Abstract
Kluyveromyces lactis zymocin, a trimeric (αβγ) protein toxin complex, inhibits proliferation of Saccharomyces cerevisiae cells. Here we present an analysis of kti6 mutants, which resist exogenous zymocin but are sensitive to intracellular expression of its inhibitory γ-toxin subunit, suggesting that KTI6 encodes a factor needed for toxin entry into the cell. Consistent with altered cell surface properties, kti6 cells resist hygromycin B, syringomycin E, and nystatin, antibiotics that require intact membrane potentials or provoke membrane disruption. KTI6 is allelic to IPT1, coding for mannosyl-diinositolphospho-ceramide [M(IP)2C] synthase, which produces M(IP)2C, the major plasma membrane sphingolipid. kti6 membranes lack M(IP)2C and sphingolipid mutants that have reduced levels of M(IP)2C precursors, including the sphingolipid building block ceramide survive zymocin. In addition, kti6/ipt1 cells allow zymocin docking but prevent import of its toxic γ-subunit. Genetic analysis indicates that Kti6 is likely to act upstream of lipid raft proton pump Kti10/Pma1, a previously identified zymocin sensitivity factor. In sum, M(IP)2C operates in a plasma membrane step that follows recognition of cell wall chitin by zymocin but precedes the involvement of elongator, the potential toxin target.
Kluyveromyces lactis killer strains prevent Saccharomyces cerevisiae cells from undergoing G1 cell cycle exit via zymocin, a trimeric (αβγ) toxin complex (8, 52, 57). Based on conditional expression, toxicity resides within the γ-subunit (γ-toxin) and screens for growth in the presence of exozymocin or endogenously expressed γ-toxin have distinguished nontarget (class I) from toxin target (class II) kti (K. lactis toxin insensitive) mutants in Saccharomyces cerevisiae (9, 11, 62). Ten class II KTI genes suggest the existence of a complex toxin response pathway or, alternatively, the involvement of a multifactorial target complex (11). In favor of the latter, analysis of tot (γ-toxin target) mutants has identified the TOT function of Elongator, a six-subunit partner complex of RNA polymerase II (19, 31, 48, 65). Like Elongator defects, mutations in genes coding for Elongator partners or Elongator relevant factors (KTI11, KTI12/TOT4, KTI13/ATS1, and KTI14/HRR25) elicit class II resistance (16-18, 42).
As judged from recent data that kti14 casein kinase I mutants express Elongator-like defects and that hyperphosphorylation of Elongator in sit4 phosphatase mutants coincide with zymocin survival, TOT function seems to be kept in check by Elongator phosphorylation (32, 34, 42). In support of Elongator's role as a key zymocin effector, its TOT function is further suppressed by Elongator proteolysis and by lack of protein urmylation (18, 21). Notably, without Elongator, protein urmylation is affected and removal of TOT partner Kti11 enhances Elongator instability and defeats diphtheria toxicity (18, 23, 40).
Despite these advances towards identifying components of the toxin target process, the early steps of the zymocin response are poorly understood (53). Consistent with an impact on early events, class I mutations in KTI2/CHS3 and KTI10/PMA1 protect solely against exozymocin and affect chitin synthesis and plasma membrane H+-ATPase function, respectively (11, 33, 43). In line with this, holozymocin binds chitin in vitro and has exochitinase activity (10, 33). As judged from the finding that hygromycin B, whose antibiotic action requires an intact membrane potential established by H+-ATPase Pma1/Kti10, is inert against kti10 cells, plasma membrane energization is likely to be required for zymocin action (43, 50). Given the strongly hydrophobic nature of the β-subunit, to which the active γ-subunit is linked via a disulfide bond, a tentative picture that emerges for early zymocin-induced events includes chitin docking followed by delivery of the γ-toxin subunit to the membrane and subsequent subcellular import (33, 43, 56).
In support of class I zymocin protection, we present in this report evidence that kti6 cells express a membrane defect that allows them to survive antifungal drugs whose actions require an intact plasma membrane potential or that work by disrupting membranes. Consistently, KTI6 is allelic to IPT1, coding for mannosyl-diinositolphospho-ceramide [M(IP)2C] synthase, the terminal sphingolipid biosynthetic enzyme in yeast. In line with this, membranes of kti6 cells lack M(IP)2C and, in contrast to what occurs in Elongator tot-mutants, γ-toxin import is blocked in kti6 cells. Presumably, early during the zymocin response, M(IP)2C acts in a step at the cell membrane that follows zymocin docking but precedes both the engagement of plasma membrane H+-ATPase and Elongator. This suggests a possible function of M(IP)2C as a secondary membrane receptor required for γ-toxin uptake.
MATERIALS AND METHODS
Strains, media, vectors, and K. lactis zymocin methods.
Cultivation of yeast strains (Table 1) used rich YP and minimal S media (55). Phenotypic studies involved drug (Sigma) supplementation with caffeine (5 to 7.5 mM), hygromycin B (100 μg/ml), nystatin (50 μg/ml), Calcofluor White (50 μg/ml), or 6% (vol/vol) ethanol. Recovery from heat shock and syringomycin E assays followed previous protocols (26, 58). Zymocin responses by killer eclipse assays involved K. lactis killer AWJ137 (Table 1) and growth for 1 day at 30°C (36). Zymocin plate assays used partially purified zymocin from AWJ137 filtrates (40 to 65% [vol/vol]) and serial dilutions of S. cerevisiae tester strains (34). Growth was for 2 days at 30°C. Galactose-induced expression of γ-toxin involved transformation with pHMS14 and control vector pHMS22 carrying just the GAL1 promoter (19). Growth on 2% (vol/vol) galactose S medium was for 3 days at 30°C. pIPT1, a pRS415 vector carrying IPT1/SYR4 (58), was used for complementing kti6 cells. Multicopy PMA1 and AST1 involved pCM10.4 and pAC49, respectively (3, 12, 43). ISC1 overexpression used the GAL1 fusion in pYES2 (51).
