Abstract
Mental disorders, such as schizophrenia or Alzheimer’s disease, are associated with impaired synaptogenesis and/or synaptic communication. During development, neurons assemble into neuronal networks, the primary supracellular mediators of information processing. In addition to the orchestrated activation of genetic programs, spontaneous electrical activity and associated calcium signaling have been shown to be critically involved in the maturation of such neuronal networks. We established an in vitro model that recapitulates the maturation of neuronal networks, including spontaneous electrical activity. Upon plating, mouse primary hippocampal neurons grow neurites and interconnect via synapses to form a dish-wide neuronal network. Via live cell calcium imaging, we identified a limited period of time in which the spontaneous activity synchronizes across neurons, indicative of the formation of a functional network. After establishment of network activity, the neurons grow dendritic spines, the density of which was used as a morphological readout for neuronal maturity and connectivity. Hence, quantification of neurite outgrowth, synapse density, spontaneous neuronal activity, and dendritic spine density allowed to study neuronal network maturation from the day of plating until the presence of mature neuronal networks. Via acute pharmacological intervention, we show that synchronized network activity is mediated by the NMDA-R. The balance between kynurenic and quinolinic acid, both neuro-active intermediates in the tryptophan/kynurenine pathway, was shown to be decisive for the maintenance of network activity. Chronic modulation of the neurotrophic support influenced the network formation and revealed the extreme sensitivity of calcium imaging to detect subtle alterations in neuronal physiology. Given the reproducible cultivation in a 96-well setup in combination with fully automated analysis of the calcium recordings, this approach can be used to build a high-content screening assay usable for neurotoxicity screening, target identification/validation, or phenotypic drug screening.
Electronic supplementary material
The online version of this article (doi:10.1007/s10571-014-0057-6) contains supplementary material, which is available to authorized users.
Keywords: Neuronal network activity, Calcium imaging, Primary hippocampal neurons, Dendritic spines, NMDA receptor, Kynurenine pathway
Introduction
Current theories on brain function emphasize the significance of functional networks underlying cognitive functions such as learning and memory. During fetal development and early postnatal life, newborn neurons interconnect via synaptic contacts to form functional neuronal networks. The sporadic electrical activity exhibited by these neurons synchronizes among interconnected neurons and gives rise to robust neuronal network activity (Cohen et al. 2008). Synchronized oscillatory activity has been described in intact cortex, hippocampus, retina, spinal cord, cerebellum, and brainstem of rodents and humans and is thought to be genuinely intrinsic (Ben-Ari 2001; Katz and Shatz 1996; Khazipov and Luhmann 2006; Zhang and Poo 2001). Such network activity is thought to facilitate the fine-tuning of synapses and the formation of dendritic spines (Ben-Ari 2001; Mohajerani et al. 2007).
Cumulative evidence indicates that schizophrenia, a neurodevelopmental disorder, is associated with impaired neuronal maturation, leading to altered connectivity within and between neuronal networks (Balu and Coyle 2011; Melom and Littleton 2011; Stephan et al. 2006). Many of the schizophrenia risk genes exert important functions during the development of the central nervous system (CNS) (Bradshaw and Porteous 2012; Hayashi-Takagi and Sawa 2010), and early-life environmental insults such as maternal immune activation can induce or exacerbate schizophrenia-like symptoms in the offspring (Kneeland and Fatemi 2013; Hida et al. 2013). As such, genetic predisposition in combination with early-life environmental factors are thought to impair neuronal maturation and connectivity, leading to schizophrenia symptoms that emerge later in life. In addition to neurodevelopmental diseases, neurodegenerative disorders like Alzheimer’s disease are also associated with impaired functional connectivity between neurons and neuronal networks (Bokde et al. 2009). Even before neurofibrillary tangle formation and gross neuronal degeneration, soluble amyloid beta oligomers can impair synaptic connectivity and plasticity, leading to mild cognitive impairment (Sivanesan et al. 2013). Current therapies are most often insufficient to relieve all symptoms and modify the disease progression, and only a small number of patients respond to these treatments. Consequently, continuous efforts are made to unravel the etiology and find new disease modifying treatments. The validation of an in vitro application that allows both fundamental research and drug screening should improve our understanding and the treatment of these diseases. Furthermore, this in vitro model should ideally be relevant to study neuronal network maturation (neurodevelopment, at early timepoints) and neurodegeneration, at later timepoints.
The NMDA-R is of particular importance in hippocampal synaptic communication. Unlike most neurotransmitter receptors, NMDA-Rs are both ligand gated and voltage dependent, making them coincidence detectors for membrane depolarization and synaptic transmission. The binding of glutamate and co-agonist glycine or d-serine needs to coincide with membrane depolarization, which is mostly achieved by AMPA receptor activation, and removal of the Mg2+ block, before the ion channel opens. Once opened, this ion channel is permeable to Na+, K+, and Ca2+, the latter being an important regulator of synaptic plasticity-related processes (Coultrap and Bayer 2012). NMDA-Rs are heterotetramers composed of 2 obligatory GluN1 subunits and 2 other subunits (Paoletti 2011). GluN1 subunits comprise the glycine/d-serine binding site and are subdivided into 8 subtypes which are derived from one gene (Grin1) via alternative splicing. Four GluN2 subunits are encoded by four separate genes (Grin2A-D) and show cell type-specific expression. GluN2 subunits contain the glutamate/NMDA binding site at the extracellular N-terminal domain and define the electrophysiological properties of the NMDA-R, including calcium permeability. Each of the 4 GluN2 subtypes has a different intracellular C-terminal domain that interacts with different sets of signaling molecules. During early maturation, a developmental switch is seen from GluN2B to GluN2A, which is believed to control synaptogenesis and synapse stabilization (Gambrill and Barria 2011; Williams et al. 1993). In 1995, a third group of glutamate receptor subunits was discovered: GluN3 (Ciabarra et al. 1995; Sucher et al. 1995), which bind glycine and d-serine with much higher affinity than GluN1 (Nilsson et al. 2007). GluN3A and GluN3B, which are encoded by two separate genes, are believed to be dominant-negative modulators of the “classical” NMDA-Rs that are composed of GluN1 and GluN2 subunits (Pachernegg et al. 2012). The expression of GluN3A peaks in the early postnatal life, while GluN3B expression only starts in adulthood.
Many complex neurological diseases are associated with light to moderate neuroinflammation. Inflammatory mediators such as interferon-γ can induce indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme of the kynurenine pathway (Stone et al. 2013). This pathway, which is mainly active in glial cells, catabolizes the essential amino acid tryptophan, a precursor for serotonin. Excessive activation of this pathway is seen in diseases such as schizophrenia (Erhardt et al. 2001; Schwarcz et al. 2001), Alzheimer’s disease (Gulaj et al. 2010), Huntington’s disease (Guidetti et al. 2004) and mood disorders (Erhardt et al. 2012). The pathway has gained a lot of interest since it was shown that many of the intermediate catabolites (the so-called Trycats) exert biological activity. For example, quinolinic acid (QA) is an NMDA-R agonist (Misztal et al. 1996; Stone and Perkins 1981) and a pro-oxidant (Stipek et al. 1997), has immunomodulatory actions (Guillemin et al. 2003) and promotes tau hyperphosphorylation (Rahman et al. 2009). Kynurenic acid (KA), on the other hand, antagonizes multiple excitatory amino acid receptors, including the NMDA-R (Elmslie and Yoshikami 1985) and the homomeric α7-nicotinic receptor (Hilmas et al. 2001). In Alzheimer’s disease patients, plasma concentrations of QA are elevated while KA is decreased, indicating that pathological overactivation of the kynurenine pathway aggravates the primary brain damage mainly via glutamate excitotoxicity and oxidative stress (Gulaj et al. 2010; Plangar et al. 2011). In contrast, schizophrenia patients show increased levels of KA in the CSF, causing NMDA-R hypofunction and disconnection (Erhardt et al. 2001; Lavebratt et al. 2013).
Cultures of dissociated neurons are known to preserve the capacity to form an ad random neuronal network, and develop functional neuronal networks in which the spontaneous electrical activity synchronizes over time (Cohen et al. 2008). In these cultures, maturation takes place within a limited amount of time and the relative proportions of neurons and glial cells can be controlled. The neurons are directly accessible for manipulation, be it pharmacologically or with molecular biological techniques such as RNAi or expression of a transgene. Morphological studies have demonstrated that cultured hippocampal neurons undergo defined stages of maturation, including neurite sprouting, outgrowth and branching, and establishment of synaptogenesis (Dotti et al. 1988; Fletcher et al. 1994). Later stages are marked by the appearance of dendritic spines, which are thought to be shaped by neuronal activity (Fortin et al. 2012; Papa et al. 1995). Malfunctioning of neuronal networks is often associated with changes in the stability of dendritic spines (Penzes et al. 2011).
