Abstract
MTHFR (methylenetetrahydrofolate reductase) catalyses the synthesis of 5-methyltetrahydrofolate, the folate derivative utilized in homocysteine remethylation to methionine. A severe deficiency of MTHFR results in hyperhomocysteinaemia and homocystinuria. Betaine supplementation has proven effective in ameliorating the biochemical abnormalities and the clinical course in patients with this deficiency. Mice with a complete knockout of MTHFR serve as a good animal model for homocystinuria; early postnatal death of these mice is common, as with some neonates with low residual MTHFR activity. We attempted to rescue Mthfr−/− mice from postnatal death by betaine supplementation to their mothers throughout pregnancy and lactation. Betaine decreased the mortality of Mthfr−/− mice from 83% to 26% and significantly improved somatic development from postnatal day 1, compared with Mthfr−/− mice from unsupplemented dams. Biochemical evaluations demonstrated higher availability of betaine in suckling pups, decreased accumulation of homocysteine, and decreased flux through the trans-sulphuration pathway in liver and brain of Mthfr−/− pups from betaine-supplemented dams. We observed disturbances in proliferation and differentiation in the cerebellum and hippocampus in the knockout mice; these changes were ameliorated by betaine supplementation. The dramatic effects of betaine on survival and growth, and the partial reversibility of the biochemical and developmental anomalies in the brains of MTHFR-deficient mice, emphasize an important role for choline and betaine depletion in the pathogenesis of homocystinuria due to MTHFR deficiency.
Keywords: betaine supplementation, brain development, hyperhomocysteinaemia, methylenetetrahydrofolate reductase (MTHFR), remethylation
Abbreviations: BHMT, betaine–homocysteine methyltransferase; DMG, dimethylglycine; E18, embryonic day 18 (etc.); MTHFR, methylenetetrahydrofolate reductase; P0, postnatal day 0 (etc.); SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine
INTRODUCTION
Homocysteine is a sulphur amino acid which serves as the carbon backbone in methyl group metabolism (via the remethylation pathway) and as a precursor for the synthesis of cysteine, taurine and glutathione (via the trans-sulphuration pathway). The enzyme MTHFR (5,10-methylenetetrahydrofolate reductase; EC 1.5.1.20) catalyses the irreversible reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which functions as a methyl donor for the remethylation of homocysteine to methionine by 5-methyltetrahydrofolate–homocysteine S-methyltransferase (methionine synthase; EC 2.1.1.13). The flux through MTHFR determines the amount of newly formed one-carbon units that are directed towards nucleotide synthesis or towards the de novo synthesis of transferable methyl groups, following the activation of methionine to SAM (S-adenosylmethionine). Other sources of transferable methyl groups are the dietary preformed donors – methionine, betaine and choline [1]. Choline can be metabolized to betaine, which serves as an alternative methyl donor for the remethylation of homocysteine to methionine, by the enzyme BHMT (betaine–homocysteine methyltransferase; EC 2.1.1.5) [2].
Bi-allelic defects in the human MTHFR gene cause severe MTHFR deficiency, the most common inherited disorder of folate metabolism, with residual enzyme activities between 0% and 20% of those of controls in cultured fibroblasts [3]. This enzymic defect is one of the contributors to the inborn error of metabolism, homocystinuria. Although homocystinuria is a relatively rare disorder, the features seen in these patients with marked impairment of enzyme function can provide some insight into the clinical consequences of the frequently encountered milder MTHFR deficiency due to homozygosity for the C→T substitution at cDNA position 677 [4,5]. The phenotype of severe MTHFR deficiency has two major features: (1) various neurological and psychiatric symptoms caused by encephalopathy with neuronal loss and demyelination; and (2) recurrent thrombotic or thromboembolic events. Other symptoms relate to connective tissue anomalies and liver pathology [6]. Newborns with MTHFR deficiency are homocystinuric but have non-specific symptoms or are asymptomatic. The diagnosis of MTHFR deficiency is usually made in infants or older children, after the onset of symptoms such as psychomotor developmental delay [6].
Betaine supplementation has proven effective in ameliorating the biochemical abnormalities and the clinical course in homocystinuria due to severe MTHFR deficiency. It lowers the elevated plasma homocysteine levels associated with this disease and increases plasma methionine concentrations [7,8]. Betaine supplementation is believed to directly enhance homocysteine remethylation and, consequently, to increase the availability of methionine for protein synthesis and transmethylation. However, high-dose betaine treatment does not normalize homocysteine metabolism in homocystinuric patients, as indicated by plasma homocysteine levels that remain 5–10-fold elevated [7].
We have created a murine model for severe and mild MTHFR deficiency by generating mice with homozygous and heterozygous knockout of Mthfr alleles [9]. The Mthfr−/− and Mthfr+/− mice are good animal models for human homocystinuria and moderate hyperhomocysteinaemia respectively, based on residual enzyme activities and degree of hyperhomocysteinaemia. We recently reported the effects of betaine supplementation on homocysteine and choline metabolism in adult Mthfr+/+ (wild-type) mice and in their littermates with mild (Mthfr+/−) or severe (Mthfr−/−) deficiency. We demonstrated that MTHFR deficiency in mice is associated with a higher demand for betaine-dependent remethylation, and that betaine can lower plasma homocysteine levels in mice with all three Mthfr genotypes. However, remethylation through BHMT could compensate only partly for MTHFR deficiency, even with high-dose betaine treatment [10].
Homocystinuria due to severe MTHFR deficiency in humans can cause early lethality in the first year of life. There is a good correlation between the degree of residual enzyme activity and the clinical course in these patients [3]. It is not surprising, therefore, that there is considerable early postnatal loss of Mthfr−/− mice. In this paper, we report the results of our attempts to rescue nullizygous Mthfr mice from early postnatal death by betaine supplementation of their mothers, and describe the impact of this treatment on homocysteine metabolism and brain development of the pups.
EXPERIMENTAL
Animal husbandry and experiments
All mice were produced in our own breeding facility and housed in adequate shoebox cages with free access to food and water. The animal experiments were approved by the Animal Care Committee of the Montreal Children's Hospital and complied with the guidelines of the Canadian Council for Animal Care.