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| K. lactis | ||
| AWJ137 | MATα leu2 trp1 [k1+ k2+] zymocin producer, immune | 19 |
| IFO1267 | MATa [k1+ k2+] zymocin producer, immune | 25 |
| S. cerevisiae | ||
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | EUROSCARF |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | EUROSCARF |
| Y04007 | BY4741 but ipt1Δ::kanMX4 | EUROSCARF |
| Y14007 | BY4742 but ipt1Δ::kanMX4 | EUROSCARF |
| Y05281 | BY4741 but sur4Δ::kanMX4 | EUROSCARF |
| Y03656 | BY4741 but sur2Δ::kanMX4 | EUROSCARF |
| Y04667 | BY4741 but acb1Δ::kanMX4 | EUROSCARF |
| LL20 | MATα leu2-3,112 his3-11,15 can1 | 11 |
| KY117 | MATaura3-52 trp1-Δ1 lys2-801am ade2-101 his3Δ200 | 11 |
| ARB6 | LL20 but kti6-1 | 11 |
| ARB27 | LL20 but kti6-2 | 11 |
| ARBK10 | KY117 but kti6-3 | 11 |
| ARBK28 | KY117 but kti6-4 | 11 |
| ARBK30 | KY117 but kti6-5 | 11 |
| ARB68 | LL20 but kti10 | 11 |
| FFY3 | LL20 but tot3/elp3Δ::KILEU2 ura3 | 19 |
| LFY12 | LL20 but tot4/kti12Δ::KILEU2 ura3 | 19 |
| DJY3 | LL20 but chs3/kti2Δ::KILEU2 ura3 | 33 |
| DG106 | LL20 but kti10 kti6 | This study |
| DG107 | LL20 but Sphis5+::GAL1::KTI6/IPT1 | This study |
| DG108 | LL20 but kti6Δ/ipt1Δ::KILEU2 | This study |
| YCC1 | BY4741 (MATaIPT1) × LL20 (MATα KTI6) | This study |
| YCC2 | BY4741 (MATaIPT1) × ARB27 (MATα kti6-2) | This study |
| YCC3 | Y04007 (MATaipt1Δ) × LL20 (MATα KTI6) | This study |
| YCC4 | Y04007 (MATaipt1Δ) × ARB27 (MATα kti6-2) | This study |
| YCC5 | BY4742 (MATα IPT1) × KY117 (MATaKTI6) | This study |
| YCC6 | BY4742 (MATα IPT1) × ARBK10 (MATakti6-3) | This study |
| YCC7 | Y14007 (MATα ipt1Δ) × KY117 (MATaKTI6) | This study |
| YCC8 | Y14007 (MATα ipt1Δ) × ARBK10 (MATakti6-3) | This study |
| W303-1A | MATaura3-1 leu2-3/112 his3-11/15 trp1-1 ade2-1 can1-100 | 60 |
| DM1 | W303-1A but ipt1W93STOP | 60 |
| WBY286 | W303-1A but lac1Δ::ADE2 | H. Riezman, Geneva |
| WBY283 | W303-1A but lag1Δ::HIS3 | H. Riezman, Geneva |
| WBY616 | W303-1A but lag1Δ::HIS3 lac1Δ::ADE2 | H. Riezman, Geneva |
| KA31-1A | MATα ura3 leu2 trp1 his3 | 63 |
| SUY05 | KA31-1A but csg1Δ::HIS3 | 63 |
| SUY06 | KA31-1A but csg2Δ::URA3 | 63 |
| SUY07 | KA31-1A but csg1Δ::HIS3 csg2Δ::URA3 | 63 |
| SUY08 | KA31-1A but ipt1Δ::kanMX4 | 63 |
| SUY41 | KA31-1A but csh1Δ::kanMX4 | 63 |
| SUY42 | KA31-1A but csg1Δ::HIS3 csh1Δ::LEU2 | 63 |
| SUY43 | KA31-1A but csg2Δ::HIS3 csh1Δ::LEU2 | 63 |
| SUY44 | KA31-1A but csg1Δ::HIS3 csg2Δ::URA3 csh1Δ::LEU2 | 63 |
Identification of KTI6/IPT1 and sphingolipid measurements.
Zymocin was concentrated from 1.3 liters of YPD culture broth of K. lactis killer strain IFO1267 (Table 1) to 13 ml by hydroxyapatite column chromatography as described previously (59). We screened the EUROSCARF deletion set of 4,898 nonessential S. cerevisiae gene disruptions in the BY4741 background (Table 1) for altered sensitivity against K. lactis zymocin by transfer onto 96-well microplates using an 8 by 6 array stainless steel replicator. Approximately 103 mutant cells were grown in 60 μl of 1 M sorbitol-YPD liquid medium with 1:300 or 1:1,500 dilution zymocin in round-bottomed 96-well microplates at 30°C. Observation for 3 days revealed that the wild-type strain failed to grow in the presence of a 1:1,500 zymocin dilution.
Two hundred six resistant mutants which grew on 1:300-diluted zymocin were retested to confirm their phenotype. KTI6 was isolated as follows. Zymocin-resistant mutants isolated from the BY4741 deletion set (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 kanMX4 [G418r]) were grown for 18 h at 30°C and mixed with ARB27 (kti6-2), a killer-resistant mutant strain derived from LL20 (MATα leu2 his3 URA3) (11) (Table 1). After overnight incubation at 30°C, the mixed cells were suspended in 100 μl sterilized water in a 96-well microplate and transferred onto YPD/G418 plates. Cells were resuspended in water and then transferred into SD medium without uracil. Upon replica plating twice on selective medium, diploids were concentrated by single colony isolation on SD medium without uracil and reselected on YPD/G418 medium.
Using killer strain K. lactis IFO1267, the killer eclipse assay (36) was performed on all diploids selected. A zymocin-resistant (zymR) diploid obtained by crossing ipt1Δ (Y04007 [Table 1]) and kti6-2 cells was analyzed further. The phenotypic behavior of the cross was reconfirmed by reversing the cross. ARBK28 (MATa kti6-3 zymR) was mated with Y14007, a BY4742 ipt1Δ knockout (MATα ipt1Δ::kanMX4 zymR) (Table 1). MATa/MATα kti6-3/ipt1Δ::kanMX4 homozygous, recessive diploids were compared with all other diploid genotypes (Fig. 3A). Correct integration of the kanMX4 cassette in the sphingolipid synthesis gene IPT1 from strain Y04007 was verified by PCR analysis of genomic DNA.
FIG. 3.
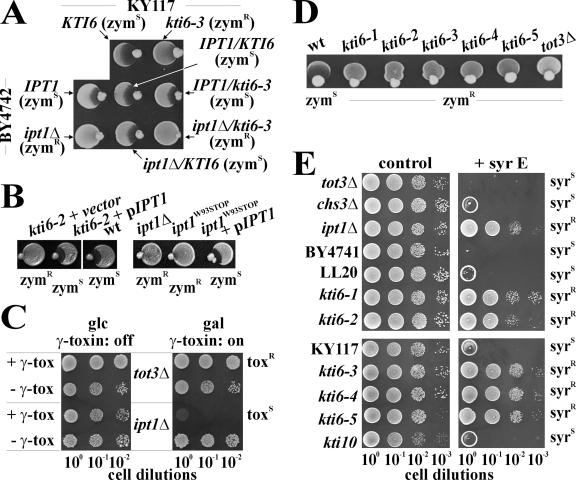
KTI6 is allelic with IPT1. A. Complementation studies on diploids obtained from crossing kti6-3 and ipt1Δ mutants. Haploid KTI6 (KY117), kti6-3 (ARBK10), IPT1 (BY4742), and ipt1Δ (Y14007) cells and homo- and heterozygous KTI6/IPT1 (YCC5), kti6-3/IPT1 (YCC6), KTI6/ipt1Δ (YCC7), and kti6-3/ipt1Δ (YCC8) diploids obtained after mating were subjected to killer eclipse assays (see Fig. 1A). Note that zymocin resistance (zymR) is unique to the haploid kti6-3 (ARBK10) and ipt1Δ (Y14007) cells as well as the kti6-3/ipt1Δ diploid (YCC8), while other crosses express sensitivity (zymS). B. pIPT1 complements kti6 mutants. Mutant kti6-2 (ARB27) and ipt1W93STOP (DM1) cells carrying empty pRS415 (vector) or pRS415-IPT1 (pIPT1) were tested in killer eclipse assays with wild-type (LL20) or ipt1Δ (Y04007) cells. C. An IPT1 deletion induces class I resistance. Serial dilutions of tot3Δ (FFY3) and ipt1Δ (Y04007) cells were subjected to conditional expression of γ-toxin (see Fig. 1B). D. Mutations in KTI6 phenocopy zymR. Using killer eclipse assays (see Fig. 1A), five independent kti6 alleles (ARB6, ARB27, ARBK10, ARBK28, and ARBK30 [Table 1]) were compared to tot3Δ cells (FFY3). E. kti6 cells resist syringomycin E. YPD plates containing zero (control) or 5 μg/ml syringomycin E (syr E) were incubated with five kti6 isolates (see D), toxin-resistant kti10 (ARB68), tot3Δ (FFY3), ipt1Δ (Y04007), and chs3Δ (DJY3) mutants and parental strains (LL20, KY117, and BY4741). Syringomycin E-sensitive (syrS) and -resistant (syrR) responses are shown.
Combining kti10 and kti6 mutations involved yeast matings between ARB68 (kti10) and ARBK10 (kti6-3) (Table 1) or PCR disruption of KTI6 by the K. lactis LEU2 gene (see below) using previously described protocols (33). Sphingolipid analysis essentially followed the protocol for yeast radiolabeling with [3H]myo-inositol followed by 5% (vol/vol) trichloroacetic acid extraction (14). Sphingolipids were separated by thin-layer chromatography on Whatman HP-K plates and 3H-labeled sphingolipids were detected by using a BioScan apparatus (14).