Calcium imaging of primary neuronal cultures constitutes a powerful and robust tool to study neuronal network functionality, since it has proven to be a reliable readout for electrical activity (Herzog et al. 2011; Smetters et al. 1999). Following a burst of action potentials, depolarization of the neuron opens different voltage-gated channels, including voltage-gated calcium channels, resulting in a transient calcium influx that can be visualized with fluorescent calcium indicators. In the present setup, 30–40 neurons are viewed, allowing to study neuronal physiology at the network level with good spatial resolution and a time resolution of 2 frames/s.
This work aims to validate a new in vitro setup for studying neuronal maturation and connectivity. It describes the detailed time course of neuronal network formation via morphological and functional readouts. We show that synchronized neuronal activity is mediated by the NMDA-R and that the ongoing network activity is influenced by the neurotransmitters acetylcholine and serotonin, which are known to modulate hippocampal activity in vivo (Drever et al. 2011; Gulyas et al. 1999). Modulation of the trophic support, positively by increasing the astrocyte density and negatively by lowering the nerve growth factor (NGF) concentration, clearly influences neuronal network formation. We further show that neuro-active catabolites of the kynurenine pathway exert acute modulatory functions on neuronal network activity. The approach presented in this paper allows to study neurodevelopmental processes, as well as synaptic connectivity in mature neuronal networks. The validation of this in vitro neuronal network model based on primary hippocampal neurons in 96-well plates, in combination with a custom-made algorithm to automatically analyze the calcium fluxes (Cornelissen et al. 2013), is an important step toward the integration in a high-content screening environment. High-content screening assays could be used in neurotoxicity screening, target identification/validation or phenotypic drug screening.
Experimental Procedures
Preparation of Primary Hippocampal Cultures
Hippocampi were dissected (Kaech and Banker 2006) from wild-type E18 FVB mouse embryos in HEPES (7 mM) buffered Hanks balanced salt solution (HBSS), followed by trypsin digestion (0.05 %; 10 min; 37 °C) and mechanical dissociation by trituration through 2 fire-polished glass pipettes with decreasing diameter. After centrifugation (5 min at 200×g), the cell pellet was resuspended in minimal essential medium (MEM) supplemented with 10 % heat-inactivated normal horse serum (Innovative Research, MI, USA) and 30 mM glucose (MEM-horse medium). Cells were plated in poly-d-lysin-coated 96-well plates (Greiner Cell coat, µClear, Wemmel, Belgium), at 30,000 cells/cm2, and kept in an humidified CO2 incubator (37 °C; 5 % CO2). After 4 h, the medium was replaced with B27 supplemented Neurobasal medium, containing sodium pyruvate (1 mM), glutamax (2 mM), and glucose (30 mM). To suppress proliferation of non-neuronal cells, arabinosylcytosine (AraC; 2 µM; Sigma-Aldrich, Bornem, Belgium) was added at the fourth day after plating. All cell culture supplies were purchased from Life Technologies (Merelbeke, Belgium), unless stated otherwise.
Preparation of Primary Astrocyte Cultures
Cerebral cortex from wild-type E18 FVB mouse embryos was dissected (Booher and Sensenbrenner 1972) and cells were further processed as described for the hippocampal cultures. After plating, the cells were kept in MEM-horse medium for 12 days, until the astrocytes formed a nearly confluent monolayer, with minimal neuronal presence. 2 days before splitting the astrocytes, the medium was replaced by B27 supplemented Neurobasal medium, allowing it to be conditioned for 48 h. Afterward, the conditioned medium was collected, the astrocytes were trypsinized (0.05 %; 10 min; 37 °C) and seeded in 96-well plates at different plating densities. After 24 h of recovery in MEM-horse medium, hippocampal cultures were plated on top of the pre-plated astrocytes. Conditioned medium was diluted 1:2 in fresh B27 supplemented Neurobasal medium, and used after 4 h in MEM-horse medium. Cultures grew in the absence of AraC.
Characterization of Distinct Cell Types in Primary Neuronal Cultures
At different days in vitro (DIV), cultures were fixed [4 % paraformaldehyde in 0.1 M phosphate buffer, 10 min at room temperature (RT)] and immunostained for a neuronal marker, HuC/HuD neuronal protein, and an astrocyte marker, glial fibrillary acidic protein (GFAP), followed by DAPI staining to obtain the total number of nuclei. Briefly, after pre-incubation with blocking buffer containing 1 % Triton X-100 for 10 min, the primary antibodies were applied for 4 h at RT (mouse monoclonal against HuC/HuD neuronal protein, 1/1,000, Life Technologies; rabbit polyclonal against GFAP, 1/400, Dako, Heverlee, Belgium). After washing with PBS, secondary antibodies were added for 2 h (Cy3-conjugated donkey-anti-mouse and FITC-conjugated goat-anti-rabbit Fab fragments, Jackson Immunoresearch, Suffolk, UK). Finally, DAPI was applied to the cultures for 2 min at a concentration of 2.5 µg/ml, followed by a PBS wash.
Stained cultures were automatically imaged for DAPI, HuC/HuD and GFAP in a BD pathway 435 bioimager (20× magnification NA 0.75, Becton–Dickinson, Erembodegem, Belgium). An overview of the well was created using 4 × 4 tiles with an inter-frame gap of 500 µm. Images were subsequently exported to Cell P software (Olympus, Münster, Germany) and nuclei, neurons and astrocytes were manually counted on 4 × 4 tiles, using the touch-count function. Results were expressed as # cells/cm2.
Live Cell Calcium Imaging
Cell cultures were loaded with 2 µM Fluo-4-AM in Neurobasal B27 medium at 37 °C and 5 %. After 30 min, the medium was replaced with recording medium, containing (in mM): CaCl2 0.9; MgCl2 0.5; KCl 2.67; NaCl 138; KH2PO4 1.47; Na2HPO4·7H2O 8; glucose 10. To study the sensitized NMDA-R, the same recording medium was used with exclusion of MgCl2 and addition of 10 µM glycine (Sigma-Aldrich). Cells were imaged on an inverted dual spinning disk confocal microscope (UltraVIEW ERS, PerkinElmer, Seer Green, UK), for 5 or 8 min, with a 25× objective lens (NA 0.80). To distinguish neurons from non-neuronal cells, 30 µM glutamate was added during the last minute of calcium recording (Pickering et al. 2008). Immediately after finishing the calcium recording, DAPI (2.5 µg/ml DAPI, 1 % TritonX-100, 4 % paraformaldehyde) was added to obtain an image of the nuclei in the recorded field.
Automated Analysis of Calcium Recordings
The resulting calcium recordings were automatically analyzed with a custom-made analysis algorithm (Cornelissen et al. 2013), that provides multiple parameters that reflect the activity of the neurons. Briefly, the neurons were recognized based on a nucleus image and the response to 30 µM glutamate. For each region of interest, traces of the fluorescence intensity over time were generated and used for subsequent signal analysis. The average bursting frequency and amplitude were measured from the normalized (F/F 0) traces of individual neurons, as was the average decay time, which is the time needed to go from a peak amplitude to 50 % of the baseline fluorescence. Neurons with at least 1 peak during the 4 min recording were counted and expressed as the percentage of active neurons. Whole fluorescence signals of individual neurons were compared and an average correlation score was calculated. Neurons that showed similar calcium patterns (coincident peaks and valleys) returned a high correlation score (Pearson correlation; between −1 and 1). To measure the synchronous bursting frequency and amplitude, all neuronal traces were combined into 1 average trace depicting only the correlated network bursts, while uncorrelated peaks were averaged out. For acute experiments, calcium recordings were analyzed in 2 or 3 separate stretches, corresponding to different treatments.
Dendritic Spine Density
Cultures were fixed (4 % paraformaldehyde in 0.1 M phosphate buffer, 10 min at RT) and stained with the carbocyanine dye CM-DiI (1 µg/ml; 20 min; Life Technologies). At least 24 h passed between staining and imaging, allowing the hydrophobic dye to spread throughout the plasma membrane. High-resolution confocal images were acquired using a dual spinning disk microscope (UltraVIEW VoX, PerkinElmer, Seer Green, UK) with a 63× oil objective lens (NA 1.4), followed by image deconvolution (resolution ≈ 0.25 µm). Dendritic spine density was calculated by manually counting the dendritic protrusions and correcting for the length of the dendrite under investigation (Volocity image analysis software, PerkinElmer, Seer Green, UK). At least 1,500 µm of dendrite length was considered for each condition, on multiple non-overlapping images.
Neurite Outgrowth and Synapse Density
After fixation, cultures were immunostained for the neurite marker β-III-tubulin (rabbit polyclonal against β-III-tubulin, 1/1,000, Covance, Brussels, Belgium) and the synaptic protein synaptophysin-I (guinea pig polyclonal against synaptophysin-I, 1/1,000, Synaptic Systems, Göttingen, Germany). FITC-conjugated goat-anti-rabbit Fab fragments and Cy3-conjugated donkey-anti-guinea-pig (Jackson immunoresearch) secondary antibodies were used. Images of the stained cultures were automatically acquired using a BD pathway 435 bioimager (4 × 4 tiles with 500 µm inter-frame gap, 20× objective) and exported to Cell P software.
