Abstract
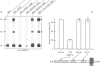
Vesicle-associated membrane protein (VAMP) (or synaptobrevin), a type II membrane protein of small synaptic vesicles, is essential for neuroexocytosis because its proteolysis by tetanus and botulinum neurotoxins types B, D, F and G blocks neurotransmitter release. The addition of cross-linking reagents to isolated small synaptic vesicles induces the formation of 30 and 50 kDa complexes containing the isoform 2 of VAMP (VAMP-2). Whereas the 30 kDa band is a VAMP-2 homodimer, the 50 kDa species results from the cross-linking of VAMP-2 with synaptophysin. This heterodimer also forms in detergent-solubilized vesicles and involves the N-terminal part of VAMP-2. The implications of the existence of a synaptophysin-VAMP-2 complex in the processes of vesicle docking and fusion with the presynaptic membrane are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. K., Calakos N., Kreiner T., Scheller R. H. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992 Feb;116(3):761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Yamasaki S., Binz T., Niemann H., Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993 Dec;12(12):4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980 Oct;87(1):297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L. A., Trimble W. S., Scheller R. H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989 Jul 5;264(19):11061–11064. [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fykse E. M., Takei K., Walch-Solimena C., Geppert M., Jahn R., De Camilli P., Südhof T. C. Relative properties and localizations of synaptic vesicle protein isoforms: the case of the synaptophysins. J Neurosci. 1993 Nov;13(11):4997–5007. doi: 10.1523/JNEUROSCI.13-11-04997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa H. P., Saisu H., Ishizuka T., Sekine Y., Tsugita A., Odani S., Abe T. A complex of rab3A, SNAP-25, VAMP/synaptobrevin-2 and syntaxins in brain presynaptic terminals. FEBS Lett. 1993 Sep 13;330(2):236–240. doi: 10.1016/0014-5793(93)80281-x. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Südhof T. C. The multisubunit structure of synaptophysin. Relationship between disulfide bonding and homo-oligomerization. J Biol Chem. 1990 May 25;265(15):8869–8873. [PubMed] [Google Scholar]
- Knaus P., Marquèze-Pouey B., Scherer H., Betz H. Synaptoporin, a novel putative channel protein of synaptic vesicles. Neuron. 1990 Oct;5(4):453–462. doi: 10.1016/0896-6273(90)90084-s. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Fernandez J. M. The exocytotic fusion pore and neurotransmitter release. Neuron. 1994 Apr;12(4):707–716. doi: 10.1016/0896-6273(94)90325-5. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994 Jul;13(1):1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G. Tetanus and botulism neurotoxins: a new group of zinc proteases. Trends Biochem Sci. 1993 Sep;18(9):324–327. doi: 10.1016/0968-0004(93)90065-u. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994 Mar 1;4(3):220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Malizio C., Trimble W. S., Polverino de Laureto P., Milan G., Sugiyama H., Johnson E. A., Montecucco C. Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J Biol Chem. 1994 Aug 12;269(32):20213–20216. [PubMed] [Google Scholar]
- Schiavo G., Rossetto O., Catsicas S., Polverino de Laureto P., DasGupta B. R., Benfenati F., Montecucco C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem. 1993 Nov 15;268(32):23784–23787. [PubMed] [Google Scholar]
- Schiavo G., Shone C. C., Rossetto O., Alexander F. C., Montecucco C. Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J Biol Chem. 1993 Jun 5;268(16):11516–11519. [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Thomas L., Hartung K., Langosch D., Rehm H., Bamberg E., Franke W. W., Betz H. Identification of synaptophysin as a hexameric channel protein of the synaptic vesicle membrane. Science. 1988 Nov 18;242(4881):1050–1053. doi: 10.1126/science.2461586. [DOI] [PubMed] [Google Scholar]
- Weiss J. H., Hartley D. M., Koh J. Y., Choi D. W. AMPA receptor activation potentiates zinc neurotoxicity. Neuron. 1993 Jan;10(1):43–49. doi: 10.1016/0896-6273(93)90240-r. [DOI] [PubMed] [Google Scholar]
- Williamson M. P. The structure and function of proline-rich regions in proteins. Biochem J. 1994 Jan 15;297(Pt 2):249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]