Reverse transcription-PCR and PCR-based gene manipulations.
For IPT1/KTI6 expression studies, total RNA from yeast was isolated with RNAeasy columns (QIAGEN, Hilden, Germany). Following DNase I treatment, 4 μg RNA was subjected to first-strand cDNA synthesis at 42°C for 1 h using 20 μl reverse transcription-PCR mixtures (RevertAid kit; MBI Fermentas, Lithuania). Next, 1/10 was used in 25 PCR cycles using Taq polymerase and IPT1/KTI6 (5′-TCC TCT TTG GGG ACT ATT GG-3′ and 5′-CCA AGC CAG TAA ATC CTT GG-3′) or ACT1 (5′-CTT CCG GTA GAA CTA CTG GT-3′ and 5′-CCT TAC GGA CAT CGA CAT CA-3′) primers to amplify 0.38-kb IPT1 and 0.44-kb ACT1 fragments.
KTI6 in vivo deletion used KTI6 knockout primers (koIPT1-FW, 5′-TTA TCA TTT CTG CTA AGA ATC ACC TAA AGT CTT TCA ACG TCT AAG AAA GCC GAC GGC CAG TGA ATT CCC GG-3′, and koIPT1-RV, 5′-TTA TTT AAA TTA TCC GAA ATT ACT TTT ATT ACA TTA TGA CAT TCT ATA GTA GCT TGG CTG CAG GTC GAC GG-3′) and template YDpKlLEU2 to amplify a KTI6/IPT1 deletion cartridge (33). ipt1Δ was confirmed by PCR on genomic ipt1Δ DNA using primers IPT1-FW (5′-AAT GTG AAC GCC ACG GGA AAA AAGC-3′) and IPT1-RV (5′-AAG CAA GCC GAT TTC AGG TTA ACCC-3′), which amplified a 0.5-kb deletion (compared to the 2.2-kb IPT1 DNA). Promoter swapping by PCR involved template pFA6a-TRP1-pGAL1-3HA (41)-and IPT1/KTI6-specific primers (F4-IPT1, 5′-AAA AAT GCC TAG AAA TGC AGA CCT CCG GCC ACA ACA TTT TTA TAT AAT ACG AAT TCG AGC TCG TTT AAAC-3′, and R3-IPT1: 5′-CGA TTA TAC ATG TTT TTG ACG AAA CTC GCC AAA GAA AAT ATG ACA TTC ATG CAC TGA GCA GCG TAA TCTG-3′).
Immunological techniques.
Protein extraction for use in Western blot studies was as described previously (19). Protein loadings were checked using an antibody against the yeast Pfk1 α and β subunits (a gift of J. J. Heinisch, University of Osnabrück, Germany) and Cdc19, pyruvate kinase (kindly provided by J. Thorner, University of California at Berkeley). Cell fractionation was done by sucrose ultracentrifugation (38). Separation of yeast cytoplasmic, soluble fractions from insoluble cell wall material followed a previous protocol (49). Rather than quantifying protein content from the insoluble and clotty pellet fractions, protein quantity was checked in the parallel supernatants by immuoprobing for phosphofructokinase content (see above). Zymocin treatment of S. cerevisiae cells prior to protein extraction or cell fractionation involved growth in 100 ml YPD to an optical density at 600 nm of ≈1.0 followed by addition of 50% (vol/vol) zymocin (see above) and further incubation for 6 h.
RESULTS
Phenotypic analysis of the kti6 mutant.
Like kti2 and kti10 cells, the kti6 mutant was found to resist holozymocin (zymR) in killer eclipse assays (Fig. 1A) (11, 33, 43). Similar to the toxin-sensitive (toxS) response of wild-type and class I kti10 cells, GAL1-promoter driven expression of zymocin's γ-toxin caused kti6-2 cells to cease growth, while class II Elongator tot3Δ cells resisted γ-toxin (toxR) (Fig. 1B). This typical class I response predicts kti6 cells to be affected early in the zymocin response rather than being altered in Elongator's toxin target (TOT) capacity. Based on further phenotypic studies, kti6-2 cells performed like the wild type, with viability not detectably being affected (Fig. 1C and D).
FIG. 1.
kti6 phenotypes. A. Killer eclipse assay. Zymocin-sensitive (zymS) wild-type (wt; LL20), resistant (zymR) kti10 (ARB68), and tot3Δ (FFY3) and kti6-2 cells (ARB27) were tested against K. lactis killer AWJ137. Eclipse formation indicates zymS. B. γ-Toxin assay. Serial dilutions of the indicated strains transformed with GAL1-γ-toxin vector pHMS14 (+ γ) or empty pHMS22 (− γ) were spotted onto glucose (glc) and galactose (gal) medium. A Gal+ phenotype distinguishes γ-toxin resistance (toxR) from sensitivity (toxS). C. Growth curves. Optical densities (at 600 nm) of cells grown in liquid YPD at 30°C were compared over twelve hours. D. Sensitivity to drugs and ethanol. Serial dilutions of the above strains, including the chs3Δ chitin mutant (DJY3), were spotted on YPD plates (control) or plates supplemented with Calcofluor White (CFW; 50 μg/ml), caffeine (7.5 mM), or 6% (vol/vol) ethanol (EtOH). E. Heat shock recovery. Strains were shocked at 55°C for a given time in minutes and recovered at 30°C on YPD for 1 day. F. Resistance to antibiotics. Strains were grown on unsupplemented YPD plates (control) and on plates containing hygromycin B (hyg B; 100 μg/ml) or nystatin (nys; 50 μg/ml).
In contrast to a class II Tot− mutant (tot3Δ), kti6-2 cells expressed wild-type tolerance to caffeine and Calcofluor White (Fig. 1D). The latter contrasted with the pronounced resistance of class I chitin-deficient (kti2Δ/chs3Δ) cells to the chitin indicator drug Calcofluor White (Fig. 1D), suggesting that kti6-2 cells have normal chitin levels and do not resist zymocin by preventing chitin docking. In contrast to the ethanol sensitivities of chitin and Elongator defective strains, kti6-2 cells expressed ethanol tolerance like the wild type (Fig. 1D). Unlike the latter, however, kti6-2 cells recovered less well from heat shock although significantly better than kti10 cells (Fig. 1E). Together with strong resistance of kti6-2 cells to hygromycin B (Fig. 1F), an antibiotic that requires membrane energization through the H+-ATPase Pma1/Kti10 (50), these phenotypes suggest that KTI6 is relevant for cell surface integrity. Consistently, kti6-2 cells resisted the membrane disruptor nystatin (Fig. 1F) (35). As for the zymocin response, kti6-2 cells are therefore expected to express a plasma membrane defect that affects a step following chitin docking but that does not change Elongator's TOT capacity.
KTI6 is epistatic to KTI10, the plasma membrane H+- ATPase gene.
Since underassembly of the H+-pump Kti10/Pma1 causes class I zymR (43), we investigated the relationship between the KTI10 and KTI6 genes. In common with class I zymocin resistance, both kti10 and kti6-2 cells survived the antibiotic hygromycin B, with protection being more pronounced in kti6-2 cells (Fig. 1F). However, while the kti10 H+-pump defect caused cell death at pH 3 (43), kti6-2 cells were pH insensitive (not shown). Studying pH effects on the zymocin response, kti6-2 cells remained zymR at pH 7 (Fig. 2A) and were fairly resistant to zymocin at pH 5 (Fig. 2A). In contrast, the zymR phenotype of kti10 cells was prominent at pH 7 but suppressed at pH 5 (Fig. 2A). At pH 4, a condition inactivating zymocin, all strains tested were insensitive, indicating that the pH effect on kti10 was not due to impaired viability (not shown).