To measure neurite outgrowth, images were first processed with a differential contrast enhancement filter, followed by automatic thresholding and finally the surface of the β-III-tubulin-positive structures was measured. To adequately measure the number of synaptophysin-I-positive puncta, images were first treated with a Laplace filter, followed by automated thresholding and counting of the puncta. Synapse density was expressed as the number of synaptophysin-I puncta per µm2 β-III-tubulin surface.
Culture Treatment and Pharmacology
To study acute effects on neuronal network activity, tetrodotoxin (TTX; 2 µM), forskolin (FSK; 10 nM–10 µM), MK-801 (1–100 µM; in-house J&J Pharmacy), ketamine (1–100 µM; in-house J&J Pharmacy), KA (10–150 µM, QA (10–150 µM), acetylcholine (10 µM), muscarine (10 µM), nicotine (1 µM), or serotonin (10 µM) was dissolved in the recording medium and added to distinct wells, 3 min after initiation of baseline calcium recording. Effects were further followed for 4 min before the glutamate stimulus was given. All compounds were purchased from Sigma-Aldrich, unless stated otherwise. For successive experiments, 3 min of baseline recording were followed by 3 min in the presence of ketamine (50 µM), KA (100 µM) or QA (100 µM), in turn followed by another 3 min of ketamine (50 µM), KA (100 µM) or QA (100 µM) without washing the previous treatment away. Acute effects were studied on 6 and 7 DIV cultures.
For NGF deprivation experiments, cultures were incubated from the fourth day in vitro with neutralizing mouse monoclonal antibodies against NGF (3–9 µg/ml; Peprotech, London, UK). A nonsense mouse monoclonal antibody (anti-BAX; 9 µg/ml; Abcam, Cambridge, UK) was included as negative control. Neither NGF nor nonsense antibodies contained preservatives.
RT qPCR for NMDA-R Subunits
Hippocampal cultures were grown under control conditions until the time of cell lysis at 4, 7 or 10 DIV. Cells were lysed in 100 µl/well RLT buffer (Qiagen, Germantown, MD, USA), supplemented with 1 % β-mercaptoethanol. RNA was extracted using a RNeasy mini kit (Qiagen), combined with DNase treatment (RNase-free DNase set, Qiagen) using prescribed procedures. RNA concentration and integrity were assessed with the nanodrop spectrophotometer (Thermo scientific, Wilmington, DE, USA) and the BioAnalyzer (Agilent Technologies, Diegem, Belgium), typically yielding highly intact RNA (RIN ≥ 9) with a concentration of about 60 ng/µl. cDNA was synthetized starting from 150 ng RNA, using a SuperScript III First-Strand Synthesis SuperMix (Life Technologies, Merelbeke, Belgium), yielding 20 µl cDNA of which 5 µl (1:10) was used in the subsequent qPCR reaction. Primers and probes were purchased from Tib molbiol (Berlin, Germany) for 5 target genes: Grin1, Grin2A, Grin2B, Grin2D, and Grin3A (for primer sequences, see Table 1). qPCR was conducted in triplo in a LightCycler 480 instrument, using LightCycler 480 Probes Master and LightCycler 96-well plates (95 °C for 10′; 40 cycles of 95 °C for 10″/60 °C for 15″/72 °C for 1″). Primers were used at 0.5 µM and probes at 0.1 µM. The efficiency (E) of each primer/probe mix was measured by the determination of the slope of a standard curve on a serial dilution of initial cDNA.
Table 1.
Nucleotide sequences of primers and probes for 5 target genes (Grin1, Grin2A, Grin2B, Grin2D,, and Grin3A) and 1 reference gene (eIF3) 6FAM: 6-Carboxyfluorescein BBQ: BlackBerry Quencher
| Target | Sequence (5′->3′) |
|---|---|
| Grin1 F | GAGGATACCAGATGTCCACCAGAC |
| Grin1 R | GATGTGTCATTAGGCCCCGT |
| Grin1 probe | 6FAM-TGGTGACCCTGTCAAGAAGGTGATCT-BBQ |
| Grin2A F | GCTACACACTCTGCACCAATTTATG |
| Grin2A R | CGGTCCTTATTCAGCACGATCA |
| Grin2A probe | 6FAM-CAATGTGACTTGGGATGGCAAGGACTT-BBQ |
| Grin2B F | GGGATTGCCATCATCACCACT |
| Grin2B R | CCTTCAAAAGTGACGTTGATCAGATA |
| Grin2B probe | 6FAM-TGCTCTTGGGCTCAGGGATGAAACTGT-BBQ |
| Grin2D F | AGCAATACCCACCCCTGAAGT |
| Grin2D R | GTCCAGTTTCCCTGCCTTGA |
| Grin2D probe | 6FAM-TGGAACGGTGCCCAATGGGT-BBQ |
| Grin3A F | AAACCCCCTAAATGCTGGACT |
| Grin3A R | CAAACGAAAACCTTGAGAAGGA |
| Grin3A probe | 6FAM-AGAAAGGCAAAACATACAGAAAATGGCCCA-BBQ |
| eIF3 F | ATGATAGAGATCGGAGAGGACC |
| eIF3 R | CTCTTCAGTCCGGTCATCTTTC |
| eIF3 probe | 6FAM-AGCAAGCTCTTGGAGACGCACT-BBQ |
In total, 5 reference genes were included in the PCR (PSMD1, eIF3, GAPDH, RPL38, eEF2), of which a combination of PSMD1 (Taqman gene expression assay Mm01295362_m1; Life Technologies), eIF3 (for sequence, see Table 1) and GAPDH (Taqman gene expression assay Mm99999915_g1; Life Technologies) was used to normalize the experimental data, based upon analysis by geNorm software (M value <0.5; qBasePlus, Biogazelle, Zwijnaarde, Belgium). The PCR products were run on a 2 % agarose gel to confirm the right amplicon length.
Cycle number was measured using the baseline-independent second derivative maximum method. Normalized relative gene expression was determined by the method and was rescaled to the expression level at 4 DIV.
Statistics
The number of biological and technical replicates of each experiment is listed in supplemental Table 1, and is mentioned in the respective figure legends. A biological replicate refers to 1 dissection and plating procedure. A technical replicate refers to 1 well of a 96-well plate from within a biological replicate.
For statistical analysis of normally distributed data from calcium imaging (Fig. 1f), a linear mixed effects model was used to model the data of each analysis parameter. The nested structure of the data was accounted for using a mixed effects analysis to cope with the heterogeneity between and within biological replicates: several technical replicates were measured within one biological replicate (mouse) and there were repeated measures of the mice on the different DIV. In order to accommodate the dependency of the measurements within the biological replicate and the repeated measurements, a random intercept for mouse has been included in the model. The DIV entered the model as fixed effect term (as a categorical covariate) to allow for modeling flexible patterns over time (instead of imposing a linear trend). The model also accounted for the unequal variances over time. For synchronous bursting frequency and correlation score, data were log-transformed prior to analysis. For synchronous bursting frequency, a value of 0.25 (corresponding to the detection limit) was assigned in case of no occurrence. Post hoc t tests were conducted from the model to detect significant changes between consecutive days. For visual representation, means and standard deviations from the raw data were plotted.
Fig. 1.
Primary hippocampal neurons develop synchronous network activity within a short time frame. a Representative image of a 7 DIV culture. All nuclei are stained with DAPI (blue). Neurons are recognized by the cytoplasmic marker HuC/HuD neuronal protein (red), while astrocytes are identified by means of GFAP labeling (green). Astrocytes display either a stellate-shaped (active) phenotype (arrow) or a resting/protoplasmic (arrowhead) morphology. b Quantification of the distinct cell types over time. DAPI-positive/HuC,D-negative/GFAP-negative structures are indicated as “other”. On 4 DIV, AraC is added as a mitotic inhibitor. Mean ± SD; n = 2 isolations ×8 technical replicates. c Representative image of Fluo-4 loaded cells at 7 DIV. This image was taken immediately after application of 30 µM glutamate. Neurons are circled. d Normalized fluorescence over time traces of 5 DIV neurons. Each trace represents 1 neuron. At the fourth minute, the cells are exposed to 30 µM glutamate (Glu), resulting in a large and prolonged calcium influx. Traces of non-neuronal cells were filtered out based on this response. e Normalized fluorescence over time traces of a 7 DIV culture. Traces corresponding to neurons (arrows) are distinguished from non-neuronal cells (arrowheads) since the latter return to baseline after the glutamate stimulus. The spontaneous activity of neurons shows faster kinetics than the calcium waves of non-neuronal cells. f Graphs representing distinct analysis parameters from 3 to 7 DIV. Data from the upper graphs were obtained from individual neurons, while the lower graphs represent parameters which are measured at the network level. Mean ± SD, n = 8 isolations × 3 technical replicates *p ≤ 0.05; § p ≤ 0.005 in post hoc t tests between consecutive days (Color figure online)
For morphological parameters (Fig. 2b, d), each analysis parameter was modeled using an ANOVA model containing time of maturation of the cells as covariate. Only two biological replicates were measured per DIV. Therefore, the nested structure of the data was not accounted for. The presence of significant differences over time was tested using an overall F-test for each analysis parameter. Conditional on the overall significance of the time effect, post hoc t tests were conducted from the model to detect significant changes between consecutive days. For visual representation, means and standard deviations were plotted from the original data.