Mice, heterozygous for a disruption of the Mthfr gene (Mthfr+/−) and from F6 or F7 generations of backcrosses to the BALB/cAnNCrlBR strain, were used for breeding. Female Mthfr+/− mice on regular rodent chow (Purina laboratory rodent diet 5001; Purina Mills) were supplemented with anhydrous betaine (Sigma) at 2% (w/v) in the drinking water before mating them to Mthfr+/− males. Water intake was monitored. Betaine supplementation was continued until weaning of the pups at 3 weeks of age. Untreated matings served as controls. Cages were checked daily. The day of birth was designated as P0 (postnatal day 0). Newborn mice were examined daily for viability and body weight. The body weights of homozygous mutant (Mthfr−/−) mice were compared with those of their wild-type (Mthfr+/+) and heterozygous (Mthfr+/−) littermates. A few mice who died before P2 were excluded from weight and mortality calculations. This mortality, due to non-specific causes, was gender- and genotype-independent.
A total of 15 Mthfr+/+ and Mthfr+/− mice of betaine-supplemented mothers were supplemented with 2% betaine in the drinking water from weaning until 7–9 weeks of age for measurements of betaine and DMG (dimethylglycine) in plasma and tissues. Results were compared with those from 20 untreated mice of the same age whose mothers had not been supplemented with betaine.
At different prenatal [E18 (embryonic day 18)] and postnatal (P6, P9, P12 and adult) time points, mice were killed. The blood of older mice was collected by heart puncture, anticoagulated with EDTA (MicrovetteR 500; Sarstedt) and immediately placed on ice. Plasma was separated by centrifugation at 10000 g for 5 min and frozen immediately at −70 °C until analysis. Livers and brains were dissected and weighed. They were then frozen on solid CO2 and stored in aliquots at −70 °C, or fixed by immersion in 10% (v/v) formalin and processed for 5 μm paraffin sections and haematoxylin/eosin staining.
Genotyping
Mice were genotyped for the Mthfr disruption by PCR of genomic DNA extracted from tail biopsies, as described previously [9].
Metabolites
Plasma total homocysteine concentrations were measured after reduction by a HPLC method, as described previously [11]. Betaine and DMG concentrations in plasma and tissue extracts were analysed by HPLC as reported in [12]. Betaine and DMG were extracted from tissues by adding 1 ml of 100 mM KH2PO4 (pH 4.5) to 100–150 mg of freeze-dried tissues in 2 ml Eppendorf tubes. After addition of an equal volume of clean glass beads, the tubes were mixed vigourously to homogenize the contents. The mixture was incubated for 10 min with intermittent mixing and vortexing. At the end of the incubation time, the tubes were centrifuged (5000 g, 10 min) and the supernatant was collected. The extraction procedure was repeated twice and the combined supernatants were frozen until analyses.
Tissue aminothiols were measured as reported [13], and tissue SAM and SAH (S-adenosylhomocysteine) concentrations were measured according to the method described by Melnyk et al. [14]. Global DNA methylation was assessed by a cytosine-extension assay as described by Pogribny et al. [15]. Adenosine in tissues was measured according to Nithipatikom et al. [16] after adaptation for electrochemical detection.
BHMT activity
BHMT activity in crude liver extracts was analysed as reported previously [17].
Statistical analyses
Standard software was used for all analyses. Arithmetric means±S.E.M. are provided. Genotype and gender distributions were compared using the χ2 test. Weights or metabolite levels were compared between groups using one-way ANOVA and, if a significant difference was found, single parameters were compared with the unpaired, two-sided t test. If values were not normally distributed, the Kruskal–Wallis test and the non-parametric Wilcoxon test were used as appropriate. Linear correlations between two parameters were calculated and expressed using Spearman's coefficient. Throughout all analyses, a P value of <0.05 was considered significant.
RESULTS
Betaine supplementation increases the survival rate of Mthfr−/− mice
A total of 314 mice in 50 litters (mean litter size 6.28±0.31) were born after mating of untreated Mthfr+/− mice. Mthfr genotype frequencies were 25.1% Mthfr+/+, 48.0% Mthfr+/− and 26.9% Mthfr−/−. This genotype distribution was not significantly different from expected values using the χ2 test. Gender proportions showed a small but significant preponderance of female (56.4%) compared with male (43.6%) mice for the Mthfr+/+ and Mthfr+/− genotypes. The genders of the 45 Mthfr−/− mice were evenly distributed (23 female, 22 male; with five surviving females and six surviving males). One-third of the Mthfr−/− mice could not be classified by gender because of early death and/or incomplete recovery of their bodies. Of the homozygous mutants, 83% died, at an average age of 9.1±0.7 days (median 7.0 days; n=53). Only 11 out of 64 (17%) Mthfr−/− mice survived (Figure 1).
Figure 1. Survival of mice homozygous for a disruption of the Mthfr gene (−/−).
Data are derived from 64 Mthfr−/− mice without (▪) and 62 Mthfr−/− mice with (○) a betaine supplement to their mothers.
Betaine supplementation in the drinking water was tolerated without apparent side effects. A mean daily betaine intake in water of approx. 3 g/kg body weight was observed during mating and pregnancy, which increased to a variable extent during lactation. Matings of betaine-treated heterozygotes yielded a total of 249 mice in 38 litters. The litter size (mean 6.38±0.44) was not different from that of untreated matings. Genotype frequencies were 27.3% Mthfr+/+, 44.9% Mthfr+/− and 27.8% Mthfr−/−. Gender (51.1% female, 48.9% male) and genotype distributions were not significantly different from expected values. The mortality of homozygous mutant pups from betaine-treated mothers decreased significantly compared with that of pups from untreated mothers, to 26% (average day of death 16.3±3.4; median 11.0 days; n=16). Of 62 Mthfr−/− mice, 46 (74%) survived. Withdrawal of betaine supplementation after weaning of homozygous mutant pups, at the age of 3 weeks, had no adverse effects on survival (Figure 1).
Betaine supplementation improves the somatic development of Mthfr−/− mice
Body weight
Body weights were followed for 480 pups, 253 from untreated dams and 227 from betaine-supplemented dams. These included 55 untreated and 62 betaine-supplemented Mthfr−/− mice (Figure 2).
Figure 2. Postnatal weight development of mice wild-type (+/+), heterozygous (+/−) or homozygous (−/−) for a disruption of the Mthfr gene and stratified for treatment with (B) or without (C) a betaine supplement.
Body weights are depicted as arithmetric means. The numbers of Mthfr−/− pups evaluated were 62 from mothers with a betaine supplement and 55 from mothers without a supplement. Pups from the latter group of untreated mothers were separated into two groups: survivors (n=12; Δ) and non-survivors (n=43; ○). The number of the latter group decreased with age due to their high postnatal mortality.
The weight development of Mthfr+/− and Mthfr+/+ pups of untreated mothers was not different. The mean body weight of untreated Mthfr−/− mice at P0 was 87.9% of that of heterozygous and wild-type littermates, corresponding to 1.00±0.34 S.D.s. Starting from P1, the body weights of untreated Mthfr−/− mice were significantly decreased. The trough of the weight development curve occurred at P9–P13, together with a peak of mortality at this age.