FIG. 2.
KTI6 is epistatic to KTI10/PMA1. A and B. The pH modulation of zymocin protection associated with kti10 (ARB68), kti6-2 (ARB27), and kti6kti10 (DG106) mutants was compared to that of the wild type (wt; LL20) and a chs3Δ chitin mutant (DJY3) using YPD zymocin (45% [vol/vol]) plates (A) or killer eclipse (B) assays. Sensitivity to zymocin (zymS) is distinguished from partial protection (zymPR) and full resistance (zymR). C. Zymocin resistance associated with kti6 cells outcompetes the kti10 mutant. The indicated strains (see B) were subjected to killer toxin assays using no (−) zymocin or 60%(vol/vol) toxin complex (+). D. Zymocin resistance of kti6 cells is unaffected by high-copy KTI10/PMA1 or the kti10 suppressor ATS1. Zymocin responses of strains carrying AST1 and KTI10/PMA1 in multicopy or 2μm vector on its own were assessed as in B. E. kti6-2 cells suppress synthetic sickness of multicopy (2μ) AST1 or KTI10/PMA1. Indicated plasmids in KTI6 or kti6-2 cells were probed on YPD plates without (−) or with (+) 45% (vol/vol) zymocin.
Since low pH did not affect chitin-deficient (chs3Δ) or Tot− (tot3Δ) zymR mutants (Fig. 2A and not shown), but operated mildly on kti6-2 and severely on kti10 cells, KTI6 and KTI10 ought to operate after chitin docking and prior to Elongator. Consistent with KTI6-KTI10 epistasis, a double kti6kti10 mutant behaved like kti6-2 cells on their own, i.e., without showing low pH suppression (Fig. 2B). Similarly, using toxin assays towards increased zymocin doses, kti10 resistance became drastically weakened by 60% (vol/vol) zymocin while protection of kti6-2 cells or the kti6kti10 double mutant was unaffected (Fig. 2C). Also, neither the pma1/kti10 multicopy suppressor AST1 (3, 12) nor high-copy KTI10 itself (43) could reinstall zymS in kti6-2 cells (Fig. 2D). Genetic interaction was further evidenced by suppression of a kti6-2 mutation onto simultaneous expression of PMA1/KTI10 and AST1 from multicopy vectors. While in wild-type KTI6 cells, overexpression of both genes induced synthetic sickness (Fig. 2E) and hypersensitive zymocin phenotypes (not shown), kti6-2 cells completely restored cell viability and zymocin resistance (Fig. 2E). Collectively, order of function analysis suggests that KTI6 has a role in the toxin response that is likely to be upstream of KTI10 function.
KTI6 is allelic with IPT1.
To identify KTI6 we crossed zymR kti6-2 (MATα) cells to a preselected zymR pool of the BY4741 (MATa) knock-out collection. Diploids obtained were subjected to killer assays to identify noncomplementing crosses. We observed zymR in kti6-2/ipt1Δ YCC4 diploids (not shown; Table 1) and reconfirmed this cross by mating zymR kti6-3 (MATa) cells with zymR BY4742 (MATα) ipt1Δ cells. Again, the resulting kti6-3/ipt1Δ diploids (YCC8) expressed zymR (Fig. 3A), indicating failure of ipt1Δ to complement the kti6 mutations. Consistently, the zymR phenotype of haploid MATα or MATa ipt1Δ cells compared to kti6-3/ipt1Δ diploids (Fig. 3A and B), while haploid MATα IPT1 and MATa KTI6 cells behaved as zymS as homozygous KTI6/IPT1 (YCC5) or heterozygous KTI6/ipt1Δ (YCC7) and kti6-3/IPT1 (YCC6) diploids (Fig. 3A). So, complementation of kti6 mutations by IPT1 implies that kti6 represents a recessive IPT1 mutation.
In support of this, the kti6-2 defect was complemented by a plasmid-coupled IPT1 gene on pITP1 (Fig. 3B) (58). Similarly, a zymR ipt1W93STOP allele isolated in a screen for plant defensin DmAMP1 tolerance and shown to truncate the IPT1 product (60) was complemented by pIPT1, too (Fig. 3B).
Consistent with the properties of kti6 cells, intracellular γ-toxin expression blocked growth of ipt1Δ cells (Fig. 3C), reinforcing KTI6/IPT1 allelism. IPT1 encodes mannosyl-diinositolphospho-ceramide [M(IP)2C] synthase, the yeast terminal sphingolipid biosynthetic enzyme (14). In line with a plasma membrane function for M(IP)2C, ipt1Δ/syr4Δ cells survive syringomycin E, a membrane disrupting fungicidal (58). Consistently, five independent zymR kti6 alleles also resisted syringomycin E, whereas other zymR mutants (chs3Δ, kti10, tot3Δ and mutants) became growth-inhibited by syringomycin E (Fig. 3D and E). Again, this stresses KTI6/IPT1 allelism, a notion supported by 4:0 (zymR:zymS) segregation of spores obtained from a cross between ipt1Δ::kanMX and kti6-2 cells (not shown). Furthermore, as judged from DNA sequence analysis, we found Ipt1 allozymes with single amino acid substitutions in kti6-2 (G292R) and kti6-3 (H298N) cells and a frameshift mutation in kti6-5 that alters the Ipt1 C terminus at position F523FDPLA-Stop into F523LIRLHRL-Stop. In sum, M(IP)2C synthase Ipt1 or its product, sphingolipid M(IP)2C, is vital for the antifungal activity of zymocin.
K. lactis zymocicity requires sphingolipid biosynthesis and M(IP)2C.
Using phytosphingosine and a C26 fatty acid, yeast ceramide synthase (LAG1, LAC1) forms ceramide, the sphingolipid building block (Fig. 4) (54). C26 fatty acid elongation involves FEN1, SUR4, ACB1, and TSC13, while phytosphingosine formation requires condensation of palmitoyl-coenzyme A and serine to 3-ketohydrosphingosine by the LCB1, LCB2, and TSC3 products and SUR2-dependent hydroxylation (Fig. 4) (22, 24, 37, 45, 47). In yeast, ceramide decoration yields three complex sphingolipids. First, inositol-phosphoceramide (IPC) is made by addition of inositol phosphate via the AUR1 product (28). Second, mannosyl-inositol-phosphoceramide (MIPC) requires mannosylation by the CSG1, CSG2 (5, 6), and CSH1 products (63). Third, the IPT1 product adds inositol phosphate to give M(IP)2C (Fig. 4) (14).
FIG. 4.
Zymocin response profiles of sphingolipid defects. The simplified flow chart of sphingolipid biosynthesis is modified from previous reviews (13, 46). A to D. Zymocin responses of the indicated (see below) mutant, conditional, and wild-type (wt) strains were assessed on YPD zymocin plates (A and C) or in killer eclipse assays (B and D) (see Fig. 1A). Indicated are sensitive (zymS), partially protected (zymPR), and resistant (zymR) scenarios. The strains tested were as follows. A: wt (BY4741), sur2Δ (Y03656), sur4Δ (Y05281), acb1Δ (Y04667), and ipt1Δ (Y04007). B: lag1Δ (WBY283), lac1Δ (WBY286), and lag1Δlac1Δ(WBY616). C: ISC1 (BY4741 carrying pYES2), GAL1-ISC1 (BY4741 carrying pYES2-GAL1-ISC1 vector), and ipt1Δ (Y04007). D: csg1Δ (SUY05), csg2Δ (SUY06), csh1Δ (SUY41), csg1Δcsg2Δ (SUY07), csg2Δcsh1Δ (SUY43), csg1Δcsh1Δ (SUY42), and csg1Δcsg2Δcsh1Δ (SUY44).
