Abstract
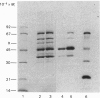
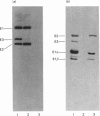
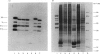
The production of high-titre monospecific polyclonal antibodies against the purified pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase multienzyme complexes from ox heart is described. The specificity of these antisera and their precise reactivities with the individual components of the complexes were examined by immunoblotting techniques. All the subunits of the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes were strongly antigenic, with the exception of the common lipoamide dehydrogenase component (E3). The titre of antibodies raised against E3 was, in both cases, less than 2% of that of the other subunits. Specific immunoprecipitation of the dissociated N-[3H]ethylmaleimide-labelled enzymes also revealed that E3 alone was absent from the final immune complexes. Strong cross-reactivity with the enzyme present in rat liver (BRL) and ox kidney (NBL-1) cell lines was observed when the antibody against ox heart pyruvate dehydrogenase was utilized to challenge crude subcellular extracts. The immunoblotting patterns again lacked the lipoamide dehydrogenase band, also revealing differences in the apparent Mr of the lipoate acetyltransferase subunit (E2) from ox kidney and rat liver. The additional 50 000-Mr polypeptide, previously found to be associated with the pyruvate dehydrogenase complex, was apparently not a proteolytic fragment of E2 or E3, since it could be detected as a normal component in boiled sodium dodecyl sulphate extracts of whole cells. The low immunogenicity of the lipoamide dehydrogenase polypeptide may be attributed to a high degree of conservation of its primary sequence and hence tertiary structure during evolution.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Ching E. Biogenesis of mitochondrial proteins in HeLa cells. Methods Enzymol. 1979;56:66–79. doi: 10.1016/0076-6879(79)56010-8. [DOI] [PubMed] [Google Scholar]
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bleile D. M., Hackert M. L., Pettit F. H., Reed L. J. Subunit structure of dihydrolipoyl transacetylase component of pyruvate dehydrogenase complex from bovine heart. J Biol Chem. 1981 Jan 10;256(1):514–519. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. G., Lawson R., Yeaman S. J. Multi-site phosphorylation of bovine kidney branched-chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1983 Jun 27;157(1):59–62. doi: 10.1016/0014-5793(83)81116-8. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Hamada M., Otsuka K. I., Tanaka N., Ogasahara K., Koike K. Purification properties and subunit composition of pig heart lipoate acetyltransferase. J Biochem. 1975 Jul;78(1):187–197. [PubMed] [Google Scholar]
- Hu C. W., Utter M. F., Patel M. S. Induction of pyruvate dehydrogenase in 3T3-L1 cells during differentiation. J Biol Chem. 1983 Feb 25;258(4):2315–2320. [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Koike M., Koike K. Structure, assembly and function of mammalian alpha-keto acid dehydrogenase complexes. Adv Biophys. 1976:187–227. [PubMed] [Google Scholar]
- Kresze G. B., Dietl B., Ronft H. Mammalian lipoate acetyltransferase: molecular weight determination by gel filtration in the presence of guanidinium chloride. FEBS Lett. 1980 Mar 24;112(1):48–50. doi: 10.1016/0014-5793(80)80124-4. [DOI] [PubMed] [Google Scholar]
- Kresze G. B., Ronft H., Dietl B., Steber L. Limited proteolysis of 2-oxoglutarate dehydrogenase multienzyme complex from bovine kidney. FEBS Lett. 1981 May 5;127(1):157–160. doi: 10.1016/0014-5793(81)80364-x. [DOI] [PubMed] [Google Scholar]
- Kresze G. B., Steber L. Inactivation and disassembly of the pyruvate dehydrogenase multienzyme complex from bovine kidney by limited proteolysis with an enzyme from rat liver. Eur J Biochem. 1979 Apr;95(3):569–578. doi: 10.1111/j.1432-1033.1979.tb12998.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson R., Cook K. G., Yeaman S. J. Rapid purification of bovine kidney branched-chain 2-oxoacid dehydrogenase complex containing endogenous kinase activity. FEBS Lett. 1983 Jun 27;157(1):54–58. doi: 10.1016/0014-5793(83)81115-6. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C. Studies on the apparent instability of bovine kidney alpha-ketoglutarate dehydrogenase complex. Arch Biochem Biophys. 1974 Apr 2;161(2):505–514. doi: 10.1016/0003-9861(74)90333-6. [DOI] [PubMed] [Google Scholar]
- Lynen A., Sedlaczek E., Wieland O. H. Partial purification and characterization of a pyruvate dehydrogenase-complex-inactivating enzyme from rat liver. Biochem J. 1978 Feb 1;169(2):321–328. doi: 10.1042/bj1690321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machicao F., Wieland O. H. Subunit structure of dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex from bovine kidney. Hoppe Seylers Z Physiol Chem. 1980 Jul;361(7):1093–1106. doi: 10.1515/bchm2.1980.361.2.1093. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Matuda S., Shirahama T., Saheki T., Miura S., Mori M. Purification and immunochemical studies of pyruvate dehydrogenase complex from rat heart, and cell-free synthesis of lipoamide dehydrogenase, a component of the complex. Biochim Biophys Acta. 1983 Oct 13;741(1):86–93. doi: 10.1016/0167-4781(83)90013-1. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Phosphorylation-dephosphorylation cycles and the regulation of fuel selection in mammals. Curr Top Cell Regul. 1981;18:107–129. doi: 10.1016/b978-0-12-152818-8.50013-x. [DOI] [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. Structure-function relationships in pyruvate and alpha-ketoglutarate dehydrogenase complexes. Adv Exp Med Biol. 1982;148:231–241. doi: 10.1007/978-1-4615-9281-5_19. [DOI] [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Stanley C. J., Perham R. N. Purification of 2-oxo acid dehydrogenase multienzyme complexes from ox heart by a new method. Biochem J. 1980 Oct 1;191(1):147–154. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp L. R., Pettit F. H., Yeaman S. J., Reed L. J. Purification and properties of pyruvate dehydrogenase kinase from bovine kidney. J Biol Chem. 1983 Aug 10;258(15):9454–9458. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]