Fig. 2.
Morphological readouts reflecting the maturation of primary hippocampal neurons. a Representative images of primary neurons, immunostained for the neurite marker β-III-tubulin (green) and the synapse marker synaptophysin I (red) at 7 and 14 DIV. b Evolution of neurite outgrowth and synapse density from 4 to 21 DIV. Mean ± SD; n = 2 isolations × 6 technical replicates *p ≤ 0.05; § p ≤ 0.005 in post hoc t tests between consecutive days. c Representative images of DiI-stained neurons (1 µg/ml, 20 min) with dendritic spines at 7 and 14 DIV. d Evolution of dendritic spine density from 4 to 21 DIV in primary hippocampal cultures. Mean ± SD; n = 2 isolations. For each isolation, 750 µm of dendrite was analyzed on 5 non-overlapping images *p ≤ 0.05; § p ≤ 0.005 in post hoc t tests between consecutive days (Color figure online)
For astrocyte conditioning and NGF deprivation experiments, normally distributed calcium imaging parameters were analyzed using an ANOVA model with DIV and treatment as fixed effects (Figs. 3a, 4a). No significant interactions were found between DIV and treatment for any of the calcium imaging parameters. The nesting was not accounted for due to the limited number of biological replicates (n = 2). First, an overall F test was performed to assess any differences over DIV and treatment. Conditional to the overall F tests, post hoc tests were carried out within DIV; for the astrocyte conditioning experiments, post hoc Dunnett’s t tests were carried out for each analysis parameter (equal variances assumed), while Dunnett’s T3 tests were used in the NGF deprivation experiment (equal variances not assumed). For amplitude and synchronous bursting frequency, a log-normal distribution was applied. For synchronous bursting frequency, a value of 0.25 (corresponding to the detection limit) was assigned in case of no occurrence. Morphological parameters (Figs. 3c, 4b) were analyzed using a one-way ANOVA. Conditional to the overall F test, a post hoc Dunnett’s T3 test was carried out to detect differences in the spine density in the astrocyte conditioning experiment. Again, the nesting was not accounted for due to the limited number of biological replicates (n = 2).
Fig. 3.
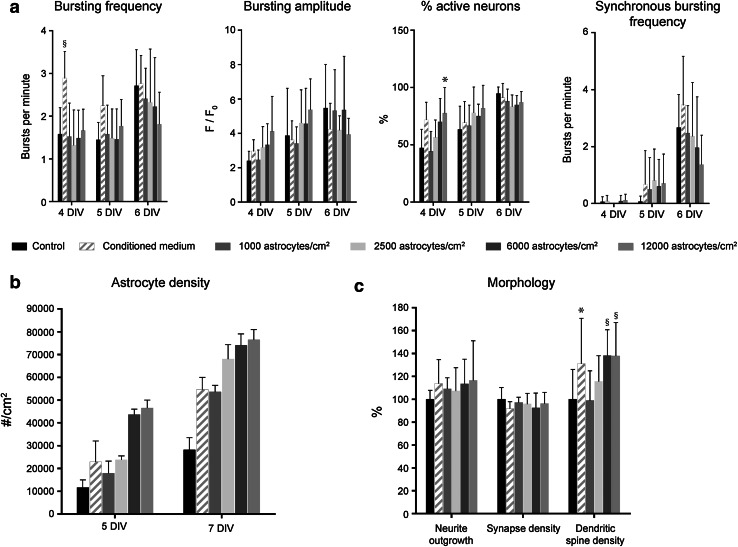
The development of neuronal network activity is enhanced by chronic trophic support of astrocytes. a Early neuronal network formation is accelerated by a feeder layer of astrocytes and by astrocyte-conditioned medium, as measured via calcium imaging on 4, 5 and 6 DIV. Mean ± SD; n = 2 isolations × 3 technical replicates ANOVA results: bursting frequency differences between DIV: p < 0.001; between treatments: p = 0.002; bursting amplitude differences between DIV: p < 0.001, between treatments: p = 0.303; % active neurons differences between DIV: p < 0.001; between treatments: p = 0.031; synchronous bursting frequency differences between DIV: p < 0.001, between treatments: p = 0.806. *p ≤ 0.05; § p ≤ 0.005 versus control (within DIV). b Astrocyte density at 5 and 7 DIV. Mean ± SD; n = 2 isolations × 2 technical replicates. c Effect of astrocytes and conditioned medium on neurite outgrowth, synapse density, and dendritic spine density. Neurite outgrowth and synapse density: Mean ± SD; n = 2 isolations × 6 technical replicates. Dendritic spine density: Mean ± SD; n = 2 isolations. For each isolation, 1,250 µm of dendrite was analyzed on 8 non-overlapping images ANOVA results: neurite outgrowth p = 0.455; synapse density p = 0.179; dendritic spine density p < 0.001 *p ≤ 0.05; § p ≤ 0.005 versus control
Fig. 4.
A decreased NGF concentration impairs functional, but not morphological network development. a Anti-NGF treatment impairs the formation of a functional neuronal network, as measured via calcium imaging at 5, 6, and 7 DIV. Mean ± SD; n = 2 isolation × 3 technical replicates ANOVA results: bursting frequency differences between DIV: p < 0.001, between treatments: p < 0.001; bursting amplitude differences between DIV: p = 0.067, between treatments: p < 0.001; synchronous bursting frequency differences between DIV: p < 0.001, between treatments: p < 0.001; correlation score differences between DIV: p < 0.001, between treatments p < 0.001 *p ≤ 0.05; § p ≤ 0.005 versus control (within DIV). b Effect of anti-NGF treatment on neurite outgrowth, synapse density and dendritic spine density. Neurite outgrowth and synapse density: Mean ± SD; n = 2 isolations × 4 technical replicates. Dendritic spine density: Mean ± SD; n = 2 isolations. For each isolation, 750 µm of dendrite was analyzed on 5 non-overlapping images. ANOVA results: neurite outgrowth p = 0.372; synapse density p = 0.106; dendritic spine density p = 0.072
To detect the influence of the recording medium on spontaneous network activity (Fig. 6b, f), an unpaired t test was performed. For dose–response experiments of ketamine, KA and QA (Fig. 6c–e, g–i), data on synchronous bursting frequency were log-transformed prior to analysis to cope with the log-normal nature of the data. For synchronous frequency, a value of 0.25 (corresponding to the detection limit) was used in case of no occurrence. No transformation was performed on the correlation parameter. For each compound, a linear model was estimated where the relevant parameter (synchronous bursting frequency or correlation) is modeled as a function of concentration (categorical covariate), recording medium (categorical covariate) and the interaction of concentration and recording medium. Contrasts were estimated to compare normal and sensitizing medium at each individual concentration and (back-transformed) least-squares means were generated (online document: Lenth 2013) for convenient display of the data (LS mean ± 95 % CI) and were investigated for significant changes from before treatment (the first 3 min of recording in the absence of the compound). The p values associated to the estimated contrasts (differences at each concentration level) are used to designate significance on the plot.
Fig. 6.