While phytosphingosine (sur2Δ) or C26 fatty acid elongation defects (sur4Δ) yielded partial zymocin protection (Fig. 4A), a ceramide synthase double knockout (lag1Δ lac1Δ) expressed zymR comparable to that of ipt1Δ cells (Fig. 4B). Since AUR1 is essential, it was not possible to assay an IPC block, but upregulation of IPC breakdown by GAL1-driven overexpression of ISC1 coding for IPC phospholipase C (51) protected partially against zymocin (Fig. 4C). Multiple mutant csg1Δ csh1Δ and csg1Δcsh1Δcsg2Δ cells in which MIPC synthesis is blocked survived zymocin just like ipt1Δ cells (Fig. 4D), while single CSG1, CSG2 and CSH1 deletions and double mutants (csg1Δcsg2Δ, csh1Δcsg2Δ) remained zymS (Fig. 4D). Likewise, csg1Δcsh1Δ cells phenocopied resistance of ipt1Δ cells to hygromycin B, whereas the single mutants were killed by the antibiotic (Fig. 5A).
FIG. 5.
Hygromycin B tolerance of sphingolipid mutants and reverse transcription-PCR-based IPT1 expression analysis. A. As indicated, cells were grown on YPD (control) or hygromycin B (hyg B; 75 μg/ml) YPD plates for 2 days at 30°C. Sensitivity and resistance are indicated by hygS and hygR. B. Gene expression studies of zymocin-resistant sphingolipid mutants (for strains, see Fig. 4). Reverse transcription-PCR was carried out to probe for expression of the actin (ACT1, top panel) and the M(IP)2C synthase (IPT1/KTI6, bottom panel) genes.
As csg1Δcsh1Δ, lag1Δlac1Δ, and ipt1Δ cells all lack M(IP)2C and survive zymocin, toxicity depends on M(IP)2C rather than its synthase Ipt1 per se. Reverse transcription-PCR studies revealed that IPT1/KTI6 expression was, indeed, intact in wild-type, csg1Δcsh1Δ and lag1Δlac1Δ cells and, as expected, absent from ipt1Δ cells while all strains expressed ACT1, an internal control (Fig. 5B). In conclusion, profiling sphingolipid defects reinforces the importance of IPT1/KTI6 and the sphingolipid M(IP)2C in governing a plasma membrane event required for zymocin toxicity and whose block in kti6 cells abrogates zymocin action.
kti6 cells lack M(IP)2C and IPT1/KTI6 repression protects against zymocin.
The amount of M(IP)2C in kti6-2 cells was determined by long term labeling with [3H]myo-inositol. Following lipid extraction and separation by thin-layer chromatography, radiolabeled sphingolipids were detected and compared with sphingolipids in wild-type KTI6 cells and kti6-2 mutant cells transformed with pIPT1. kti6-2 cells (Fig. 6A, bottom panel) completely lacked the radioactive fraction representing alkali-stable M(IP)2C species typical of wild-type cells (Fig. 6A, top panel). Consistent with previous reports on M(IP)2C-minus strains (14), mutant kti6-2 cells apparetly compensated for the lack of M(IP)2C by up-regulating the level of MIPC (Fig. 6A, bottom panel).
FIG. 6.
kti6 cells lack M(IP)2C and a conditional IPT1/KTI6 allele modulates zymocin action. A. Extraction of 3H-labeled lipids. kti6-2 cells (ARB27) (lower panel), kti6-2 cells (ARB27) with pIPT1; (middle panel), KTI6 wild-type cells (LL20) (upper panel) and thin-layer chromatography were as described previously (14). Peak sphingolipid frac-tions IPC, MIPC, and M(IP)2C are shown. B. Conditional IPT1/KTI6 expression affects zymocin responses. Glucose repression (lower panel, right) of a GAL1-IPT1 allele (DG107) induces resistance (zymR), and galactose induction (upper panel) increases sensitivity (zymHS). Other strains: ipt1Δ (DG108) and IPT1 (LL20).
Notably, introduction of pIPT1 into kti6-2 cells restored M(IP)2C and down-regulated MIPC (Fig. 6A, middle panel). We conclude from these data that kti6-2 cells fail to make M(IP2)C just like ipt1Δ cells. These results are consistent with the phenotypic assays (Fig. 3 and Fig. 4) and reinforce that KTI6 and IPT1 are allelic. In line with the importance of M(IP)2C for zymocin toxicity was the effect of conditional KTI6/IPT1 expression. Here, a GAL1-IPT1 fusion was used for controlled Ipt1 expression. Glucose repression of the conditional allele yielded zymR and phenocopied ipt1Δ cells lacking M(IP)2C (Fig. 6B), while galactose induced hypersensitivity (Fig. 6B). Thus, the level of IPT1 expression is inversely correlated with the zymocin response. Again, this shows the importance of Ipt1-mediated formation of M(IP)2C for zymocin action.
kti6 cells allow zymocin to bind but prevent import of γ-toxin.
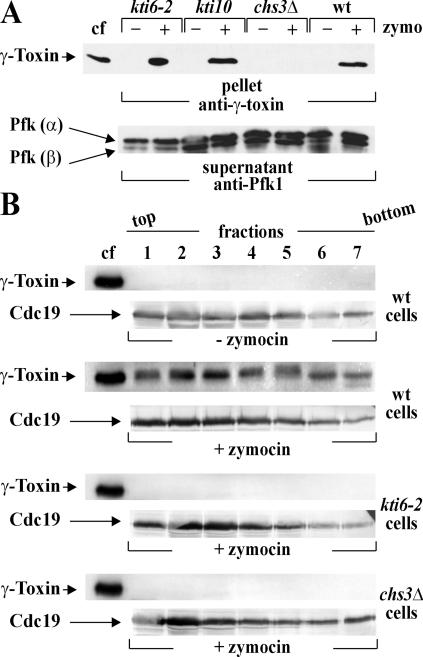
Taking into account that kti6-2 cells resist exozymocin without protecting against endogenous γ-toxin, we probed for a kti6-2 defect in the primary contact with holozymocin. Together with other class I (chs3Δ and kti10) mutants, protein extracts of zymocin-treated kti6-2 and wild-type cells were subdivided into soluble cytosolic and insoluble cell wall fractions. Following immunoprobing with anti-γ-toxin antibody, γ-toxin was absent from cell wall fractions of chitin-deficient chs3Δ cells (Fig. 7A), consistent with the known binding of holozymocin to chitin in vitro (33). Equivalent cell wall fractions from wild-type, kti10 and kti6-2 cells, however, produced reactive anti-γ-toxin signals that corresponded to a γ-toxin control from culture filtrates of K. lactis killer cells (Fig. 7A). Hence, holozymocin is able to dock onto the cell wall of kti6 cells in a manner similar to sensitive wild-type or resistant Tot− and kti10/pma1 mutant cells. Moreover, indistinguishably from what occurs with wild-type sensitive or resistant kti10/pma1 cell walls, holozymocin is able to dock onto the surface of kti6-2 cells. This strongly suggests that the sphingolipid M(IP)2C is required after zymocin has attached to its chitin receptor.
FIG. 7.
Zymocin docking onto kti6 cells is unaffected while γ-toxin import is denied. A. Zymocin docking studies. Protein extracts from zymocin-treated (+) or nontreated (−) cells of the wild-type (wt; LL20) and resistant kti10 (ARB68), chs3Δ (DJY3) or kti6-2 (ARB27) strains were fractionated into soluble (supernatant) and insoluble cell wall/debris (pellet) material. Following SDS-PAGE, supernatants were immunoprobed by anti-Pfk1 antibody (arrows indicate α and β subunits of Pfk1), while pelleted cell wall material was immunoprobed by an anti-γ-toxin antibody. Concentrated culture filtrate (cf) of the zymocin producer K. lactis strain AWJ137 served as a positive γ-toxin control. B. γ-Toxin import studies. Following sucrose gradient ultracentrifugation, cell fractions obtained from wild-type (LL20) cells or kti6-2 (ARB27) and chs3Δ (DJY3) mutants that were treated (+) or not treated (−) with zymocin were separated by SDS-PAGE and subjected to Western analysis using anti-γ-toxin and anti-Cdc19 antibodies. Concentrated killer culture filtrate (cf) served as a positive γ-toxin reference (see A). Arrows indicate the positions of γ-toxin and pyruvate kinase Cdc19.
