Abstract
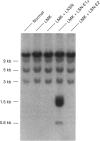
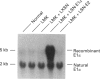
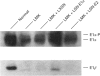
We have successfully used retroviral gene transfer to correct the deficiency of the branched-chain alpha-oxo acid dehydrogenase complex in lymphoblasts from a homozygous Mennonite maple syrup urine disease (MSUD) patient. The mutation in Mennonites is a Tyr-393 to Asn substitution in the branched-chain alpha-oxo acid decarboxylase (E1)alpha subunit of the enzyme complex. This promotes improper assembly of mutant E1 alpha with E1 beta subunits, leading to degradation of both polypeptides. For transduction studies, a full-length human E1 alpha CDNA was inserted into the retroviral vector LXSN to produce the recombinant LSN-E1 alpha. High-titre [6 x 10(5) colony-forming units/ml] amphotropic retroviral preparations free of helper viruses were obtained by co-cultivation of infected GP+E86 with PA317 cells. Transduction of MSUD lymphoblasts from the Mennonite patient with LSN-E1 alpha viruses restored the decarboxylation of alpha-oxo[1-14C]isovalerate to the normal level. The normal decarboxylation activity in transduced MSUD cells remained stable without G418 selection during the 14 weeks studied. Southern-blot analysis indicated that the recombinant E1 alpha cDNA was integrated into the host genome. Northern and Western blotting showed that both the normal E1 alpha mRNA and the subunit were properly expressed in transduced MSUD cells. However, the level of E1 beta subunits is lower than that of normal cells, suggesting competition of the recombinant E1 alpha with the mutant form for assembly with E1 beta. The results provide a paradigm for the development of somatic gene therapy for disorders involving mitochondrial multienzyme complexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. D., Wojcinski Z. W., Wise A. P., Godkin M. A. Maple syrup urine disease in five hereford calves in ontario. Can Vet J. 1987 Aug;28(8):505–511. [PMC free article] [PubMed] [Google Scholar]
- Chowdhury J. R., Grossman M., Gupta S., Chowdhury N. R., Baker J. R., Jr, Wilson J. M. Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science. 1991 Dec 20;254(5039):1802–1805. doi: 10.1126/science.1722351. [DOI] [PubMed] [Google Scholar]
- Chuang D. T., Cox R. P. Enzyme assays with mutant cell lines of maple syrup urine disease. Methods Enzymol. 1988;166:135–146. doi: 10.1016/s0076-6879(88)66020-4. [DOI] [PubMed] [Google Scholar]
- Chuang J. L., Cox R. P., Chuang D. T. Characterization of the promoter-regulatory region and structural organization of E1 alpha gene (BCKDHA) of human branched-chain alpha-keto acid dehydrogenase complex. J Biol Chem. 1993 Apr 15;268(11):8309–8316. [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- DANCIS J., HUTZLER J., LEVITZ M. Metabolism of the white blood cells in maple-syrup-urine disease. Biochim Biophys Acta. 1960 Sep 23;43:342–343. doi: 10.1016/0006-3002(60)90448-0. [DOI] [PubMed] [Google Scholar]
- Dariush N., Fisher C. W., Cox R. P., Chuang D. T. Structure of the gene encoding the entire mature E1 alpha subunit of human branched-chain alpha-keto acid dehydrogenase complex. FEBS Lett. 1991 Jun 17;284(1):34–38. doi: 10.1016/0014-5793(91)80755-r. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Wynn R. M., Cox R. P., Chuang D. T. Expression and assembly of a functional E1 component (alpha 2 beta 2) of mammalian branched-chain alpha-ketoacid dehydrogenase complex in Escherichia coli. J Biol Chem. 1992 Aug 15;267(23):16601–16606. [PubMed] [Google Scholar]
- DiLella A. G., Woo S. L. Cloning large segments of genomic DNA using cosmid vectors. Methods Enzymol. 1987;152:199–212. doi: 10.1016/0076-6879(87)52021-3. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Lau K. S., Randle P. J. Activation of phosphorylated branched chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1982 Oct 4;147(1):35–39. doi: 10.1016/0014-5793(82)81006-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher C. R., Chuang J. L., Cox R. P., Fisher C. W., Star R. A., Chuang D. T. Maple syrup urine disease in Mennonites. Evidence that the Y393N mutation in E1 alpha impedes assembly of the E1 component of branched-chain alpha-keto acid dehydrogenase complex. J Clin Invest. 1991 Sep;88(3):1034–1037. doi: 10.1172/JCI115363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. R., Fisher C. W., Chuang D. T., Cox R. P. Occurrence of a Tyr393----Asn (Y393N) mutation in the E1 alpha gene of the branched-chain alpha-keto acid dehydrogenase complex in maple syrup urine disease patients from a Mennonite population. Am J Hum Genet. 1991 Aug;49(2):429–434. [PMC free article] [PubMed] [Google Scholar]