NMDA-R functionality controls neuronal network activity. a Five NMDA-R subunits are expressed in primary hippocampal cultures, as detected with RT qPCR at 4, 7, and 10 DIV. The expression level is shown as the normalized relative quantity, rescaled to the expression level at 4 DIV. Mean ± SD; n = 3 isolations × 3 technical replicates for each DIV. b Basal neuronal network activity is altered when recorded in Mg2+-free and glycine containing medium. The frequency of synchronous bursts is significantly increased in the case of NMDA-R sensitization. c–e Ketamine (non-competitive NMDA-R antagonist), KA (competitive NMDA-R antagonist) and QA (competitive NMDA-R agonist) induce distinct effects on network activity. Backtransformed least square means from the statistical model are plotted with 95 % CI. The frequency of synchronous bursts is expressed as the ratio after/before treatment. f The correlation score is significantly decreased in NMDA-R sensitizing medium. g–i. The change in synchronicity is expressed as the difference in correlation score (after–before) and shows acute alteration after addition of ketamine, KA or QA. Least square means from the statistical model are plotted with 95 % CI. j–m Neuronal network activity of 6/7 DIV cultures was followed for 9 min in standard recording medium. A first (ant)agonist was added after 3 min of baseline recording, followed by a second one at 6 min without washing the first one away. Three interconnected points in the lower panels represent 1 recording, as shown in the upper panels. KA and QA: 100 µM; ketamine: 50 µM; n = 3 isolations × 2–3 technical replicates. b, f Mean ± SD; n = 16 isolations × 5 technical replicates; § p < 0.005 in unpaired t test. c–e, g–i Mean ± 95 % CI; n = 3 isolations × 1–2 technical replicates *p ≤ 0.05; § p ≤ 0.005 in ANOVA based model compared to absence of change (contrasts; 100 % for synchronous bursting frequency and 0 for correlation score)
Results
Neuronal Networks Develop Synchronized Activity Within a Well-Defined Time-Window
Since the dissection procedure of hippocampi from mouse embryos is crucial for the reproducibility of the cultures and the density of the neurons in vitro is decisive for their connectivity and spontaneous activity (Ivenshitz and Segal 2010), we assessed the proportion of the most prominent cell types in the cultures over time. Nuclei, neurons, and astrocytes were quantified at specific timepoints from 1 to 21 days in vitro (DIV; Fig. 1a, b). In addition to the neurons (HuC/HuD neuronal protein-positive) and astrocytes [glial fibrillary acidic protein-positive (GFAP+)], the number of cells immunonegative for HuC/HuD and GFAP were classified as “other” and include fibroblasts and a small number of pre-oligodendrocytes (Neural/Glial Antigen 2-positive/2′,3′-cyclic nucleotide 3′-phosphodiesterase-negative/myelin basic protein-negative; data not shown). At these timepoints, no microglia could be identified through immunostaining for CD11b, ionized calcium binding adaptor molecule 1, purinergic receptor P2X4 or Major Histocompatibility Complex class II, both on cultures and on cytospin preparations of culture medium, leading us to conclude that microglia were absent in these cultures.
The total number of nuclei increased during the first week in vitro, followed by a decrease after 2 weeks. The number of neurons, which were post-mitotic at the time of isolation, showed a slight decrease over 3 weeks in vitro. However, the neuronal population was relatively stable within the experimental window (4–14 DIV). The initial rise in nuclei represented the proliferation of astrocytes and other cells. At 4 DIV, arabinosylcytosine (AraC) was added to the medium inhibiting the proliferation of dividing cells. Other non-neuronal cells showed a higher sensitivity to AraC than astrocytes, the proliferation of which ceased from 7 DIV onwards. However, this AraC concentration decreased astrocyte proliferation markedly between 4 and 7 DIV compared to cultures that were not treated with AraC (data not shown).
Spontaneous calcium peaks could be observed in the neuronal cultures after loading with Fluo-4 (Fig. 1c–e). Both neurons and non-neuronal cells showed calcium fluxes, with different kinetics. Neurons exhibited relatively short (about 5 s) and steep calcium peaks, while the other cells showed slower waves (about 30 s), which often spread to neighboring cells. In order to distinguish neurons from non-neuronal cells, 30 µM of glutamate was added to the well during the last minute of each calcium recording. The applied glutamate concentration was neurotoxic, and resulted in a sustained rise of calcium in the neurons only (Pickering et al. 2008) (Fig. 1e, arrows). Non-neuronal cells responded with a slow and transient calcium wave (Fig. 1e, arrowheads), and were discarded from further analysis. The spontaneous neuronal activity evolved from asynchronous at 5 DIV (Fig. 1d) to synchronized activity at 7 DIV (Fig. 1e), reminiscent of the maturation of a neuronal network.
Calcium recordings were analyzed with a custom-made algorithm (Cornelissen et al. 2013), extracting different parameters, as shown in Fig. 1f. Synchronization of the activity was evident from 2 parameters: the correlation score, which expresses the amount of synchrony between neurons in a given calcium recording, and the frequency of synchronous bursts. At 5 DIV, the first synchronous bursts arose, but the activity was still largely asynchronous. 2 days later, at 7 DIV, the activity became mostly synchronous, as reflected in the correlation score and synchronous bursting frequency. The formation of the network coincided with a significant increase in the fraction of active neurons. At 3 and 4 DIV, before the onset of synchronous bursting, only 45 % of the neurons were active during a 4-min recording, while more than 90 % of the neurons showed activity at 7 DIV, demonstrating that the growing network incorporates and activates new neurons, since the total number of neurons was nearly constant between 4 and 7 DIV (Fig. 1b). The frequency of individual bursts remained rather constant over the different DIV while the proportion of bursts that occurred in synchrony increased. The amplitude of the calcium bursts of individual neurons showed an upward trend, whereas the decay time decreased, i.e., by day 7, more calcium entered the neuron compared to day 4, but at the same time, the neuron was better equipped to buffer the excess intracellular calcium. Though Fluo-4 is a calcium indicator of the non-ratiometric type, we found a linear relationship between fluorescence intensity and intracellular calcium concentration in the dynamic range that was typical for our experiments using the calcium ionophore ionomycin (data not shown).
Network Formation Coincides with the Outgrowth of Neurites and the Formation of Synapses, While Dendritic Spines Appear only After Synchronization of the Activity
Neurite outgrowth started after the first contact of the neuron with the bottom of the 96-well plate. At the fourth day after plating, the neurons already contained some presynaptic synaptophysin-I-immunoreactive puncta (Fig. 2b), which are thought to form functional synapses after contacting a postsynaptic partner. Synaptophysin is present on synaptic vesicle membranes and is believed to be involved in regulating vesicle endocytosis (Gordon et al. 2011).
A striking increase in neurite outgrowth was observed between 4 and 7 DIV (Fig. 2b). This expansion slowed down between 7 and 11 DIV after which the neurite surface remained stable, as the bottom of the plate was largely covered by neurites. The number of synaptophysin-I-positive puncta, on the other hand, kept on increasing until 14 DIV. A significant increase in synapse density, expressed as the number of puncta per surface unit (µm) neurite, was seen from 4 to 14 DIV (Fig. 2a, b). As such, the synchronization of neuronal activity (5–7 DIV, Fig. 1d–f) coincided with an important increase in neurite outgrowth and synapse density. Although synaptogenesis continued afterward, it can be assumed that the critical number of functional synapses to obtain synchronous network activity was reached by 7 DIV.
Dendritic spines were visualized via labeling with the lipophilic dye DiI and subsequent confocal microscopy (Fig. 2c). The number of dendritic spines per µm of dendrite showed a marked increase over time, reflecting the maturation process of the neurons (Fig. 2d). While synchronization of the bursting occurred between 5 and 7 DIV, spine density started to increase only at 7 DIV and was most pronounced between 11 and 21 DIV. These data suggest that robust network activity aids in the formation of dendritic spines, which offer electrical and biochemical compartmentalization of intensively used synapses.
Chronic and Acute Modulation of Neuronal Network Activity
The presented in vitro model holds great potential to facilitate fundamental and drug discovery-related research on 2 levels. The first one is the neurodevelopmental level, which is likely to be disturbed in mental diseases such as schizophrenia, autism spectrum disorders, intellectual disability, etc. To validate the proposed setup as a tool for studying neurodevelopment, it is essential to show that the formation of the neuronal network is responsive to chronic modulatory treatments. The second level is synaptic communication in the mature network, which is compromised in diseases like Alzheimer’s disease, schizophrenia, mood disorders, etc. Therefore, we explored if mature neuronal network activity can acutely be influenced by known neuromodulatory compounds.
The Development of Neuronal Network Activity is Enhanced by Chronic Trophic Support of Astrocytes
Astrocytes exert multiple functions to sustain the growth and functionality of neurons. Next to providing metabolic support, they are now known to be directly involved in synaptic transmission via the so-called tripartite synapse. To study the effect of astrocytes on neuronal network formation, hippocampal neurons were seeded on top of a feeder layer of astrocytes, 24 h after seeding this feeder layer in different densities. Calcium imaging was performed at 4, 5 and 6 DIV (Fig. 3a). At 4 DIV, the percentage of active neurons was elevated in the cultures grown on a feeder layer when compared to control cultures. The magnitude of this effect was astrocyte density-dependent. However, this effect diminished at 5 DIV. By 6 DIV, the untreated cultures caught up and the positive effect of the astrocytes was neutralized. Neurons that received the highest density of astrocytes tended to show higher bursting amplitudes at 4 DIV, an effect that also diminished over time. Our data indicate that synchronized bursting in the treated cultures was initiated a little earlier (5 DIV) than in control cultures. However, this effect did not reach statistical significance and disappeared 1 day later. These findings suggest that astrocytes accelerated the early phases of neuronal maturation up to the point where synchronous activity was introduced. Alternatively, as there was no medium change, the excessive presence of astrocytes might have depleted the nutrients in the medium faster than in control cultures, even though visual inspection did not show acidification of the culture medium.
