Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) can be induced by a sudden inflammatory burst, causing loss of the endothelial–epithelial barrier integrity; pulmonary edema; extensive cell death; and, ultimately, lung architectural damage (1, 2). Pulmonary endothelial cells play a central role, controlling chemotaxis, maintaining the endothelial barrier while regulating permeability of cell junctions for leukocytes to reach sites of infection, and maintaining vascular tone for adequate perfusion and gas exchange (1, 2). Despite advances in clinical management, ALI/ARDS continues to be associated with high morbidity and mortality. A better understanding of the cellular mechanisms alongside the development of novel therapeutics is a critical need. In this issue of the Journal, Shaheen and colleagues (pp. 307–317) address the possibility of using indole-3-acetic acid (IAA) as a therapy for ALI/ARDS conditions, with a focus on pulmonary endothelial cell biology and endothelial barrier permeability (3).
IAA, an abundant plant hormone, reportedly has antioxidant and antiinflammatory properties (3). It has been detected in multiple human body compartments and may derive from the enteric flora in mammals (4, 5). Its effect seems to be dose-dependent in various organ systems, with recent disparate controversial outcomes related to cardiovascular physiology (6). IAA’s mechanism of action is not known; however, because of its purported beneficial effects, Shaheen and colleagues investigated the potential of IAA as a pretreatment for ALI/ARDS. Through a series of elegant and well-designed experiments, the researchers found IAA pretreatment decreases of proinflammatory cytokine secretion and improvement of transendothelial electrical resistance, a measure of endothelial barrier function. These results were supported by experiments in a murine model of ALI/ARDS in which IAA pretreatment decreased pulmonary edema, measured using a lung wet-to-dry ratio, and extravasation assays. The authors report that the dose-dependent effects of IAA decrease endothelial barrier disruption and increase endothelial integrity.
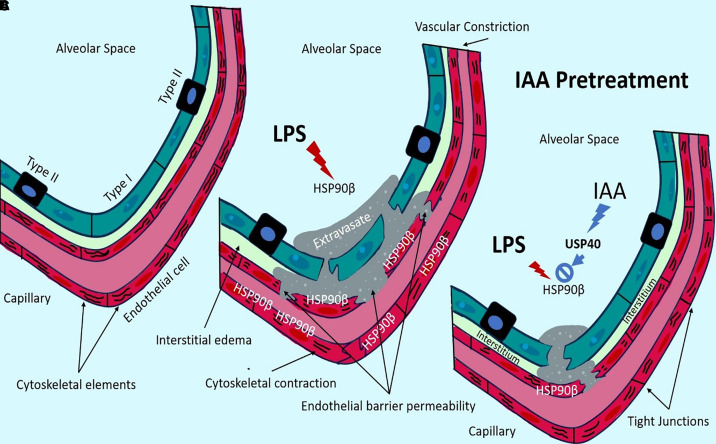
Regulation of lung endothelial cell barrier integrity is complex, with the organization and contraction of cytoskeletal elements being a critical and tightly orchestrated process. Heat Shock Protein 90 (HSP90) is a proinflammatory chaperone protein involved in numerous pathologic processes but chiefly studied in cancer migration, metastases, and blood–barrier disruption (7). HSP90 activation leads to endothelial cell contraction with a net effect of increasing endothelial barrier permeability. This seems to hold true in the pulmonary endothelium, where HSP90 activation in models of ALI/ARDS is associated with pulmonary edema and disruption of endothelial barrier integrity. In fact. HSP90 inhibitors are a focus of current studies testing their potential to hasten the resolution of inflammatory states that can lead to ALI/ARDS (8). Using sophisticated knockout murine models and vector-induced models of ubiquitin-specific peptidase 40 (USP40) expression inhibition, Shaheen and colleagues demonstrate a mechanism of action for IAA to induce USP40, which increased the deubiquitination of the HSP90β isomer.
Collectively, the studies by Shaheen and colleagues provide convincing evidence that IAA pretreatment leads to decreased acute inflammatory pulmonary responses through the activation and stabilization of USP40. This ultimately reduces the action of HSP90β and promotes the maintenance of endothelial barrier integrity, with decreased lung injury (Figure 1). Although immune histopathologic correlation would have provided additional strong validation, the findings reinforce the importance of a gut–lung axis in which commensal bacteria indirectly affect the health of the lung by conferring inflammatory protection through IAA, a gut bacterial byproduct (9). This further highlights a mechanism for the role of microbiotic gut dysregulation in human lung diseases.
Figure 1.
