundant in the vascular system, kidney cortex and MCs [11–13,32–34]. However, the biological role(s) of Nox4 is not well understood still. In the present study, we show that Rac1 and Nox4 are required for Ang II- and AA-induced ERK1/ERK2 activation. These results support the concept that Nox4 is coupled with Ang II redox signalling in MCs.
MC hypertrophy accompanies diseases characterized by the activation of the renin–angiotensin system [35]. However, the signalling pathways involved in Ang II-induced protein synthesis, a critical step in cell hypertrophy, remain poorly defined. Recently, we have shown that AA is a key mediator of Ang II-induced hypertrophy in MCs [2]. Our results indicate that ERK1/ERK2 and Rac1 play an important role in Ang II- and AA-mediated protein synthesis. This is consistent with the observations that AA, Rac1 and ROS production act as signal transducers in the regulatory pathway leading to stimulation of ERK1/ERK2 by Ang II. Importantly, inhibition of ERK1/ERK2 only attenuates Ang II-induced protein synthesis. We have previously reported that Rac1- and redox-dependent activation of Akt/PKB contributes to the stimulation of protein synthesis by Ang II [2,12]. In the present study, we provide evidence that full hypertrophic response to Ang II requires parallel activation of both ERK1/ERK2 and Akt/PKB. The observations that inhibition of Rac1 or ROS generation abrogated Ang II-induced protein synthesis, combined with the findings that ERK1/ERK2 or Akt/PKB activation by Ang II are Rac1- and ROS-mediated (present results and [12]), indicate that Rac1-stimulated ROS generation is quite probably an upstream activator of both ERK1/ERK2 and Akt/PKB pathways. Thus our studies are consistent with an ERK1/ERK2 activation pathway that contributes to MC hypertrophy as depicted in Figure 8 Note that the effects of Ang II on protein synthesis exceed those of exogenous AA. It is probable that endogenous AA generated by Ang II interacts more effectively with target molecules, emphasizing the importance of local pools of AA and clustering in signalling events. Furthermore, additional Ang II-induced pathway(s) necessary, but not sufficient, to elicit protein synthesis may be required to obtain optimal response to AA. Importantly, ERK1/ERK2 is part of the redox-signalling cascade involved in the hypertrophic response to Ang II and AA. These observations are in contrast with those in VSMCs. Although Ang II robustly activates ERK1/ERK2 and p38-MAPK, only p38-MAPK activation is mediated by ROS [18]. This suggests that the mechanism of ERK1/ERK2 activation by Ang II, as well as its redox dependence, is cell specific. Interestingly, in MCs, ERK1/ERK2 is clearly activated by H2O2 and its redox sensitivity has been documented (see [19,25] and present study), whereas in VSMCs the sensitivity of ERK1/ERK2 to H2O2 remains controversial, with some reports showing no effect [18,36] and other reports demonstrating a stimulatory effect of H2O2 [20,37–39]. A similar controversy exists regarding the redox dependence of ERK1/ERK2-mediated hypertrophic response to Ang II in VSMCs [18,38], confirming the cell specificity of the redox regulation of ERK1/ERK2 in response to Ang II. The observations described in the present study are consistent with the reports showing the role of Rac in the effects of Ang II and AA products on cell growth [40,41] as well as with the recent results of Clerk et al. [15], demonstrating that the activation of Rac1 contributes to the hypertrophic response of cardiac myocytes by modulating ERK cascade. Similarly, in cultured renal proximal tubular cells, Hannken et al. [42] have shown that Ang II-stimulated ERK1/ERK2 activation is essential for the hypertrophic response of the cells to this vasoactive peptide. Overall, the results presented in this study and in our previous report [2] are in agreement with the observation that endothelin-1-induced MC hypertrophy requires activation of ERK and Akt/PKB [43]. Moreover, our results correlate well with those recently described by Greene et al. [19], who showed that ROS generated by 5-hydroxytryptamine mediate ERK activation in MCs, supporting the view that stimulation of ERK by G-protein-coupled receptors is dependent on ROS in MCs. Our findings highlight the importance of activation of ERK1/ERK2 through ROS by G-protein-coupled receptors. Since Ang II receptors are expressed in glomerular endothelial and epithelial cells, it would be of major interest to compare the signalling pathways utilized by Ang II in the various cell types that constitute the glomerular microvascular bed.
Figure 8. Proposed model of MC protein synthesis and hypertrophy stimulation by Ang II.
Acknowledgments
We thank Sergio Garcia for help with the cell culture. This work was supported in part through a VA Research Enhancement Award Program (G.G.C. and H.E.A.), VA Merit Review Award (G.G.C.), National Institutes of Health grants DK 43988 and DK 33665 (H.E.A.), DK 55815 (G.G.C.), the NIH/NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) George O'Brien Kidney Center (G.G.C. and H.E.A.) and the Southern Arizona Foundation (Y.G.). Y.G. was supported by a Scientist Development Grant from the American Heart Association, National. B.W. was supported by a Jack C. Kent Research Fellowship from the National Kidney Foundation.