To ask whether M(IP)2C-minus kti6-2 cells survive zymocin as a result of reduced γ-toxin uptake, equal amounts of protein extracts from zymocin-treated kti6-2 and reference cells were subjected to cell fractionation and Western blots using anti-γ-toxin and anti-Cdc19 antibodies. As expected, we found that in the absence of zymocin, γ-toxin was not detectable in wild-type cells (Fig. 7B), while zymocin-treated cells significantly accumulated the toxic γ-subunit intracellularly (Fig. 7B). In contrast, however, γ-toxin import by M(IP)2C-minus kti6-2 cells was completely absent and copied the behavior of chitin-deficient chs3Δ cells (Fig. 7A), which are unable to establish the primary contact with holozymocin (33, 43) (Fig. 7A). Given that kti6-2 cells allow zymocin contact, while endogenous γ-toxin induces kti6-2 cell death (Fig. 1B), nondetectable γ-toxin levels suggest an import or uptake defect of kti6-2 cells. Whether this reflects a role for sphingolipid M(IP)2C in γ-toxin binding to or translocation across the membrane is currently being investigated.
DISCUSSION
Zymocin resistance of kti6 cells.
Based on chitin affinity purification of zymocin and the refractory nature of chitin-deficient chs3Δ cells, zymocin's primary cell contact requires chitin interaction (33). Searching for nonchitin factors required for zymocin binding and/or uptake, kti6 cells are shown here to survive exozymocin but to be killed by a chitin poison (Calcofluor White) or intracellular γ-toxin expression. These data indicate a response defect distinct from Elongator or chitin mutants and are consistent with our findings (Fig. 7) that γ-toxin binds kti6 cells with normal efficiency, but does not enter their cytosol. Based on heat shock sensitivity and nystatin resistance, phenotypes shared by kti6 and kti10 plasma membrane H+-ATPase mutants (50), KTI6 is possibly involved in membrane function. In support of this idea, resistance to zymocin and antifungals (hygromycin B, syringomycin E, and defensin DmAMP1) that require and alter membrane function and permeability suggests that kti6 cells deny the docking or uptake of antimycotics (15, 58, 60). This reinforces a plasma membrane role of KTI6 and its function for antizymosis.
Consistently, kti6 cells are complemented by the plasma membrane sphingolipid M(IP)2C synthase gene IPT1, fail to form M(IP)2C, and accumulate precursor sphingolipid MIPC. Together with our data that removal, truncation, or underproduction of Ipt1 phenocopies zymocin protection, while IPT1 overexpression enhances zymocin toxicity, it is clear that M(IP)2C is an integral zymocin sensitivity determinant. Yeast sphingolipids primarily populate the plasma membrane, with M(IP)2C representing the major fraction (27). They associate in lipid rafts (30) with proteins such as major H+-pump Pma1/Kti10 (2, 3). Based on reports that sphingolipids influence lipid raft protein content, M(IP)2C-minus kti6 cells may impact the H+-pump Pma1/Kti10 and thus evoke zymocin resistance similar to kti10/pma1 mutants (43).
Indeed, our finding that zymR of kti10 cells, in contrast to kti6 single and kti6kti10 double mutants, is suppressed by H+ suggests that KTI6 functions before KTI10. Also, the fact that kti6-2 cells suppress synthetic sickness associated with cooverexpression of Pma1/Kti10 and Ast1, a plasma membrane protein and suppressor of defective Pma1 H+-pumps (3, 12), shows that M(IP)2C sphingolipids are required to mediate membrane associated communication between Ast1 and Pma1/Kti10 that impairs cell performance and viability. However, green fluorescent protein studies indicated normal Pma1/Kti10 distribution in ipt1/kti6 membranes (4), ruling out the option that Kti6 acts solely by Kti10 localization.
Role of M(IP)2C in zymocin action.
Consistent with a role for sphingolipid biosynthesis in the zymocin response pathway, ceramide synthase lag1Δlac1Δ double knockouts and csh1Δ csg1Δcsg2Δ mutants with reduced MIPC and M(IP)2C levels are shown here to be zymocin resistant (54, 63). As a major constituent of lipid rafts, M(IP)2C may influence raft-embedded proteins such as End6, Pma1/Kti10, or Ggp1 (2, 4). Indeed, cell surface defects of a ggp1Δ mutant elicited hypersensitivity to zymocin, while kti10/pma1 cells were shown to resist the toxic complex in a class I fashion (33, 43). Based on the tolerance of kti6 and kti10 cells to antifungals that require membrane energization by the H+ pump Pma1/Kti10, there may be raft communication between M(IP)2C and Pma1/Kti10. Consistently, synthesis of sphingoid base and ceramide is required for Pma1/Kti10 function and exogenously applied phytosphingosine does rescue Pma1/Kti10 defects (39, 64).
Based on our findings that kti6 protection against zymocin is barely affected by low pH in contrast to the pH-suppressible resistance of kti10, H+-ATPase is likely to follow M(IP)2C function in promoting zymocin sensitivity. In spite of intact zymocin docking, lack of M(IP)2C in kti6 cells may prevent zymocin from binding to a secondary receptor in the plasma membrane required for γ-toxin uptake. Whether M(IP)2C allows contact between γ-toxin and such a receptor or represents this receptor, as speculated for toxicity of syringomycin E (58) and demonstrated for several plant defensins (60, 61), remains open. However, the zymocin-relevant function of M(IP)2C cannot be replaced by its precursor sphingolipids MIPC or IPC, and this specificity may relate to the terminal myo-inositol phosphate moiety that distinguishes M(IP)2C from MIPC and IPC. A M(IP)2C-dependent membrane protein may serve as a second receptor, and although recently ruled out for lethality of plant defensin DmAMP1 (61), this scenario applies to yeast killer toxin K1. This ionophore hijacks, in a two-step-docking process, β-1,6-d-glucan and the glycosylphosphatidylinositol (GPI)-anchored protein Kre1 as primary cell surface and secondary membrane receptors, respectively (7, 29). Similarly, aerolysin, an Aeromonas hydrophilia toxin, binds GPI-anchored membrane proteins prior to multimerization (20).
Finally, as membrane traffic and endocytosis are impacted by lipid rafts and sphingoid bases, M(IP)2C may rather be involved in internalization of γ-toxin (30, 66). Intriguingly, the phenotypes associated with loss of END6 are suppressed by mutations in IPT1, suggesting that M(IP)2C relates to the performance of End6, a lipid raft protein with roles in endocytosis and actin polarization (4, 44). Although green fluorescent protein studies failed to demonstrate mislocalization of End6 in ipt1Δ membranes, biogenesis and sorting of lipid raft proteins do require sphingoid base synthesis (4). M(IP)2C-minus kti6/ipt1 cells may nonetheless impair compartmentalization of membrane proteins to lipid rafts. Indeed, cell surface clustering of lipid rafts at the tips of mating projections has been shown to correlate with a 50% increase of raft-associated M(IP)2C content in response to pheromones (1). Whether kti6/ipt1 cells have mating defects comparable to those of other lipid biosynthetic mutants (1) will be important to know; also important will be studying whether sphingolipid mutants interfere with endocytosis of proteins other than γ-toxin and whether this involves membrane docking or internalization steps.
Acknowledgments
We thank K. Thevissen (Ghent, Belgium), J. Heinisch (Osnabrück, Germany), A. Kihara, (Sapporo, Japan), J. Takemoto (Logan, Utah), H. Riezman (Geneva, Switzerland), A. Chang (Ann Arbor, Michigan), Y. Hannun (Charleston, South Carolina), and J. Thorner (Berkeley, CA) for providing biologicals and antifungals. We thank D. Gündel for help.