Cultures that received astrocyte conditioned medium also showed faster maturation. The percentage of active neurons and the synchronous bursting frequency were enhanced in these cultures, as was the bursting frequency of individual neurons, an effect that was not present in cultures grown on a feeder layer of astrocytes. The bursting amplitude, on the other hand, was not affected by the conditioned medium, which was different from the situation in neurons that were cultured on a feeder layer of astrocytes. These data suggest that part of the positive effect that astrocytes have on neuronal maturation is mediated by soluble factors. However, it should be noted that the conditioned medium promoted astrocytic proliferation, as counted in 5 and 7 DIV cultures (Fig. 3b). Both in control cultures and those that received conditioned medium, the number of astrocytes almost tripled between 5 and 7 DIV, but the absolute number was always higher in cultures grown in conditioned medium, indicating that conditioned medium enhanced the proliferation of astrocytes at early timepoints. The proliferation of astrocytes is limited through contact inhibition, as seen in 2,500, 6,000 and 12,000 astrocytes/cm2 at 7 DIV (Fig. 3b).
From a morphological point of view, neurite outgrowth in cultures that were grown on astrocytes or in conditioned medium was only slightly enhanced (Fig. 3c), and did not reach statistical significance. Likewise, synapse density remained unchanged. However, although the total number of synapses remained unchanged, calcium imaging data suggested that a larger part of the synapses became functional when additional neurotrophic support was provided. In addition, a strong effect was found at the level of the dendritic spines (Fig. 3c), whose density was significantly increased in cultures grown on a feeder layer of astrocytes, as well as in cultures grown in conditioned medium.
Together, these data indicate that the formation of a neuronal network is accelerated by chronic elevation of the neurotrophic support. Such effects were revealed by assessing the network activity and the density of dendritic spines. Neurite outgrowth and synapse density seem less sensitive readouts and failed to detect subtle changes in network formation.
A Decreased NGF Concentration Impairs the Development of Synchronized Network Activity
Enhancing neurotrophic support resulted in faster neuronal network formation, as shown in Fig. 3. Consequently, we hypothesized that decreased neurotrophic content would induce defects in the formation of the neuronal network. This hypothesis was tested by introducing a neutralizing antibody against NGF in the culture medium at 4 DIV. As the culture medium did not contain exogenous NGF, this antibody reduced the paracrine effect of endogenous NGF, released by astrocytes.
The calcium imaging data of the first 3 days of anti-NGF treatment revealed a clear, dose-dependent defect in neuronal network formation when compared to naive cultures or cultures that received a non-binding antibody (nonsense AB, Fig. 4a). The amplitude of the neuronal bursts was reduced but the bursting frequency was increased in NGF-deprived cultures, reminiscent of ectopic neuronal bursting. Both synchronous bursting frequency and correlation scores were significantly reduced in anti-NGF-treated cultures, indicating that synapse functionality and the maturation of the neuronal network were compromised. Cultures that received the lowest concentration of the antibody (3 µg/ml) seemed to recover from the insult, presumably by antibody depletion or by a sufficient concentration of unbound NGF.
Unlike the pronounced defects that were detectable by functional imaging, morphological changes were less prominent (Fig. 4b). The degree of neurite outgrowth and synaptic density remained largely unchanged at 7 DIV, while functional calcium imaging indicated that these synapses were largely dysfunctional. Dendritic spine density was slightly, but not significantly, decreased in the cultures that received the highest concentrations of the anti-NGF antibody. This slight decrease might be the result of impaired activity-dependent spine formation.
Together, these data indicate that serious defects in neuronal network formation were induced by reduced neurotrophic support. Tempering the NGF concentration clearly perturbed synapse functionality and the synchronization of neuronal activity, which led to a slightly decreased dendritic spine density at later stages. In contrast, we failed to find any sign of impaired neurite outgrowth or synapse density, thereby showing that calcium imaging of neuronal network activity is the most sensitive readout for detecting subtle changes in neuronal network functionality.
Neuronal Network Activity Responds to Acute Pharmacological Intervention
In addition to neurodevelopmental studies, the presented model has the potential to contribute to the study of synaptic connectivity in mature neuronal networks. Therefore, a number of well-known compounds were tested for their acute effects on neuronal network activity.
The calcium oscillations were abruptly stopped in response to tetrodotoxin (TTX, 2 µM), a potent blocker of Na+ channels (Fig. 5a), indicating that the observed calcium bursts are indeed a secondary event in response to action potentials. Acute application of forskolin (FSK, 5 µM) enhanced spontaneous bursting frequency in a dose-dependent way (Fig. 5b, c). As a potent activator of adenylate cyclase, FSK increases the intracellular cyclic adenosine monophosphate (cAMP) concentration, resulting in phosphorylation of downstream effectors by protein kinase A (PKA).
Fig. 5.
Neuronal network activity responds to acute pharmacological intervention. a Addition of 2 µM TTX after 3 min results in the abrupt abolition of spontaneous activity in 6 DIV cultures. After 7 min, glutamate (Glu) is added to recognize the neurons. b Exposure to 5 µM FSK increased the synchronous bursting frequency in these 6 DIV neurons. c Dose–response curve depicting the effect of FSK (6/7 DIV) on the synchronous bursting frequency. Mean ± SD; n = 3 isolations × 2 technical replicates. d 50 µM of the selective NMDA-R antagonist MK-801 was added to the recording medium, resulting in the disappearance of synchronized activity in this 7 DIV culture, showing that synchronized network activity is mediated by the NMDA-R. e MK-801 dose-dependently reduces the synchronicity of bursting across neurons. Mean ± SD; n = 3 isolations × 2 technical replicates. f–h Modulatory neurotransmitter acetylcholine (10 µM) and cholinergic agent muscarine (10 µM) induce a partial desynchronization of the network activity, while nicotine (1 µM) reduces the frequency but increases the bursting amplitude. i Modulatory neurotransmitter serotonin (5-HT; 10 µM) increases the synchronous bursting frequency
The non-competitive, selective NMDA-R antagonist MK-801 (50 µM) acutely reduced correlated network bursts in 7 DIV cultures, and dose dependently decreased the synchronicity of bursting, as reflected in the correlation score (Fig. 5d, e). The effect of MK-801 clearly indicates that synchronous network activity is largely dependent on NMDA-R function.
Network activity was also acutely altered by adding neurotransmitters known to exert modulatory activity in the hippocampus (Drever et al. 2011; Gulyas et al. 1999). Acetylcholine caused partial desynchronization of the activity (10 µM; Fig. 5f), an effect which was also observed with the cholinergic agonist muscarine (10 µM; Fig. 5g). Nicotine, on the other hand, induced a decrease in synchronous bursting frequency but an increase in bursting amplitude, leading to more robust activity (1 µM; Fig. 5h). Concentrations of nicotine that exceeded 5 µM led to over-excitation and the complete destruction of synchronized activity (data not shown). Application of serotonin (5-HT; 10 µM) led to a dose-dependent increase in synchronous bursting frequency (Fig. 5i). These data suggest that various functional neurotransmitter receptors are present on primary hippocampal cells.
It can be concluded that ongoing neuronal network activity can be modulated by addition of neuroactive substances. As such, the obtained data indicate that monitoring network activity in primary hippocampal cultures can be used as a valid tool to discover neuromodulatory compounds.
Neuronal Network Activity is Perturbed by NMDA-R (Ant)agonists, Including Disease-Relevant Kynurenic (KA) and Quinolinic Acid (QA)
RT qPCR data of 4, 7, and 10 DIV cultures confirmed that 5 NMDA-R subunits were expressed in primary hippocampal cultures: Grin1, Grin2A, Grin2B, Grin2D, Grin3A (Fig. 6a). All of the detected subunits showed increased expression over time, reminiscent of the neuronal maturation process and development of synchronous network activity. Grin2A expression increased slightly faster than Grin2B, reminiscent of the developmental switch from GluN2B to GluN2A.
Before, we showed that neuronal network activity is mediated by the NMDA-R, since it was dose-dependently blocked by the specific NMDA-R antagonist MK-801 (Fig. 5d, e). Here, we report that spontaneous neuronal network activity was altered in medium that contained the NMDA-R co-agonist glycine and was free of Mg2+ (NMDA-R sensitizing medium, Fig. 6b, f). The bursting properties of individual neurons were altered as the bursting amplitude was decreased while the frequency and decay time were increased compared to neurons residing in standard recording medium (data not shown). On the network level, the synchronous bursting frequency was increased (Fig. 6b), while the correlation score was decreased (Fig. 6f). These changes confirmed that the NMDA-R is implicated in the maintenance of neuronal network activity and showed that NMDA-R sensitization induces bursting activity reminiscent of mild excitotoxicity.