The plant hormone indole-3-acetic acid (IAA) improves endothelial barrier after lung injury. (Α) Under physiological conditions, the endothelial barrier is maintained with decreased permeability. (Β) Under significant inflammatory conditions, such as those induced by LPS, Heat Shock Protein 90 (HSP90) increases vascular leakage, interstitial edema, and alveolar edema. This resembles conditions seen in acute lung injury/acute respiratory distress syndrome. (C) Pretreatment with IAA prevents the endothelial barrier disruption caused by HSP90. Shaheen and colleagues demonstrated that IAA action leads to the promotion of UPS40, which increases the deubiquitination of HSP90β (active isomer of HSP90), effectively inactivating the HSP90 protein and decreasing the inflammatory cascade.
Although the pretreatment protocols used in this work are ideal for assessing prophylactic therapy in numerous conditions, this is not necessarily directly applicable in a clinical setting, as it is not feasible yet to predict which patients will develop severe complications such as ARDS. Thus, future studies with IAA as “a rescue after exposure” are needed to consider it for human use, a limitation that is acknowledged by the authors (3). Caution is also warranted because increased endothelial barrier permeability is a physiologic mechanism that contributes to immune responses that facilitate the clearance of pathogens from the lung tissue. If IAA leads to the inhibition of immune responses, it is possible that immune dysregulation can lead to ineffective pathogen elimination in cases of bacterial pneumonias.
Models of adult ALI/ARDS commonly utilize murine laboratory animals with fully developed alveoli. It would be beneficial to see the potential protective effects of IAA on the preterm lung, which is commonly exposed to inflammatory states. Preterm lungs are characterized by a capillary network that undergoes multiple transitions (progenitor to differentiated states), and are further characterized by having immature endothelial cell barrier integrity, especially at the canalicular and saccular stages of development (10, 11). Newborn murine animal models are phenotypically similar to premature stages of human lung development, although the animals are able to survive without the assistance of ventilatory support (12). Chronic ventilatory support associated with hyperoxic and hypoxic exposures that cause oxidative stress and inflammatory conditions such as chorioamnionitis are implicated in the development of chronic lung disease of prematurity that exhibit alveolar developmental arrest and vascular maldevelopment (13). Pulmonary edema caused by an immature and dysregulated endothelial barrier is a complication of these sequelae of preterm birth. Although chronic diuretic therapies are a mainstay (14), it is intriguing to consider whether IAA treatment could affect endothelial cell integrity in the context of established vascular dysfunction. Additionally, respiratory distress syndrome of the newborn is pathophysiologically distinct from adult ARDS (1, 2, 15). The effects of IAA on such a system are unknown, but further studies such as the ones conducted by Shaheen and colleagues could be revealing.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2024-0209ED on June 10, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet . 2022;400:1145–1156. doi: 10.1016/S0140-6736(22)01485-4. [DOI] [PubMed] [Google Scholar]
- 2. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers . 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaheen N, Miao J, Li D, Xia B, Baoyinna B, Zhao Y, et al. Indole-3-acetic acid protects against lipopolysaccharide-induced endothelial cell dysfunction and lung injury through the activation of USP40. Am J Respir Cell Mol Biol . 2024;71:307–317. doi: 10.1165/rcmb.2024-0159OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye X, Li H, Anjum K, Zhong X, Miao S, Zheng G, et al. Dual role of indoles derived from intestinal microbiota on human health. Front Immunol . 2022;13:903526. doi: 10.3389/fimmu.2022.903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res . 2018;46:D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol . 2015;26:876–887. doi: 10.1681/ASN.2013121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uddin MA, Akhter MS, Kubra KT, Whitaker KE, Shipley SL, Smith LM, et al. Hsp90 inhibition protects the brain microvascular endothelium against oxidative stress. Brain Disord . 2021;1:100001. doi: 10.1016/j.dscb.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barabutis N. Heat shock protein 90 inhibition in the inflamed lungs. Cell Stress Chaperones . 2020;25:195–197. doi: 10.1007/s12192-020-01069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol . 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev . 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren X, Ustiyan V, Pradhan A, Cai Y, Havrilak JA, Bolte CS, et al. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ Res . 2014;115:709–720. doi: 10.1161/CIRCRESAHA.115.304382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damianos A, Kulandavelu S, Chen P, Nwajei P, Batlahally S, Sharma M, et al. Neonatal intermittent hypoxia persistently impairs lung vascular development and induces long-term lung mitochondrial DNA damage. J Appl Physiol (1985) . 2022;133:1031–1041. doi: 10.1152/japplphysiol.00708.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers . 2019;5:78. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakaria RP, Dhanireddy R. Pharmacotherapy in bronchopulmonary dysplasia: what is the evidence? Front Pediatr . 2022;10:820259. doi: 10.3389/fped.2022.820259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitsett JA, Weaver TE. Alveolar development and disease. Am J Respir Cell Mol Biol . 2015;53:1–7. doi: 10.1165/rcmb.2015-0128PS. [DOI] [PMC free article] [PubMed] [Google Scholar]