Starting from P5, the body weights of untreated non-surviving Mthfr−/− mice were significantly lower than those of surviving nullizygotes. Surviving nullizygotes regained weight until weaning. Their relative weight decreased during adolescence, then recovered, with the adult weight being 0.97±1.12 S.D.s below that of their heterozygous and wild-type littermates.
In the betaine-supplemented group, the weight development of Mthfr+/− and Mthfr+/+ offspring was not different. Fur appearance, which is usually delayed by about 5 days in Mthfr−/− mice [8], was delayed by only approx. 2 days in Mthfr−/− mice from betaine-supplemented mothers. The body weights of Mthfr−/− pups at P0 were 91.4±3.1% (−1.02±0.34 S.D.s) of those of age- and sex-matched Mthfr+/− and Mthfr+/+ littermates. From P1, their weights were significantly decreased compared with those of their heterozygous and wild-type littermates. Weight development showed decelerations during the first 2 weeks of life and during the first 2 weeks after weaning. The adult weight of Mthfr−/− mice was 1.18±0.23 S.D.s below that of their heterozygous and wild-type littermates.
The body weight development of Mthfr+/− and Mthfr+/+ offspring was not distinguishable between litters of untreated and betaine-supplemented mothers. The mean body weights of Mthfr−/− mice were significantly higher when their mothers had been treated, compared with those from untreated mothers, starting from P1. Although the weights of untreated Mthfr−/− mice remained lower than those of betaine-treated mice throughout somatic development, statistical significance was not achieved after P16, mostly due to high inter-individual variation. Adult body weights did not differ between treatment groups.
Brain weight and morphology
Brain weights were evaluated in a subgroup of 6-day-old pups (Figure 3). At this age, Mthfr+/− mice in the untreated group had slightly but significantly lower body and brain weights than Mthfr+/+ pups. Untreated Mthfr−/− pups with severely decreased body weights also showed a severe reduction in brain weight, with a significant increase in the brain/body weight ratio compared with Mthfr+/+ mice (0.076±0.008 and 0.054±0.003 respectively, n=10, P<0.01); this observation indicates that, at P6, somatic dystrophy was more pronounced than the disturbance in brain development.
Figure 3. Influence of a betaine supplement on body weights and brain weights at P6 of wild-type (+/+) mice and their littermates with a heterozygous (+/−) or homozygous (−/−) disruption of the Mthfr gene.
Data are means and S.E.M. of eight (body weight) or five (brain weight) mice per genotype and treatment group. C, pups of control untreated mothers; B, pups of betaine-supplemented mothers.
The brain and body weights of Mthfr+/+ and Mthfr+/− pups from betaine-supplemented dams were not different from those of the corresponding untreated pups. However, Mthfr−/− pups with the betaine supplement had significantly higher body and brain weights than untreated Mthfr−/− pups, resulting in a significantly lower brain/body weight ratio (0.057±0.001), a value that was comparable with that of wild-type mice. However, the body and brain weights of treated Mthfr−/− animals each still remained significantly below those of wild-type mice. When a group of 18 adult mice (three females and three males per genotype) from litters rescued by betaine supplemention of their mothers were evaluated at age P200, we found that the brain weights of the Mthfr−/− mice (395±13 mg, n=6) were still significantly lower than those of heterozygous (477±8 mg, n=6) or wild-type (487±2 mg, n=6) littermates, whereas the body weights of these 18 mice were not significantly different.
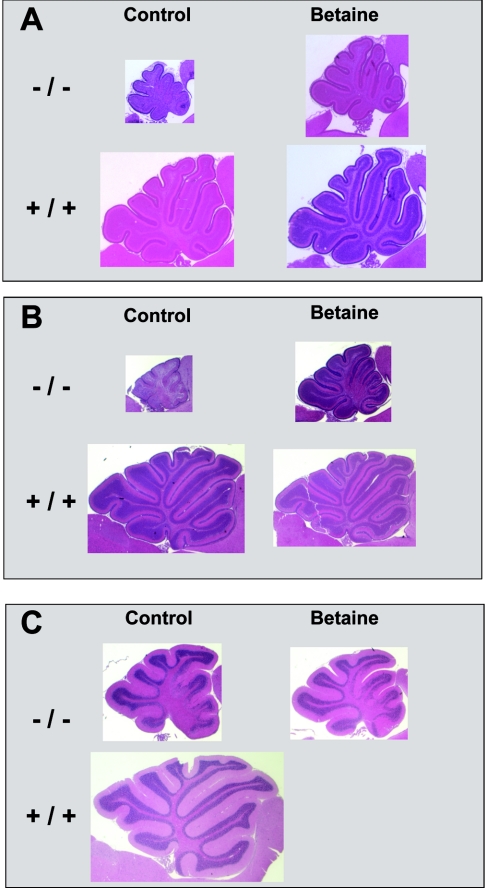
Analysis of the gross anatomy of the brains of Mthfr−/− mice revealed proportionately smaller cerebella (Figure 4). Pups from betaine-supplemented dams had a much larger cerebellar size compared with untreated mice. Histological examination showed that cerebellar development was disturbed in Mthfr−/− mice. Foliation and differentiation of the cerebellar cortex in Mthfr−/− mice at P9 and P12 appeared immature compared with Mthfr+/+ mice (Figures 4A and 4B). The internal granule cell layer was not well developed and a single Purkinje cell layer was not clearly distinguishable. These defects were observed in both anterior and posterior lobules, but were more pronounced in anterior segments. In adult Mthfr−/− mice, the defects were restricted to the anterior region of the cerebellum (Figure 4C), as observed in our previous report [9].
Figure 4. Impact of betaine supplementation to mothers on cerebellar development of their Mthfr−/− pups.
Depicted are parasagittal cerebellar sections of mice at age P9 (A), P12 (B) and adult (C). A haematoxylin/eosin stain was used, and size proportions are conserved for each age group. The slides are oriented with rostral to the right.
We also identified structural abnormalities in the hippocampus of Mthfr−/− mice (Figure 5). In addition to hypotrophy of the hippocampus with dilated ventricular spaces, the neuroepithelial layers of the dentate gyrus were only loosely assembled in comparison with wild-type or heterozygous animals. Both cerebellar and hippocampal histological abnormalities were ameliorated, but not abolished, by the betaine supplement. Maternal betaine supplementation improved the foliation defect in the anterior lobules of Mthfr−/− cerebella at P9 and P12, and led to a reduced number of affected lobules in the adult Mthfr−/− cerebellum (Figure 4). Cerebellar changes persisted until adulthood (Figure 4C); the hippocampus was not examined in adult animals.