At 6 and 7 DIV, acute addition of ketamine, a well-known non-competitive NMDA-R antagonist, suppressed ongoing network activity, as reflected in multiple parameters, including frequency of synchronous bursts (Fig. 6c) and correlation score (Fig. 6g). Ketamine concentrations of 50 µM or higher dramatically decreased both synchronous bursting frequency and correlation scores. At a concentration of 10 µM, only the networks recorded in standard recording medium were inhibited, indicating that sensitization of the NMDA-R neutralized the effect of a low dose of ketamine.
KA, a metabolite of the kynurenine pathway and competitive NMDA-R antagonist, suppressed the frequency of synchronous bursts (Fig. 6d) as seen with ketamine. However, the concentration range was higher and the effect lower when compared to the non-competitive ketamine. Correlation scores were only slightly affected after KA application (Fig. 6h). In standard recording medium, KA slightly decreased the correlation score, as seen with ketamine. However, in sensitizing medium, KA slightly increased the correlation score, as it was decreased in this medium compared to standard recording medium (Fig. 6f). Under these circumstances, KA showed a neuroprotective effect and rescued the excitotoxicity induced by the sensitizing medium (Fig. 6h).
QA, also a metabolite of the kynurenine pathway and a competitive NMDA-R agonist, caused considerable disturbance in neuronal networks’ activity. Regarding the synchronous bursting frequency (Fig. 6e), the net effect of QA in standard recording medium was zero, while the 95 % confidence intervals became very large, reflecting the divergent responses between cultures: some cultures were able to cope with additional NMDA-R activation resulting in increased bursting frequency, while others (partially) lost the calcium gradient across the plasma membrane leading to decreased bursting. The latter condition was predominant in cultures that were recorded in NMDA-R sensitizing medium and were exposed to the highest concentrations of QA. This disturbed activity was also reflected in the correlation scores (Fig. 6i). These data suggest that the competitive NMDA-R agonist QA exerts a mild excitotoxic effect that can exacerbate former excitotoxic insults, in this case the NMDA-R sensitizing recording medium.
Successive addition of these NMDA-R (ant)agonists suggested that the ratio of agonists versus antagonists (e.g., KA and QA) is crucial for the maintenance of normal network activity (Fig. 6j–m). The concentrations of the compounds in this experiment were chosen based on the result of the dose–response experiment (KA and QA 100 µM; ketamine 50 µM) and their effects were assessed in standard recording medium. Ketamine decreased synchronous bursting in an irreversible way, i.e., addition of QA did not restore normal network activity or attenuate the irreversible effect of ketamine (Fig. 6j, k). These ketamine-related effects were expected as it is a non-competitive antagonist of the NMDA-R. In contrast, addition of QA in the presence of KA led in most cases to normalization of the synchronous burst frequency, as shown in Fig. 6l. Accordingly, KA was able to revert the excitatory effect of QA on synchronous burst frequency (Fig. 6m), indicating that the relative KA/QA concentration ratio is decisive for neuronal network activity.
Together, these data demonstrate that the presented in vitro setup was suitable to study the role and functionality of the NMDA-R at the level of the neuronal network in physiological and pathological conditions.
Discussion
In this paper, we show that monitoring the maturation and spontaneous activity of in vitro neuronal networks is a valid tool to study synaptogenesis and synaptic connectivity in physiological and pathological conditions. We monitored calcium dynamics on consecutive days and identified a window, i.e., 5, 6, and 7 days after plating, during which remarkable changes in neuronal activity occurred. The initially random activity of the neurons synchronized over time, resulting in correlated network bursts. This process clearly demonstrates the maturation of the neuronal network in vitro, and can be used as a model to study synaptogenesis. At later timepoints, these cultures can be used to study the effect of pharmacological and genetic interventions on established network activity and synaptic communication.
Spontaneous electrical activity was monitored via live cell calcium imaging. This technique proved to offer a higher sensitivity in detecting subtle changes in neuronal physiology compared to monitoring neuronal morphology. Though micro-electrode arrays (MEAs) offer an excellent time resolution and are often used in neuronal network research, calcium imaging offers two main advantages: first, the activity can be registered at the single-cell level. Second, the density of the neurons can be reduced with calcium imaging, thereby limiting the number of animals that need to be sacrificed and dissected in high-content screening campaigns. The time resolution of calcium imaging was shown to be sufficiently high to detect robust activity of the neuronal network, and via acute application of TTX, we confirmed that the observed calcium fluxes occurred in response to action potentials (Herzog et al. 2011; Smetters et al. 1999).
The observed neuronal network activity is thought to be involved in activity-dependent synaptogenesis and to stimulate the fine-tuning of newly formed synapses (Ben-Ari 2001; Mohajerani et al. 2007). The synchronization of neuronal activity between 5 and 7 DIV coincided with an important increase in neurite outgrowth and synapse density. We found that the outgrowth of neurites reached a plateau at 11 DIV, whereas the density of synapses increased until 14 DIV. As such, synchronous network activity depends on the maturity of a critical number of synapses, but additional synapses are formed afterward.
Dendritic spines are highly dynamic protrusions that are present on the dendritic tree of CNS neurons, both in vivo and in vitro, and contain the postsynaptic machinery of the excitatory synapse, including neurotransmitter receptors, postsynaptic density, spine apparatus, etc. Since dendritic spines are dynamic structures that can change in shape and density in response to paradigms that influence synaptic strength, the general belief is that spines comprise the primary sites of learning and memory in the CNS (Bhatt et al. 2009; Papa et al. 1995). Consequently, dendritic spine density is often used as a morphological readout for synaptic strength and neuronal connectivity. In the presented model, the largest increase in spine density occurred from 7 DIV onwards, after establishment of the neuronal network. This observation supports the idea that robust neuronal network activity contributes to the fine-tuning of synapses and that dendritic spines provide electrical and biochemical compartmentalization of extensively used synapses (Segal 2010). Dendritic spine density was significantly increased by astrocytic support, while the effect of decreased NGF was less pronounced. Compared to neurite outgrowth and synapse density, dendritic spine density was a more sensitive readout, as the former were not affected in these long-term experiments. The functional approach whereby network activity was visualized with a calcium indicator proved to be the most sensitive readout. As such, this is the preferred technique to be incorporated into a high-content screening environment to study synaptogenesis and synaptic connectivity.
We showed that synchronized activity in cultivated hippocampal neurons is mediated by the NMDA-R, as MK-801 and ketamine potently blocked ongoing network activity. Also, the composition of the recording medium with respect to NMDA-R sensitization, influenced the bursting properties of the neuronal network and altered the sensitivity to NMDA-R (ant)agonists. KA and QA showed effects that were reminiscent of NMDA-R antagonism (KA) and agonism (QA). Successive addition of KA and QA clearly showed that the relative concentration of both compounds is an important cue that influences neuronal network activity. The concentration ratio is known to be disturbed in multiple neurological disorders with an inflammatory component, including schizophrenia, where the KA/QA ratio is increased (Erhardt et al. 2001; Lavebratt et al. 2013) contributing to NMDA-R hypofunction. In Alzheimer’s disease, the KA/QA ratio is decreased (Gulaj et al. 2010; Plangar et al. 2011) and contributes to neuronal degeneration via QA’s excitotoxic action. As such, we showed that the presented model allows to study NMDA-R function in physiological, as well as in pathological conditions.
In addition to monitoring the acute effects on neuronal network activity, the system also allows for longitudinal studies into developmental processes. Astrocytes are the principal supporting cells in the CNS and are known to exert many functions to support neuronal functionality, including structural and metabolic support, neurotransmitter uptake and release etc. Astrocytes also play an active role in synaptic transmission, via the so-called tripartite synapse (Perea et al. 2009). We showed a positive relation between the supportive functions of astrocytes and spontaneous neuronal activity. The beneficial effect on network formation was not only seen in the presence of additional astrocytes, but also following administration of conditioned medium that contained signaling molecules and growth factors released by astrocytes (Pyka et al. 2011a). In addition to the classical neurotrophic factors NGF, brain derived neurotrophic factor (BDNF) and neurotrophins (NT’s), a number of other secreted factors have been proposed to mediate such positive effect, including thrombospondins (Christopherson et al. 2005), estradiol (Hu et al. 2007) and astrocyte-derived extracellular matrix components (Pyka et al. 2011b). Unpublished data showed that recombinant NGF, BDNF, or VEGF alone do not accelerate network formation, suggesting that a combination of the above-mentioned factors is necessary to induce the effect. Although the effect is most probably attributable to soluble factors, it should be noted that the conditioned medium accelerated astrocyte proliferation, so that cultures which received conditioned medium contained more astrocytes than control cultures.
Growth factors such as NGF are essential for the maintenance of neuronal physiology and growth (Cohen and Levi-Montalcini 1957; Otal et al. 2005) and the TrkA receptor for NGF is expressed on primary hippocampal neurons (Culmsee et al. 2002). As opposed to the beneficial effect of increased neurotrophic support on network formation, lowering the NGF concentration had detrimental effects on the synchronization of network activity as measured by calcium imaging. At the same time, there was no significant decrease in neuronal morphology. The partial deprivation of NGF from 4 DIV onwards can be considered as a mild insult, since the cultures were allowed to grow under normal conditions up to 4 DIV and other neurotrophic signaling, such as BDNF and NT3/4, remained intact. These results underline the sensitivity of calcium imaging in detecting subtle changes in neuronal physiology.