We thank FEBS for a fellowship to C.M. H.K.K. acknowledges receipt of a Grant-in-Aid for Scientific Research (Ministry of Education, Culture, Sports, Science and Technology of Japan). R.S. was supported by a Deutsche Forschungsgemeinschaft grant (Scha 750/2).
REFERENCES
- 1.Bagnat, M., and K. Simons. 2002. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA 99:14183-14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagnat, M., S. Keranen, A. Shevchenko, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnat, M., A. Chang, and K. Simons. 2001. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell 12:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balguerie, A., M. Bagnat, M. Bonneu, M. Aigle, and A. M. Breton. 2002. Rvs161p and sphingolipids are required for actin repolarization following salt stress. Eukaryot. Cell 1:1021-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeler, T., K. Gable, C. Zhao, and T. Dunn. 1994. A novel protein, Csg2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J. Biol. Chem. 269:7279-7284. [PubMed] [Google Scholar]
- 6.Beeler, T. J., D. Fu, J. Rivera, E. Monaghan, K. Gable, and T. M. Dunn. 1997. SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37 degrees C, is required for mannosylation of inositolphosphorylceramide. Mol. Gen. Genet. 255:570-579. [DOI] [PubMed] [Google Scholar]
- 7.Breinig, F., D. J. Tipper, and M. J. Schmitt. 2002. Kre1p, the plasma membrane receptor for the yeast K1 viral toxin. Cell 108:395-405. [DOI] [PubMed] [Google Scholar]
- 8.Butler, A. R., J. H. White, and M. J. R. Stark. 1991. Analysis of the response of Saccharomyces cerevisiae cells to Kluyveromyces lactis toxin. J. Gen. Microbiol. 137:1749-1757. [DOI] [PubMed] [Google Scholar]
- 9.Butler, A. R., M. Porter, and M. J. R. Stark. 1991. Intracellular expression of Kluyveromyces lactis toxin gamma subunit mimics treatment with exogenous toxin and distinguishes two classes of toxin-resistant mutant. Yeast 7:617-625. [DOI] [PubMed] [Google Scholar]
- 10.Butler, A. R., R. W. O'Donnell, V. J. Martin, G. W. Gooday, and M. J. R. Stark. 1991. Kluyveromyces lactis toxin has an essential chitinase activity. Eur. J. Biochem. 199:483-488. [DOI] [PubMed] [Google Scholar]
- 11.Butler, A. R., J. H. White, Y. Folawiyo, A. Edlin, D. Gardiner, and M. J. R. Stark. 1994. Two Saccharomyces cerevisiae genes which control sensitivity. to G1 arrest induced by Kluyveromyces lactis toxin. Mol. Cell. Biol. 14:6306-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, A., and G. R. Fink. 1995. Targeting of the yeast plasma membrane [H+]ATPase: a novel gene AST1 prevents mislocalization of mutant ATPase to the vacuole. J. Cell Biol. 128:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson, R. C., and R. L. Lester. 2002. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1583:13-25. [DOI] [PubMed] [Google Scholar]
- 14.Dickson, R. C., E. E. Nagiec, G. B. Wells, M. M. Nagiec, and R. L. Lester. 1997. Synthesis of mannose-(inositol-P)2-ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene. J. Biol. Chem. 272:29620-29625. [DOI] [PubMed] [Google Scholar]
- 15.Feigin, A. M., J. Y. Takemoto, R. Wangspa, J. H. Teeter, and J. G. Brand. 1996. Properties of voltage-gated ion channels formed by syringomycin E in planar lipid bilayers. J. Membr. Biol. 149:41-47. [DOI] [PubMed] [Google Scholar]
- 16.Fichtner, L., and R. Schaffrath. 2002. KTI11 and KTI13, Saccharomyces cerevisiae genes controlling sensitivity to G1 arrest induced by Kluyveromyces lactis zymocin. Mol. Microbiol. 44:865-876. [DOI] [PubMed] [Google Scholar]
- 17.Fichtner, L., F. Frohloff, K. Bürkner, M. Larsen, K. D. Breunig, and R. Schaffrath. 2002. Molecular analysis of KTI12/TOT4, a Saccharomyces cerevisiae gene required for Kluyveromyces lactis zymocin action. Mol. Microbiol. 43:783-791. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner, L., D. Jablonowski, A. Schierhorn, H. K. Kitamoto, M. J. R. Stark, and R. Schaffrath. 2003. Elongator's toxin-target (TOT) function is NLS-dependent and suppressed by post-translational modification. Mol. Microbiol. 49:1297-1307. [DOI] [PubMed] [Google Scholar]
- 19.Frohloff, F., L. Fichtner, D. Jablonowski, K. D. Breunig, and R. Schaffrath. 2001. Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J. 20:1993-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukushima, K., Y. Ikehara, M. Kanai, N. Kochibe, M. Kuroki, and K. Yamashita. 2003. A beta-N-acetylglucosaminyl phosphate diester residue is attached to the glycosylphosphatidylinositol anchor of human placental alkaline phosphatase: a target of the channel-forming toxin aerolysin. J. Biol. Chem. 278:36296-36303. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa, K., N. Mizushima, T. Noda, and Y. Ohsumi. 2000. A protein conjugation system in yeast with homology to biosynthetic enzyme reaction of prokaryotes. J. Biol. Chem. 275:7462-7465. [DOI] [PubMed] [Google Scholar]
- 22.Gable, K., H. Slife, D. Bacikova, E. Monaghan, and T. M. Dunn. 1997. Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. J. Biol. Chem. 275:7597-7603. [DOI] [PubMed] [Google Scholar]
- 23.Goehring, A. S., D. M. Rivers, and G. F. Sprague. 2003. Urmylation: a ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol. Biol. Cell 14:4329-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grilley, M. M., S. D. Stock, R. C. Dickson, R. L. Lester, and J. Y. Takemoto. 1998. Syringomycin action gene SYR2 is essential for sphingolipid 4-hydroxylation in Saccharomyces cerevisiae. J. Biol. Chem. 273:11062-11068. [DOI] [PubMed] [Google Scholar]
- 25.Gunge, N., A. Tamaru, F. Ozawa, and K. Sakaguchi. 1981. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J. Bacteriol. 145:382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampsey, M. 1997. A review of phenotypes in Saccharomyces cerevisiae. Yeast 13:1099-1133. [DOI] [PubMed] [Google Scholar]
- 27.Hechtberger, P., E. Zinser, R. Saf, K. Hummel, F. Paltauf, and G. Daum. 1994. Characterization, quantification and subcellular localization of inositol-containing sphingolipids of the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 225:641-649. [DOI] [PubMed] [Google Scholar]
- 28.Heidler, S. A., and J. A. Radding. 1995. The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrob. Agents Chemother. 39:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchins, K., and H. Bussey. 1983. Cell wall receptor for yeast killer toxin: involvement of (1→6)-β-d-glucan. J. Bacteriol. 154:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470-477. [DOI] [PubMed] [Google Scholar]
- 31.Jablonowski, D., F. Frohloff, L. Fichtner, M. J. R. Stark, and R. Schaffrath.2001. Kluyveromyces lactis zymocin mode of action is linked to RNA polymerase II function via Elongator. Mol. Microbiol. 42:1095-1105. [DOI] [PubMed] [Google Scholar]
- 32.Jablonowski, D., A. R. Butler, L. Fichtner, D. Gardiner, R. Schaffrath, and M. J. R. Stark. 2001. Sit4p protein phosphatase is required for sensitivity of Saccharomyces cerevisiae to Kluyveromyces lactis zymocin. Genetics 159:1479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jablonowski, D., L. Fichtner, V. J. Martin, R. Klassen, F. Meinhardt, M. J. R. Stark, and R. Schaffrath. 2001. Saccharomyces cerevisiae cell wall chitin, the Kluyveromyces lactis zymocin receptor. Yeast 18:1285-1299. [DOI] [PubMed] [Google Scholar]