Figure 5. Impact of betaine supplementation to mothers on hippocampal development of their Mthfr−/− pups.
Depicted are left coronar forebrain sections from mice at age P9. A haematoxylin/eosin stain was used; original magnification ×4.
Liver morphology
Mthfr−/− mice are prone to develop fatty infiltration of the liver, especially when the choline supply is limited [9]. The livers of nine untreated Mthfr−/− mice at age P6–P9 were evaluated by histology; six of the livers showed severe fatty infiltration, while three livers did not. The livers of eight Mthfr−/− pups from betaine-supplemented mothers were evaluated at age P6–P12; five of the livers showed fatty infiltration (results not shown). There was no correlation between the degree of general dystrophy of the mice, measured as body weight relative to that of Mthfr+/− or Mthfr+/+ littermates, and the extent of fatty infiltration.
Biochemical consequences of betaine supplementation
Betaine metabolism in adult mice
The effects of a 2% (w/v) betaine supplement on betaine metabolism in adult Mthfr+/+ and +/− mice are shown in Table 1. Compared with Mthfr+/+ mice, untreated Mthfr+/− mice had lower betaine concentrations in plasma, liver and brain. Betaine concentrations in plasma and tissues were increased with a 2% betaine supplement in both groups of mice, the only exception being the brains of Mthfr+/+ mice.
Table 1. Concentrations of betaine and DMG in plasma (μmol/l) and tissues (nmol/g wet weight), and specific activity of the betaine-metabolizing enzyme BHMT, in adult wild-type mice and heterozygous Mthfr mice with and without a betaine supplement of 2% (w/v) in their drinking water from birth.
The dietary groups of the two genotypes comprised littermates of equally mixed genders. Results are provided as means±S.E.M.; *P<0.05 for difference between dietary groups of same genotype; †P<0.05 compared with wild-type genotype in the same dietary group.
| Wild type | Heterozygous | ||||
|---|---|---|---|---|---|
| Tissue | Control (n=10–14) | Betaine (n=6–8) | Control (n=6–8) | Betaine (n=7) | |
| Betaine | Plasma | 101.7±15.1 | 556.9±190.6* | 56.4±4.2† | 229.7±93.8* |
| Liver | 2139±257 | 9420±3275* | 727±43† | 3993±2227 | |
| Brain | 61±10 | 56±19 | 22±4† | 134±30*† | |
| Kidney | 1853±103 | 4716±679* | 1645±213 | 3274±647*† | |
| DMG | Plasma | 13.5±2.0 | 60.7±13.4* | 8.2±0.9† | 38.3±12.7* |
| Liver | 88±13 | 250±67* | 65±15 | 160±62 | |
| Brain | 15±5 | 2±1* | 8±3 | 3±1 | |
| Kidney | 176±81 | 81±29 | 23±10 | 71±30 | |
| BHMT (units/mg of protein) | Liver | 149±15 | 244±29* | 184±23 | 287±26* |
The concentrations of the demethylated metabolite of betaine, DMG, were variable in different organs and did not show consistent changes by genotype. Betaine supplementation increased DMG levels in plasma and liver, and decreased them in brain. The specific activity of the liver betaine-metabolizing enzyme, BHMT, increased significantly with betaine supplementation in both genotype groups.
Betaine metabolism in lactating dams and suckling pups
Plasma betaine concentrations in betaine-supplemented lactating dams ranged from 190 to 1835 μmol/l (n=3), compared with 34–124 μmol/l (n=3) in untreated dams. The corresponding plasma DMG concentrations in dams on betaine supplementation ranged from 46 to 124 μmol/l, compared with 5 to 13 μmol/l in untreated dams.
At age P12, the plasma betaine concentrations of suckling Mthfr+/− and Mthfr+/+ pups from betaine-supplemented dams were slightly but significantly higher (110.5±18.9 μmol/l, n=10, 5 female, 5 male) than those of corresponding untreated pups (73.8±4.9 μmol/l, n=16, 8 female, 8 male). Plasma DMG was almost two times and significantly higher in pups from betaine-supplemented dams (29.2±2.7 μmol/l) than in those from untreated dams (17.7±0.9 μmol/l). In this group of mice, we found no differences in concentrations of betaine or DMG between genotypes or genders (results not shown).
The specific activity of liver BHMT at age P12 was 259±10 units/mg of protein in pups from untreated dams (n=16) and 260±10 units/mg in pups from betaine-supplemented dams (n=10). No differences between genders were observed. At 3 days before birth (age E18), BHMT activity in fetal liver was 73±13 units/mg of protein (n=6).
These experiments indicate that suckling pups received adequate amounts of betaine from their mothers and were able to metabolize substantial amounts to DMG.
Liver and brain thiols and DNA methylation in pups at age P6
The impact of betaine supplementation on biochemical parameters in liver (Table 2) and brain (Table 3) was examined in littermates of the three genotypes at age P6. Several genotype effects were seen in untreated groups. These included a significant increase in total homocysteine concentration in heterozygotes (1.4-fold in liver) and nullizygotes (5-fold in liver and 10-fold in brain), and moderately decreased methionine concentrations in the livers of heterozygotes and nullizygotes and in the brains of nullizygotes. SAH was increased in livers of heterozygotes and nullizygotes and in brains of nullizygotes.
Table 2. Liver concentrations of total thiols and of SAH, SAM and adenosine in MTHFR-deficient pups at P6 stratified for genotype and maternal diet.
Controls received standard rodent chow, whereas the betaine group received an additional betaine supplement of 2% (w/v) in the drinking water. The dietary groups of the three genotypes comprised littermates of mixed genders. Results are means±S.E.M. for eight individuals; *P<0.05 for difference between dietary groups of same genotype; †P<0.05 compared with wild-type genotype in the same dietary group; symbols in parentheses indicate borderline significance (P=0.06–0.09).