References
- 1.Ardaillou R., Chansel D., Chatziantoniou C., Dussaule J. C. Mesangial AT1 receptors: expression, signaling, and regulation. J. Am. Soc. Nephrol. 1999;10:S40–S46. [PubMed] [Google Scholar]
- 2.Gorin Y., Kim N.-H., Feliers D., Bhandari B., Ghosh Choudhury G., Abboud H. E. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J. 2001;15:1909–1920. doi: 10.1096/fj..01-0165com. [DOI] [PubMed] [Google Scholar]
- 3.Schlondorff D., DeCandido S., Satriano J. A. Angiotensin II stimulates phospholipases C and A2 in cultured rat mesangial cells. Am. J. Physiol. 1987;253:C113–C120. doi: 10.1152/ajpcell.1987.253.1.C113. [DOI] [PubMed] [Google Scholar]
- 4.Alexander L. D., Cui X. L., Falck J. R., Douglas J. G. Arachidonic acid directly activates members of the mitogen-activated protein kinase superfamily in rabbit proximal tubule cells. Kidney Int. 2001;59:2039–2053. doi: 10.1046/j.1523-1755.2001.00718.x. [DOI] [PubMed] [Google Scholar]
- 5.Cui X. L., Douglas J. G. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3771–3776. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang S., Konieczkowski M., Schelling J. R., Sedor J. R. Interleukin-1 stimulates Jun N-terminal/stress-activated protein kinase by an arachidonate-dependent mechanism in mesangial cells. Kidney Int. 1999;55:1740–1749. doi: 10.1046/j.1523-1755.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- 7.Metzler B., Hu Y., Sturm G., Wick G., Xu Q. Induction of mitogen-activated protein kinase phosphatase-1 by arachidonic acid in vascular smooth muscle cells. J. Biol. Chem. 1998;273:33320–33326. doi: 10.1074/jbc.273.50.33320. [DOI] [PubMed] [Google Scholar]
- 8.Munaron L., Antoniotti S., Distasi C., Lovisolo D. Arachidonic acid mediates calcium influx induced by basic fibroblast growth factor in Balb-c 3T3 fibroblasts. Cell Calcium. 1997;22:179–188. doi: 10.1016/s0143-4160(97)90011-7. [DOI] [PubMed] [Google Scholar]
- 9.Dulin N. O., Alexander L. D., Harwalkar S., Falck J. R., Douglas J. G. Phospholipase A2-mediated activation of mitogen-activated protein kinase by angiotensin II. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8098–8102. doi: 10.1073/pnas.95.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 11.Lassegue B., Clempus R. E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 12.Gorin Y., Ricono J. M., Kim N.-H., Bhandari B., Choudhury G. G., Abboud H. E. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am. J. Physiol. Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 13.Lambeth J. D. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr. Opin. Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 15.Clerk A., Pham F. H., Fuller S. J., Sahai E., Aktories K., Marais R., Marshall C., Sugden P. H. Regulation of mitogen-activated protein kinases in cardiac myocytes through the small G protein Rac1. Mol. Cell. Biol. 2001;21:1173–1184. doi: 10.1128/MCB.21.4.1173-1184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huwiler A., Stabel S., Fabbro D., Pfeilschifter J. Platelet-derived growth factor and angiotensin II stimulate the mitogen-activated protein kinase cascade in renal mesangial cells: comparison of hypertrophic and hyperplastic agonists. Biochem. J. 1995;305:777–784. doi: 10.1042/bj3050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugden P. H., Clerk A. Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- 18.Ushio-Fukai M., Alexander R. W., Akers M., Griendling K. K. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 19.Greene E. L., Houghton O., Collinsworth G., Garnovskaya M. N., Nagai T., Sajjad T., Bheemanathini V., Grewal J. S., Paul R. V., Raymond J. R. 5-HT2A receptors stimulate mitogen-activated protein kinase via H2O2 generation in rat renal mesangial cells. Am. J. Physiol. Renal Physiol. 2000;278:F650–F658. doi: 10.1152/ajprenal.2000.278.4.F650. [DOI] [PubMed] [Google Scholar]
- 20.Guyton K. Z., Liu Y., Gorospe M., Xu Q., Holbrook N. J. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J. Biol. Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh Choudhury G., Karamitsos C., Hernandez J., Gentilini A., Bardgette J., Abboud H. E. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am. J. Physiol. 1997;273:F931–F938. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel U. O., Fouqueray B., Grandaliano G., Kim Y. S., Karamitsos C., Valente A. J., Abboud H. E. Thrombin regulates expression of monocyte chemoattractant protein-1 in vascular smooth muscle cells. Circ. Res. 1995;77:503–509. doi: 10.1161/01.res.77.3.503. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh Choudhury G., Kim Y. S., Simon M., Wozney J., Harris S., Ghosh-Choudhury N., Abboud H. E. Bone morphogenetic protein 2 inhibits platelet-derived growth factor-induced c-fos gene transcription and DNA synthesis in mesangial cells. Involvement of mitogen-activated protein kinase. J. Biol. Chem. 1999;274:10897–10902. doi: 10.1074/jbc.274.16.10897. [DOI] [PubMed] [Google Scholar]