- Fisher C. W., Chuang J. L., Griffin T. A., Lau K. S., Cox R. P., Chuang D. T. Molecular phenotypes in cultured maple syrup urine disease cells. Complete E1 alpha cDNA sequence and mRNA and subunit contents of the human branched chain alpha-keto acid dehydrogenase complex. J Biol Chem. 1989 Feb 25;264(6):3448–3453. [PubMed] [Google Scholar]
- Fisher C. W., Fisher C. R., Chuang J. L., Lau K. S., Chuang D. T., Cox R. P. Occurrence of a 2-bp (AT) deletion allele and a nonsense (G-to-T) mutant allele at the E2 (DBT) locus of six patients with maple syrup urine disease: multiple-exon skipping as a secondary effect of the mutations. Am J Hum Genet. 1993 Feb;52(2):414–424. [PMC free article] [PubMed] [Google Scholar]
- Harper P. A., Healy P. J., Dennis J. A. Maple syrup urine disease as a cause of spongiform encephalopathy in calves. Vet Rec. 1986 Jul 19;119(3):62–65. doi: 10.1136/vr.119.3.62. [DOI] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase. Biochemistry. 1983 Nov 22;22(24):5519–5522. doi: 10.1021/bi00293a011. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Vossen J. M., v Beusechem V. W., Valerio D. Treatment of patients with severe combined immunodeficiency due to adenosine deaminase (ADA) deficiency by autologous transplantation of genetically modified bone marrow cells. Hum Gene Ther. 1992 Oct;3(5):553–558. doi: 10.1089/hum.1992.3.5-553. [DOI] [PubMed] [Google Scholar]
- Indo Y., Kitano A., Endo F., Akaboshi I., Matsuda I. Altered kinetic properties of the branched-chain alpha-keto acid dehydrogenase complex due to mutation of the beta-subunit of the branched-chain alpha-keto acid decarboxylase (E1) component in lymphoblastoid cells derived from patients with maple syrup urine disease. J Clin Invest. 1987 Jul;80(1):63–70. doi: 10.1172/JCI113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. A., Ponder K. P., Woo S. L. Human gene therapy: present and future. Breast Cancer Res Treat. 1992;21(2):83–93. doi: 10.1007/BF01836954. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lau K. S., Chuang J. L., Herring W. J., Danner D. J., Cox R. P., Chuang D. T. The complete cDNA sequence for dihydrolipoyl transacylase (E2) of human branched-chain alpha-keto acid dehydrogenase complex. Biochim Biophys Acta. 1992 Oct 20;1132(3):319–321. doi: 10.1016/0167-4781(92)90169-z. [DOI] [PubMed] [Google Scholar]
- Lynch C. M., Miller A. D. Production of high-titer helper virus-free retroviral vectors by cocultivation of packaging cells with different host ranges. J Virol. 1991 Jul;65(7):3887–3890. doi: 10.1128/jvi.65.7.3887-3890.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Verma I. M. Two base changes restore infectivity to a noninfectious molecular clone of Moloney murine leukemia virus (pMLV-1). J Virol. 1984 Jan;49(1):214–222. doi: 10.1128/jvi.49.1.214-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Hock R. A., Kaleko M., Miller A. D. Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum Gene Ther. 1990 Spring;1(1):31–41. doi: 10.1089/hum.1990.1.1-31. [DOI] [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfmann R., Axelrod J. H., Verma I. M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick F. L., Harper A. E. Branched-chain amino acid oxidation by isolated rat tissue preparations. Biochim Biophys Acta. 1976 Jul 21;437(2):477–486. doi: 10.1016/0304-4165(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Cook K. G., Boyd R. W., Lawson R. Evidence that the mitochondrial activator of phosphorylated branched-chain 2-oxoacid dehydrogenase complex is the dissociated E1 component of the complex. FEBS Lett. 1984 Jun 25;172(1):38–42. doi: 10.1016/0014-5793(84)80868-6. [DOI] [PubMed] [Google Scholar]