| Wild type | Heterozygous | Nullizygous | ||||
|---|---|---|---|---|---|---|
| Metabolite | Control | Betaine | Control | Betaine | Control | Betaine |
| Homocysteine (nmol/mg) | 0.406±0.030 | 0.387±0.022 | 0.559±0.024† | 0.512±0.031† | 1.907±0.112† | 1.255±0.076*† |
| Methionine (nmol/mg) | 24.0±1.4 | 25.0±0.9 | 16.4±0.9† | 20.8±0.8*† | 12.4±0.7† | 15.9±0.7*† |
| SAH (pmol/mg) | 89±4 | 121±4* | 124±12† | 101±3(*)† | 290±28† | 144±13* |
| SAM (pmol/mg) | 467±38 | 551±24(*) | 385±27(†) | 473±28*(†) | 391±39 | 440±28† |
| Cysteine (nmol/mg) | 45.8±1.3 | 43.9±1.0 | 49.5±1.7 | 52.4±1.4† | 66.5±2.2† | 64.4±1.5† |
| Cystathionine (nmol/mg) | 1.40±0.10 | 1.23±0.06 | 2.38±0.09† | 2.10±0.13† | 4.57±0.25† | 2.77±0.27*† |
| Glutathione (nmol/mg) | 54.8±1.5 | 52.8±1.2 | 49.4±1.5† | 49.4±1.9 | 47.4±1.7† | 48.9±1.0† |
| Cysteinylglycine (nmol/mg) | 31.6±1.3 | 33.0±1.0 | 32.5±0.9 | 34.0±1.5 | 26.6±1.9† | 25.1±1.3† |
| Adenosine (pmol/mg) | 487±29 | 344±12* | 615±46(†) | 723±25(*)† | 1308±136† | 820±57*† |
Table 3. Brain concentrations of total thiols and of SAH, SAM and adenosine in MTHFR-deficient pups at P6 stratified for genotype and maternal diet.
Controls received standard rodent chow, whereas the betaine group received an additional betaine supplement of 2% (w/v) in the drinking water. The dietary groups of the three genotypes comprised littermates of mixed genders. Results are means±S.E.M. for five individuals (only four for wild-type controls); *P<0.05 for difference between dietary groups of same genotype; †P<0.05 compared with wild-type genotype of same dietary group; symbols in parentheses indicate borderline significance (P=0.06–0.09).
| Wild type | Heterozygous | Nullizygous | ||||
|---|---|---|---|---|---|---|
| Metabolite | Control | Betaine | Control | Betaine | Control | Betaine |
| Homocysteine (nmol/mg) | 0.265±0.025 | 0.271±0.028 | 0.293±0.017 | 0.261±0.021 | 2.662±0.395† | 1.991±0.347*† |
| Methionine (nmol/mg) | 30.3±1.0 | 31.8±2.4 | 28.8±1.4 | 26.2±1.2 (†) | 25.2±1.7† | 31.7±3.8 |
| SAH (pmol/mg) | 176±35 | 317±22* | 229±19 | 181±12(*)† | 357±55† | 277±27* |
| SAM (pmol/mg) | 579±31 | 624±78 | 691±51 | 543±21* | 474±27† | 583±63† |
| Cysteine (nmol/mg) | 4.9±0.2 | 4.8±0.4 | 6.0±1.2 | 4.4±0.3 | 9.6±1.0† | 5.7±0.3* |
| Cystathionine (nmol/mg) | 4.2±0.1 | 4.6±0.1* | 3.1±0.2† | 3.0±0.1† | 10.9±1.3† | 4.4±0.2* |
| Glutathione (nmol/mg) | 22.3±1.1 | 24.1±1.3 | 20.6±1.1 | 21.8±1.3 | 23.4±1.2 | 23.1±1.0 |
| Cysteinylglycine (nmol/mg) | 5.7±0.1 | 7.3±0.2* | 2.6±0.2† | 4.4±0.2*† | 2.8±0.3† | 2.6±0.2† |
| Adenosine (pmol/mg) | 557±126 | 555±124 | 448±28 | 447±53 | 455±30 | 572±97 |
Examination of homocysteine trans-sulphuration metabolites showed increased cysteine and cystathionine levels in livers and brains of nullizygotes. Cystathionine was also increased in livers, but not in brains, of heterozygous mice. Glutathione levels were lower in livers of heterozygotes and nullizygotes, whereas brain did not show any differences. Cysteinylglycine was decreased in livers of nullizygotes and in brains of heterozygotes and nullizygotes. Adenosine was increased in livers of heterozygotes and nullizygotes, but not in brain.
With betaine supplementation to lactating dams, homocysteine concentrations were decreased in Mthfr−/− pups in liver and brain compared with untreated mice. Methionine increased significantly in heterozygotes and nullizygotes in liver, but not in brain. SAH increased in livers and brains of wild-type mice, whereas it showed a decrease in livers of heterozygotes and nullizygotes, and in brains of nullizygotes.
The trans-sulphuration metabolites, cysteine and cystathionine, decreased in brains of nullizygotes from betaine-treated dams. In liver, only cystathionine decreased with betaine supplementation in nullizygotes, whereas cysteine remained unchanged. Cysteinylglycine increased in brains of wild-type and heterozygous mice, but not in nullizygotes. Adenosine levels decreased with a betaine supplement in livers of wild-type and nullizygous mice.
In untreated pups, we noticed a trend towards increased hypomethylation of DNA with impaired MTHFR function. Due to high inter-individual variation, there were no significant differences betweeen genotypes in liver or brain. Betaine supplementation did not change the extent of DNA methylation in liver. However, in brain, when the genotype groups were combined, there was a small but significant decrease in hypomethylation in the betaine-supplemented group (4352±131 d.p.m./0.5 μg of DNA for 14 untreated mice compared with 3840±119 d.p.m./0.5 μg of DNA for 15 betaine-supplemented pups; P<0.01).
DISCUSSION
Betaine supplementation improves the survival and somatic development of MTHFR deficient mice
Severe MTHFR deficiency due to homozygous disruption of the mouse Mthfr gene leads to a distinct pathology that was described in the original study of these mice when they were on a mixed genetic background [9]. In contrast with the lower mortality reported in that study (survival of 76% of nullizygotes), the majority of Mthfr−/− mice in the present study, after multiple rounds of backcrosses to the BALB/cAnNCrlBR strain, died during the first two postnatal weeks. The mixed genetic background of the initial colony presumably alleviated some of the deleterious effects of severe MTHFR deficiency in the homogeneous BALB/c background.
The expected genotype distribution of offspring from Mthfr+/− matings was observed in the present study, as well as in the study of Chen et al. [9], indicating an absence of fetal losses. However, the lower birth weights of the Mthfr−/− pups point to some degree of intrauterine onset of pathology. Nonetheless, we were not able to distinguish surviving from non-surviving nullizygotes during the first few days of extrauterine life. The most reliable indicator of imminent death was poor weight gain over a few days and eventually weight loss, due to insufficient food intake as indicated by empty stomachs. Another typical feature of nullizygotes was pronounced deceleration of weight gain after weaning.
In another dietary animal model, severe folate deficiency during pregnancy and lactation led to increased fetal resorption, small litter size, low birth weight, postnatal death and depressed food consumption and weight gain, with significantly lower body and brain weights at weaning [18]. Some of these symptoms resemble those of the nullizygous pups of our present study, although we did not observe any intrauterine mortality.