- 24.Rao G. N., Lassegue B., Alexander R. W., Griendling K. K. Angiotensin II stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem. J. 1994;299:197–201. doi: 10.1042/bj2990197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilmer W. A., Tan L. C., Dickerson J. A., Danne M., Rovin B. H. Interleukin-1 induction of mitogen-activated protein kinases in human mesangial cells. Role of oxidation. J. Biol. Chem. 1997;272:10877–10881. doi: 10.1074/jbc.272.16.10877. [DOI] [PubMed] [Google Scholar]
- 26.Brash A. R. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hack N., Tay A., Schultz A., Muzin N., Clayman P., Egan S., Skorecki K. L. Regulation of rat kidney mesangial cell phospholipase A2. Clin. Exp. Pharmacol. Physiol. 1996;23:71–75. [PubMed] [Google Scholar]
- 28.Jacobs L. S., Douglas J. G. Angiotensin II type 2 receptor subtype mediates phospholipase A2-dependent signaling in rabbit proximal tubular epithelial cells. Hypertension. 1996;28:663–668. doi: 10.1161/01.hyp.28.4.663. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Kester M., Dunn M. J. Involvement of a pertussis toxin-sensitive G-protein-coupled phospholipase A2 in lipopolysaccharide-stimulated prostaglandin E2 synthesis in cultured rat mesangial cells. Biochim. Biophys. Acta. 1988;963:429–435. doi: 10.1016/0005-2760(88)90311-6. [DOI] [PubMed] [Google Scholar]
- 30.Reddy M. A., Adler S. G., Kim Y. S., Lanting L., Rossi J., Kang S. W., Nadler J. L., Shahed A., Natarajan R. Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. Am. J. Physiol. Renal Physiol. 2002;283:F985–F994. doi: 10.1152/ajprenal.00181.2002. [DOI] [PubMed] [Google Scholar]
- 31.Leusen J. H. W., Verhoeven A. J., Roos D. Interactions between the components of the human NADPH oxidase: a review about the intrigues in the phox family. Front. Biosci. 1996;1:d72–d90. doi: 10.2741/a117. [DOI] [PubMed] [Google Scholar]
- 32.Geiszt M., Kopp J. B., Varnai P., Leto T. L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 34.Etoh T., Inoguchi T., Kakimoto M., Sonoda N., Kobayashi K., Kuroda J., Sumimoto H., Nawata H. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia. 2003;46:1428–1437. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- 35.Kim S., Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 36.Baas A. S., Berk B. C. Differential activation of mitogen-activated protein kinases by H2O2 and O2− in vascular smooth muscle cells. Circ. Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Yoshizumi M., Abe J., Haendeler J., Huang Q., Berk B. C. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J. Biol. Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- 38.Frank G. D., Eguchi S., Yamakawa T., Tanaka S., Inagami T., Motley E. D. Involvement of reactive oxygen species in the activation of tyrosine kinase and extracellular signal-regulated kinase by angiotensin II. Endocrinology. 2000;141:3120–3126. doi: 10.1210/endo.141.9.7630. [DOI] [PubMed] [Google Scholar]
- 39.Madamanchi N. R., Li S., Patterson C., Runge M. S. Reactive oxygen species regulate heat-shock protein 70 via the JAK/STAT pathway. Arterioscler. Thromb. Vasc. Biol. 2001;21:321–326. doi: 10.1161/01.atv.21.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Wen Y., Gu J., Knaus U. G., Thomas L., Gonzales N., Nadler J. L. Evidence that 12-lipoxygenase product 12-hydroxyeicosatetraenoic acid activates p21-activated kinase. Biochem. J. 2000;349:481–487. doi: 10.1042/0264-6021:3490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takemoto M., Node K., Nakagami H., Liao Y., Grimm M., Takemoto Y., Kitakaze M., Liao J. K. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J. Clin. Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannken T., Schroeder R., Zahner G., Stahl R. A., Wolf G. Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27Kip1: role in angiotensin II-mediated hypertrophy of proximal tubular cells. J. Am. Soc. Nephrol. 2000;11:1387–1397. doi: 10.1681/ASN.V1181387. [DOI] [PubMed] [Google Scholar]
- 43.Goruppi S., Bonventre J. V., Kyriakis J. M. Signaling pathways and late-onset gene induction associated with renal mesangial cell hypertrophy. EMBO J. 2002;21:5427–5436. doi: 10.1093/emboj/cdf535. [DOI] [PMC free article] [PubMed] [Google Scholar]