- 34.Jablonowski, D., L. Fichtner, M. J. R. Stark, and R. Schaffrath. 2004. The yeast Elongator histone acetylase requires Sit4-dependent dephosphorylation for toxin target capacity. Mol. Biol. Cell 15:1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerridge, D. 1986. Mode of action of clinically important antifungal drugs. Adv. Microb. Physiol. 27:1-72. [DOI] [PubMed] [Google Scholar]
- 36.Kishida, M., M. Tokunaga, Y. Katayose, H. Yajima, A. Kawamura-Watabe, and F. Hishinuma. 1996. Isolation and genetic characterization of pGKL killer-insensitive mutants (iki) from Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 60:98-801. [DOI] [PubMed] [Google Scholar]
- 37.Kohlwein, S. D., S. Eder, C. S. Oh, C. E. Martin, K. Gable, D. Bacikova, and T. Dunn. 2001. Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear-vacuolar interface in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:109-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kölling, R., and C. P. Hollenberg. 1994. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J. 13:3261-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, M. C., S. Hamamoto, and R. Schekman. 2002. Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J. Biol. Chem. 277:22395-22401. [DOI] [PubMed] [Google Scholar]
- 40.Liu, S., and S. H. Leppla. 2003. Retroviral insertional mutagenesis identifies a small protein required for synthesis of diphthamide, the target of bacterial ADP-ribosylating toxins. Mol. Cell 12:603-613. [DOI] [PubMed] [Google Scholar]
- 41.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 42.Mehlgarten, C., and R. Schaffrath. 2003. Mutant casein kinase I (Hrr25p/Kti14p) abrogates the G1 cell cycle arrest induced by Kluyveromyces lactis zymocin in budding yeast. Mol. Genet. Genomics 269:188-196. [DOI] [PubMed] [Google Scholar]
- 43.Mehlgarten, C., and R. Schaffrath. 2004. After chitin docking, Kluyveromyces lactis zymocin requires Saccharomyces cerevisiae H+-ATPase Pma1. Cell. Microbiol. 6:569-580. [DOI] [PubMed] [Google Scholar]
- 44.Munn, A. L., B. J. Stevenson, I. Geli, and H. Riezman. 1995. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 6:1721-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagiec, M. M., J. A. Altisberger, G. B. Ells, R. L. Lester, and R. C. Dickson. 1994. The LCB2 gene of Saccharomyces cerevisiae and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 91:899-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obeid, L. M., Y. Okamoto, and C. Mao. 2002. Yeast sphingolipids: metabolism and biology. Biochim. Biophys. Acta 1585:163-171. [DOI] [PubMed] [Google Scholar]
- 47.Oh, C. S., D. A. Toke, S. Mandala, and C. E. Martin. 1997. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J. Biol. Chem. 272:17376-17384. [DOI] [PubMed] [Google Scholar]
- 48.Otero, G., J. Fellows, Y. Li, T. de Bizemont, A. M. Dirac, C. M. Gustafsson, H. Erdjument-Bromage, P. Tempst, and, J. Q. Svejstrup. 1999. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3:109-118. [DOI] [PubMed] [Google Scholar]
- 49.Pereira, G., M. Knop, and E. Schiebel. 1998. Spc98p directs the yeast γ-tubulin complex into the nucleus and is subject to cell cycle-dependent phosphorylation on the nuclear side of the spindle pole body. Mol. Biol. Cell 9:775-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perlin, D. S., S. L. Harris, D. Seto-Young, and J. E. Haber. 1989. Defective H+-ATPase of hygromycin B-resistant pma1 mutants from Saccharomyces cerevisiae. J. Biol. Chem. 264:21857-21864. [PubMed] [Google Scholar]
- 51.Sawai, H., Y. Okamoto, C. Luberto, C. Mao, A. Bielawska, N. Domae, and Y. A. Hannun. 2000. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J. Biol. Chem. 275:39793-39798. [DOI] [PubMed] [Google Scholar]
- 52.Schaffrath, R., and K. D. Breunig. 2000. Genetics and molecular physiology of the yeast Kluyveromyces lactis. Fungal Genet. Biol. 30:173-190. [DOI] [PubMed] [Google Scholar]
- 53.Schaffrath, R., and F. Meinhardt. 2005. Kluyveromyces lactis zymocin and other plasmid-encoded yeast killer toxins, p. 133-155. In M. Schmitt and R. Schaffrath (ed.), Microbial protein toxins. Springer Verlag, Berlin, Germany.
- 54.Schorling, S., B. Vallee, W. P. Barz, H. Riezman, and D. Oesterhelt. 2001. Lag1p and Lac1p are essential for the Acyl-CoA-dependent ceramide synthase reaction in Saccharomyces cerevisiae. Mol. Biol. Cell 12:3417-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 56.Stark, M. J., and A. Boyd. 1986. The killer toxin of Kluyveromyces lactis: characterization of the toxin subunits and identification of the genes which encode them. EMBO J. 5:1995-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stark, M. J., A. Boyd, A. J. Mileham, and M. A. Romanos. 1990. The plasmid-encoded killer system of Kluyveromyces lactis: a review. Yeast 6:1-29. [DOI] [PubMed] [Google Scholar]
- 58.Stock, S. D., H. Hama, J. A. Radding, D. A. Young, and J. Y. Takemoto. 2000. Syringomycin E inhibition of Saccharomyces cerevisiae: requirement for biosynthesis of sphingolipids with very-long-chain fatty acids and mannose- and phosphoinositol-containing head groups. Antimicrob. Agents Chemother. 44:1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugisaki, Y., N. Gunge, K. Sakaguchi, M. Yamasaki, and G. Tamura. 1984. Characterization of a novel killer toxin encoded by a double-strand linear DNA plasmid of Kluyveromyces lactis. Eur. J. Biochem. 141:242-245. [DOI] [PubMed] [Google Scholar]
- 60.Thevissen, K., B. P. Cammue, K. Lemaire, J. Winderickx, R. C. Dickson, R. L. Lester, K. K. Ferket, F. Van Even, A. H. Parret, and W. F. Broekaert. 2000. A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from Dahlia merckii. Proc. Natl. Acad. Sci. USA 97:9531-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thevissen, K., I. E. Francois, J. Y. Takemoto, K. K. Ferket, E. M. Meert, and B. P. Cammue. 2003. DmAMP1, an antifungal plant defensin from Dahlia merckii interacts with sphingolipids from Saccharomyces cerevisiae. FEMS Microbiol. Lett. 226:169-173. [DOI] [PubMed] [Google Scholar]
- 62.Tokunaga, M., A. Kawamura, and F. Hishinuma. 1989. Expression of pGKL killer 28K subunit in Saccharomyces cerevisiae: identification of 28K subunit as a killer protein. Nucleic Acids Res. 17:3435-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uemura, S., A. Kihara, J. Inokuchi, and Y. Igarashi. 2003. Csg1p and newly identified Csh1p function in mannosylinositol phosphorylceramide synthesis by interacting with Csg2p. J. Biol. Chem. 278:45049-45055. [DOI] [PubMed] [Google Scholar]
- 64.Wang, Q., and A. Chang. 2002. Sphingoid base synthesis is required for oligomerization and cell surface stability of the yeast plasma membrane ATPase, Pma1. Proc. Natl. Acad. Sci. USA 99:12853-12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkler, G. S., T. G. Petrakis, S. Ethelberg, M. Tokunaga, H. Erdjument-Bromage, P. Tempst, and J. Q. Svejstrup. 2001. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J. Biol. Chem. 276:32743-32749. [DOI] [PubMed] [Google Scholar]
- 66.Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B. J. Stevenson, and H. Riezman. 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19:2824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]