The major pathology in Mthfr deficiency appears to occur after birth, suggesting that maternal folate pools may protect mutant pups in utero. Standard rodent chow contains very high levels of folate and preformed methyl groups, which are transferred from maternal to fetal tissues. However, the early postnatal period, with its increased need for methyl groups to maintain rapid cellular proliferation and differentiation, presents a particular challenge for Mthfr-deficient pups. Primary pathogenic factors in MTHFR deficiency may be toxicity of elevated homocysteine or its derivatives, impaired methylation or a disruption in the balance of folate species. Since betaine has not been reported to correct imbalances in folate derivatives, and betaine supplementation clearly improved survival and growth in Mthfr-deficient pups, the primary pathogenic factors presumably relate to the accumulation of homocysteine or its metabolite, SAH, and the consequent inhibition of transmethylation reactions.
Betaine supplementation of dams had a dramatic effect on the survival and growth of their nullizygous pups. With this simple nutritional intervention, most of the nullizygotes could be rescued from death. Nullizygotes of treated mothers had significantly higher body weights compared with untreated nullizygotes during the first two postnatal weeks, but not at birth. The lack of adverse effects of withdrawal of betaine 3 weeks after birth also demonstrates the importance of an adequate supply of transferable methyl groups during the critical first 2 weeks of postnatal life. The primary reason for the high mortality of untreated Mthfr nullizygotes may therefore be an insufficient intake of preformed methyl groups, leading to developmental disturbances and, beyond a point of no return, to starvation and death.
Betaine supplementation to lactating dams increases betaine availability in their pups and ameliorates biochemical anomalies in nullizygotes
Betaine-supplemented dams and their suckling offspring had elevated plasma betaine levels, demonstrating increased betaine availability in pups from treated lactating mothers. These mothers are heterozygotes for the Mthfr gene disruption, a condition that is associated with elevated plasma homocysteine [9,10], decreased 5-methyltetrahydrofolate [9] and decreased liver choline and betaine [10]. These anomalies are likely to be aggravated by pregnancy and lactation, conditions that are associated with choline and betaine depletion in rats [19].
In a previous study on the effects of 2 weeks of betaine supplementation to adult Mthfr-deficient mice [10], we observed some disturbances in choline metabolism in untreated animals. We suggested that the fatty infiltration of livers in adult nullizygous mice might be due to choline deficiency. In the present study, most of the livers of nullizygotes at age P6 also had clear fatty infiltration. Neonatal rats have a 2-fold higher intake of choline than weanling animals. Rat and human milk do not contain measurable amounts of betaine [20,21]. However, since choline can be oxidized to betaine, choline can replace betaine as a source of transferable methyl groups. Consequently, betaine supplementation could increase choline availability in the livers of nullizygous mice either by a choline-sparing effect or by provision of methyl groups for the methylation of phosphatidylethanolamine to phosphatidylcholine, a precursor of choline. However, betaine supplementation did not decrease the extent of fatty infiltration of the liver in mutant pups in the present study. This could be a dose-dependent effect, particularly if the preformed methyl groups are utilized preferentially in maintaining brain methylation reactions at the expense of the liver in early postnatal life. On the other hand, since SAH levels were still elevated compared with control levels despite a betaine-lowering effect, phosphatidylethanolamine methyltransferase might have been inhibited.
When we investigated betaine metabolism and the consequences of high-dose betaine supplementation in adult Mthfr+/+ and +/− mice in the present study, we identified betaine depletion in the plasma, liver and brain of Mthfr+/− animals. Depletion of betaine in liver is attributable to the decreased capacity of Mthfr+/− mice to meet the demands for folate-dependent homocysteine remethylation, which leads to lower SAM concentrations. The compensatory increase in betaine catabolism by BHMT to restore homocysteine remethylation results in a decrease in betaine levels in liver. Betaine concentrations in other tissues are dependent on betaine export by hepatocytes and are probably reduced because betaine availability is decreased through lowered plasma concentrations. Betaine supplementation leads to greatly enhanced betaine storage and further induction of betaine metabolism, as indicated by increased concentrations of its metabolite DMG and increased BHMT activity (Table 1). These findings are in agreement with our previous study [10].
BHMT activity in fetal rat liver is only approx. 25% of that found in adult liver, whereas the activity in the neonate is equal to or greater than that in adult rats [22]. In agreement with these earlier findings, fetal mice in our study had low BHMT activity a few days before birth, with a 3-fold increase at P12, up to adult levels. The dramatic effect of betaine on the survival of Mthfr−/− mice provides strong evidence for an important role for BHMT activity during the first days of postnatal life in mice.
SAH is the product of transmethylation reactions, which occur primarily in liver [23]. When homocysteine is elevated, the SAH hydrolase reaction (EC 3.3.1.1), which usually catalyses the breakdown of SAH to homocysteine and adenosine, can operate in the reverse direction, to generate SAH [2]. Betaine supplementation caused marked decreases in both homocysteine and SAH, and an increase in methionine, in heterozygotes and nullizygotes. In contrast, the administration of betaine to wild-type mice caused an increase in SAM and SAH levels, an observation that was also reported in another rodent study [24] and that could be interpreted being due to increased turnover of homocysteine by enhanced remethylation.
Adenosine was increased in the livers in heterozygotes and nullizygotes. This increase was diminished by betaine supplementation. Adenosine levels appeared to correlate strongly with the concentrations of homocysteine and SAH in liver; this finding may relate to the fact that SAH generates homocysteine and adenosine, and that adenosine elimination by adenosine deaminase may not have been enhanced.
Homocysteine can also be eliminated by trans-sulphuration, in which homocysteine condenses with serine to form cystathionine and subsequently cysteine. In the livers of nullizygous P6 pups, the most striking anomalies were elevations of homocysteine and SAH. In addition, cystathionine was markedly increased, whereas cysteine was only moderately elevated. This demonstrates the failure of co-ordinate regulation of homocysteine metabolism and, in particular, the inability of the two remaining pathways, betaine-dependent remethylation and homocysteine trans-sulphuration, to cope with a disruption of folate-dependent remethylation.
Homocysteine metabolism in the developing mouse brain and the impact of betaine supplementation
Studies in different species have shown that the prenatal brain utilizes homocysteine preferentially for folate-dependent remethylation and not for trans-sulphuration. Remethylation enzymes have higher activities during fetal life than postnatally [22,25,26]. During the first 2 weeks of postnatal life, there is an exponential decrease of folate in the mouse brain, with lower levels of polyglutamylated folates, indicating high turnover and demand for folates in early extrauterine life [27]. In contrast with the postnatal decreases in activity of the remethylation enzymes, the rate-limiting enzyme of the trans-sulphuration pathway, cystathionine β-synthase, shows decreased activity in the cerebrum during late fetal stages, increases after birth and peaks during cerebellar development [28]. The activity of the last enzyme of the trans-sulphuration pathway from methionine to cysteine, cystathionase, increases gradually in rat brain over 4 weeks after birth [29]. As a consequence of these temporal regulatory mechanisms, trans-sulphuration in fetal brain is limited.
In contrast with the liver, the brain does not use betaine as a methyl donor. There are low concentrations of betaine in brain [30], and BHMT is not expressed in this tissue [31]. When folatedependent remethylation is disturbed, the brain becomes dependent on preformed methyl groups delivered as SAM. Furthermore, the only means of homocysteine elimination is trans-sulphuration, a pathway that is limited in brain, as discussed above. Consequently, we found more severe biochemical disruption in the brain than in the liver of 6-day-old pups from untreated dams. Most prominent were dramatic increases of homocysteine and clear elevations of cystathionine and cysteine; these findings demonstrate the activation of the trans-sulphuration pathway, but also its inability to cope with the increased homocysteine load.
Brain GSH was unchanged in nullizygotes, despite the elevation of its precursor cysteine, whereas cysteinylglycine was decreased in heterozygotes and nullizygotes. Cysteinylglycine is a product of extracellular GSH degradation, but also provides cysteine and glycine as precursor molecules for GSH synthesis in neurons [32]. Cysteinylglycine in brain was inversely correlated with homocysteine and cysteine concentrations, which could indicate a preference for cysteinylglycine over cysteine for GSH synthesis.
With betaine supplementation, biochemical parameters in brain indicated increased remethylation of homocysteine to methionine and markedly decreased flux through the trans-sulphuration pathway. As a consequence, global DNA hypomethylation was decreased. Decreased homocysteine and SAH levels in mutant pups may have contributed to the phenomenon; DNA methylation status in another model of hyperhomocysteinaemia, the cystathionine β-synthase-deficient mouse, was largely determined by SAH concentrations [33].
The cerebella of Mthfr−/− mice are abnormally small and cerebellar development is disrupted, as first shown by Chen et al. [9]. We also identified some hypotrophy in the hippocampus, with abnormal formation of the neuroepithelium in the dentate gyrus. Betaine supplementation had a beneficial effect on the pups in both areas of the brain, but could not prevent residual structural abnormalities in the cerebellum in adult nullizygotes. Of note is the fact that a compartmentation defect (i.e. a difference in the degree of disturbance between the anterior and posterior cerebellar regions) was still evident.
The cerebellum is one of the late developing brain regions. Shortly after birth until the second week of life, the cerebellar granule neuroblasts proliferate extensively in the external granular layer of the cerebellar cortex, and undifferentiated Purkinje cells migrate to form a single cell layer underneath the external granular layer [34]. This postnatal proliferative and migratory period might be particularly vulnerable to the disruption in folate-dependent remethylation and supply of methyl groups. Other early postnatal insults to rodent brain also result in more severe cerebellar damage than later exposure and produce a pathological pattern that is similar to that in Mthfr−/− mice. Irradiation and methylazoxymethanol have been shown to be especially harmful before P8 and P4 respectively. Both cause foliation abnormalities, misalignment of Purkinje cells and continued multiple innervation of climbing fibres [35].
Intrauterine choline deficiency has been shown to result in structural defects in the hippocampus of rats [36] and mice [37–39]. Choline availability altered the timing, genesis, migration and differentiation of progenitor neuronal-type cells in the fetal hippocampus. Since we have shown previously that choline metabolites are decreased in brains of adult Mthfr−/− mice, and that this decrease can be ameliorated by betaine supplementation [10], it is likely that choline or betaine depletion may have played a role in the structural brain defects in the Mthfr−/− pups.
Conclusions
In summary, our findings demonstrate the importance of epigenetic factors, such as the intake of preformed methyl groups, for a favourable clinical outcome in severe MTHFR deficiency in mice. Very few humans with severe MTHFR deficiency have been identified prenatally; these were siblings of previously identified index patients. One such published case is remarkable in demonstrating that immediate postnatal betaine treatment may prevent symptoms until adulthood ([40]; F. Skovby, personal communication). Our results in mice emphasize the importance of early (prenatal) betaine supplementation in homocystinuria and suggest that newborn screening for hyperhomocysteinaemia would allow for the immediate therapeutic use of betaine to improve the clinical course.
Acknowledgments
This work was supported by grants from the Canadian Institutes for Health Research (CIHR) to R.R., and in part by a grant to B.C.S. from the Deutsche Forschungsgemeinschaft (SFB 575). R.R. is a recipient of a Senior Scientist Award from the CIHR.
References
- 1.Zeisel S. H., Mar M.-H., Howe J. C., Holden J. M. Content of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein J. D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt D. S. Inherited disorders of folate transport and metabolism. In: Scriver C. R., Beaudet A. L., Sly W., Valle D., editors. Metabolic and Molecular Bases of Inherited Disease. 7th edn. New York: McGraw-Hill; 1995. pp. 3111–3128. [Google Scholar]
- 4.Frosst P., Blom H. J., Milos R., Goyette P., Sheppard C. A., Matthews R. G., Boers G. J., den Heijer M., Kluijtmans L. A., van den Heuvel L. P., et al. Identification of a candidate genetic risk factor for vascular disease: a common mutation in methylentetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 5.Schwahn B., Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene. Clinical consequences. Am. J. Pharmacogenomics. 2001;1:189–201. doi: 10.2165/00129785-200101030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Erbe R. W. Inborn errors of folate metabolism. In: Blakeley R. L., Whitehead V. M., editors. Folates and Pterines, vol. 3: Nutritional, Pharmacological and Physiological Aspects. New York: Wiley; 1986. pp. 413–465. [Google Scholar]
- 7.Wendel U., Bremer H. J. Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase. Eur. J. Pediatr. 1984;142:147–150. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- 8.Holme E., Kjellman B., Ronge E. Betaine for the treatment of homocystinuria caused by methylenetetrahydrofolate reductase. Arch. Dis. Child. 1989;64:1061–1064. doi: 10.1136/adc.64.7.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z., Karaplis A. C., Ackerman S. L., Pogribny I. P., Melnyk S., Lussier-Cacan S., Chen M. F., Pai A., John S. W. M., Smith R. S., et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 10.Schwahn B. C., Chen Z., Laryea M. D., Wendel U., Lussier-Cacan S., Genest J., Jr, Mar M.-H., Zeisel S. H., Castro C., Garrow T., Rozen R. Homocysteine–betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17:512–514. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- 11.Durand P., Fortin L. J., Lussier-Cacan S., Davignon J., Blache D. Hyperhomocysteinemia induced by folic acid deficiency and methionine-load – applications of a modified HPLC method. Clin. Chim. Acta. 1996;252:83–93. doi: 10.1016/0009-8981(96)06325-5. [DOI] [PubMed] [Google Scholar]
- 12.Laryea M. D., Steinhagen F., Pawliczek S., Wendel U. Simple method for the routine determination of betaine and N,N-dimethylglycine in blood and urine. Clin. Chem. 1998;44:1937–1941. [PubMed] [Google Scholar]
- 13.Melnyk S., Pogribna M., Pogribny I., Hine R. J., James S. J. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J. Nutr. Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 14.Melnyk S., Pogribna M., Pogribny I. P., Yi P., James S. J. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin. Chem. 2000;46:265–272. [PubMed] [Google Scholar]
- 15.Pogribny I., Yi P., James S. J. A sensitive new method for rapid detection of abnormal methylation patterns in global DNA and within CpG islands. Biochem. Biophys. Res. Commun. 1999;262:624–628. doi: 10.1006/bbrc.1999.1187. [DOI] [PubMed] [Google Scholar]
- 16.Nithipatikom K., Mizumura T., Gross G. J. Determination of plasma adenosine by high-performance liquid chromatography with column-switching and fluorometric detection. Anal. Biochem. 1995;223:280–284. doi: 10.1006/abio.1994.1585. [DOI] [PubMed] [Google Scholar]
- 17.Garrow T. A. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J. Biol. Chem. 1996;271:22831–22838. doi: 10.1074/jbc.271.37.22831. [DOI] [PubMed] [Google Scholar]
- 18.Tagbo I. F., Hill D. C. Effect of folic acid deficiency on pregnant rats and their offspring. Can. J. Physiol. Pharmacol. 1976;55:427–433. doi: 10.1139/y77-060. [DOI] [PubMed] [Google Scholar]
- 19.Garner S. C., Mar M.-H., Zeisel S. H. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J. Nutr. 1995;125:2851–2858. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- 20.Rohlfs E. M., Garner S. C., Mar M.-H., Zeisel S. H. Glycerophosphocholine and phosphocholine are the major choline metabolites in rat milk. J. Nutr. 1993;123:1762–1768. doi: 10.1093/jn/123.10.1762. [DOI] [PubMed] [Google Scholar]
- 21.Davies S. E. C., Chalmers R. A., Randall E. W., Iles R. A. Betaine metabolism in human neonates and developing rats. Clin. Chim. Acta. 1988;178:241–250. doi: 10.1016/0009-8981(88)90232-x. [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein J. D., Kyle W. E., Harris B. J. Methionine metabolism in mammals. Regulation of homocysteine methyltransferases in rat tissue. Arch. Biochem. Biophys. 1971;146:84–92. doi: 10.1016/s0003-9861(71)80044-9. [DOI] [PubMed] [Google Scholar]
- 23.Mudd S. H., Poole J. R. Labile methyl balances for normal humans on various dietary regimens. Metab. Clin. Exp. 1975;24:721–735. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim S. K., Choi K. H., Kim Y. C. Effect of acute betaine administration on hepatic metabolism of S-amino acids in rats and mice. Biochem. Pharmacol. 2003;65:1565–1574. doi: 10.1016/s0006-2952(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 25.Gaull G. E., Von Berg W., Raiha N. C., Sturman J. A. Development of methyltransferase activities of human fetal tissues. Pediatr. Res. 1973;7:527–533. doi: 10.1203/00006450-197305000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Thompson H. R., Jones G. M., Narkewicz M. R. Ontogeny of hepatic enzymes involved in serine- and folate-dependent one-carbon metabolism in rabbits. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G873–G878. doi: 10.1152/ajpgi.2001.280.5.G873. [DOI] [PubMed] [Google Scholar]
- 27.McClain L. D., Bridgers W. F. Folate levels in developing mouse brain. J. Neurochem. 1970;17:763–766. doi: 10.1111/j.1471-4159.1970.tb03346.x. [DOI] [PubMed] [Google Scholar]
- 28.Robert K., Vialard F., Thiery E., Toyama K., Sinet P. M., Janel N., London J. Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J. Histochem. Cytochem. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- 29.Awata S., Nakayama K., Suzuki I., Sugahara K., Kodama H. Changes in cystathionine gamma-lyase in various regions of rat brain during development. Biochem. Mol. Biol. Int. 1995;35:1331–1338. [PubMed] [Google Scholar]
- 30.Lien Y. H. Role of organic osmolytes in myelinolysis. A topographic study in rats after rapid correction of hyponatriemia. J. Clin. Invest. 1995;95:1579–1586. doi: 10.1172/JCI117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunden S. L. F., Renduchintala M. S., Park E. I., Miklasz S. D., Garrow T. A. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 1997;345:171–174. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- 32.Dringen R., Pfeiffer B., Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caudill M. A., Wang J. C., Melnyk S., Pogribny I. P., Jernigan S., Collins M. C., Santos-Guzman J., Swendseid M. E., Cogger E. A., James S. J. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- 34.Hatten M. E. Central nervous system neuronal migration. Annu. Rev. Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson S. A. Neuroanatomical and functional alterations resulting from early postnatal insults in rodents. Pharmacol. Biochem. Behav. 1996;55:663–671. doi: 10.1016/s0091-3057(96)00253-5. [DOI] [PubMed] [Google Scholar]
- 36.Albright C. D., Mar M. H., Friedrich C. B., Brown E. C., Zeisel S. H. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev. Neurosci. 2001;23:100–106. doi: 10.1159/000048701. [DOI] [PubMed] [Google Scholar]
- 37.Albright C. D., Siwek D. F., Craciunescu C. N., Mar M. H., Kowall N. W., Williams C. L., Zeisel S. H. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr. Neurosci. 2003;6:129–134. doi: 10.1080/1028415031000084418. [DOI] [PubMed] [Google Scholar]
- 38.Craciunescu C. N., Albright C. D., Mar M. H., Song J., Zeisel S. H. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craciunescu C. N., Brown E. C., Mar M. H., Albright C. D., Nadeau M. R., Zeisel S. H. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J. Nutr. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 40.Christensen E., Brandt N. J. Prenatal diagnosis of 5,10-methylenetetrahydrofolate reductase deficiency. N. Engl. J. Med. 1985;313:50–51. doi: 10.1056/NEJM198507043130116. [DOI] [PubMed] [Google Scholar]