In recent years, the technological progress in the field of automated imaging has enabled reliable calcium imaging of living cells (Chan et al. 2005). In combination with the reproducible cultivation in 96-well plates and the custom-made image analysis algorithm (Cornelissen et al. 2013), the neuronal culture model can easily be integrated into a high-content screening platform, making it a valuable tool in a drug discovery environment. In addition to pharmacological treatment, the high accessibility of the neurons allows the use of molecular biological techniques, such as RNA interference or viral transduction (MacLaren et al. 2011). Such approaches can be used to develop new in vitro disease models, which in turn would allow phenotypic drug screening, compound profiling and neurotoxicity testing. In addition to being applicable in drug discovery, the presented model can be of value for fundamental research since it can be used, for instance, to elucidate molecular mechanisms that contribute to synaptogenesis and network synchronization, to study NMDA-R physiology, neuron-glia interactions, neuroinflammation, etc. Given the limited amount of time of about 3–4 weeks that the cultivated neurons live, this system allows to study neurodevelopment, mature network physiology, and neurodegeneration.
In conclusion, we have validated the monitoring of neuronal network formation in primary hippocampal cultures as a valuable tool to study synaptogenesis and synaptic communication. We showed that calcium imaging is a sensitive tool to assess neuronal network functionality and that synchronous network activity is mediated by the NMDA-R. This in vitro approach may contribute to our future knowledge of molecular mechanisms underlying synaptogenesis and synaptic connectivity. In addition, the presented research lays the foundation for a new high-content screening assay that may contribute to drug discovery in a broad range of mental disorders.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a Baekeland fellowship of the Agency for Innovation by Science and Technology in Flanders (IWT) to Peter Verstraelen (IWT090279). The UltraVIEW VoX microscope was funded by the Hercules Foundation (AUAH-09-001). The authors would like to thank Sofie Thys for technical assistance during the preparation of hippocampal cultures and Lieve Svensson for the breeding of timed-pregnant mice. We would like to thank Francis Terloo, Inge Keuleers and Dominique De Rijck for general technical assistance. In addition, we thank Dr. Tom Jacobs and Tobias Verbeke for assistance with the statistical analyses of our experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- 5-HT
Serotonin
- 6FAM
6-Carboxyfluorescein
- AraC
Arabinosylcytosine
- BBQ
BlackBerry quencher
- BDNF
Brain derived neurotrophic factor
- cAMP
Cyclic adenosine monophosphate
- CNS
Central nervous system
- DIV
Days in vitro
- FSK
Forskolin
- GFAP
Glial fibrillary acidic protein
- KA
Kynurenic acid
- MEA
Multi-electrode array
- NGF
Nerve growth factor
- NMDA-R
N-Methyl-d-aspartate receptor
- NT
Neurotrophin
- PKA
Protein kinase A
- QA
Quinolinic acid
- TTX
Tetrodotoxin
References
- Balu DT, Coyle JT (2011) Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci Biobehav Rev 35(3):848–870. doi:10.1016/j.neubiorev.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y (2001) Developing networks play a similar melody. Trends Neurosci 24(6):353–360 [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB (2009) Dendritic spine dynamics. Annu Rev Physiol 71:261–282. doi:10.1146/annurev.physiol.010908.163140 [DOI] [PubMed] [Google Scholar]
- Bokde AL, Ewers M, Hampel H (2009) Assessing neuronal networks: understanding Alzheimer’s disease. Prog Neurobiol 89(2):125–133. doi:10.1016/j.pneurobio.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Booher J, Sensenbrenner M (1972) Growth and cultivation of dissociated neurons and glial cells from embryonic chick, rat and human brain in flask cultures. Neurobiology 2(3):97–105 [PubMed] [Google Scholar]
- Bradshaw NJ, Porteous DJ (2012) DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology 62(3):1230–1241. doi:10.1016/j.neuropharm.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GK, Richards GR, Peters M, Simpson PB (2005) High content kinetic assays of neuronal signaling implemented on BD pathway HT. Assay Drug Dev Technol 3(6):623–636. doi:10.1089/adt.2005.3.623 [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120(3):421–433. doi:10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA (1995) Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci 15(10):6498–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Levi-Montalcini R (1957) Purification and properties of a nerve growth-promoting factor isolated from mouse sarcoma 180. Cancer Res 17(1):15–20 [PubMed] [Google Scholar]
- Cohen E, Ivenshitz M, Amor-Baroukh V, Greenberger V, Segal M (2008) Determinants of spontaneous activity in networks of cultured hippocampus. Brain Res 1235:21–30. doi:10.1016/j.brainres.2008.06.022 [DOI] [PubMed] [Google Scholar]
- Cornelissen F, Verstraelen P, Verbeke T, Pintelon I, Timmermans JP, Nuydens R, Meert T (2013) Quantitation of chronic and acute treatment effects on neuronal network activity using image and signal analysis: toward a high-content assay. J Biomol Screen. doi:10.1177/1087057113486518 [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bayer KU (2012) CaMKII regulation in information processing and storage. Trends Neurosci 35(10):607–618. doi:10.1016/j.tins.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J (2002) Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience 115(4):1089–1108 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8(4):1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drever BD, Riedel G, Platt B (2011) The cholinergic system and hippocampal plasticity. Behav Brain Res 221(2):505–514. doi:10.1016/j.bbr.2010.11.037 [DOI] [PubMed] [Google Scholar]
- Elmslie KS, Yoshikami D (1985) Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord. Brain Res 330(2):265–272 [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313(1–2):96–98 [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L (2012) Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. doi:10.1038/npp.2012.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TL, De Camilli P, Banker G (1994) Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. J Neurosci 14(11 Pt 1):6695–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Srivastava T, Soderling TR (2012) Structural modulation of dendritic spines during synaptic plasticity. Neuroscientist 18(4):326–341. doi:10.1177/1073858411407206 [DOI] [PubMed] [Google Scholar]
- Gambrill AC, Barria A (2011) NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci USA 108(14):5855–5860. doi:10.1073/pnas.1012676108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SL, Leube RE, Cousin MA (2011) Synaptophysin is required for synaptobrevin retrieval during synaptic vesicle endocytosis. J Neurosci 31(39):14032–14036. doi:10.1523/JNEUROSCI.3162-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R (2004) Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiol Dis 17(3):455–461. doi:10.1016/j.nbd.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Croitoru-Lamoury J, Dormont D, Armati PJ, Brew BJ (2003) Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 41(4):371–381. doi:10.1002/glia.10175 [DOI] [PubMed] [Google Scholar]
- Gulaj E, Pawlak K, Bien B, Pawlak D (2010) Kynurenine and its metabolites in Alzheimer’s disease patients. Adv Med Sci 55(2):204–211. doi:10.2478/v10039-010-0023-6 [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Acsady L, Freund TF (1999) Structural basis of the cholinergic and serotonergic modulation of GABAergic neurons in the hippocampus. Neurochem Int 34(5):359–372 [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Sawa A (2010) Disturbed synaptic connectivity in schizophrenia: convergence of genetic risk factors during neurodevelopment. Brain Res Bull 83(3–4):140–146. doi:10.1016/j.brainresbull.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Herzog N, Shein-Idelson M, Hanein Y (2011) Optical validation of in vitro extra-cellular neuronal recordings. J Neural Eng 8(5):056008. doi:10.1088/1741-2560/8/5/056008 [DOI] [PubMed] [Google Scholar]
- Hida H, Mouri A, Noda Y (2013) Behavioral phenotypes in schizophrenic animal models with multiple combinations of genetic and environmental factors. J Pharmacol Sci 121(3):185–191 [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001) The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 21(19):7463–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Cai WQ, Wu XG, Yang Z (2007) Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience 144(4):1229–1240. doi:10.1016/j.neuroscience.2006.09.056 [DOI] [PubMed] [Google Scholar]
- Ivenshitz M, Segal M (2010) Neuronal density determines network connectivity and spontaneous activity in cultured hippocampus. J Neurophysiol 104(2):1052–1060. doi:10.1152/jn.00914.2009 [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1(5):2406–2415. doi:10.1038/nprot.2006.356 [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274(5290):1133–1138 [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ (2006) Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci 29(7):414–418. doi:10.1016/j.tins.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Kneeland RE, Fatemi SH (2013) Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 42:35–48. doi:10.1016/j.pnpbp.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L, Nikamo P, Traskman-Bendz L, Cichon S, Vawter MP, Osby U, Engberg G, Landen M, Erhardt S, Schalling M (2013) The KMO allele encoding Arg is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. doi:10.1038/mp.2013.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV (2013) Using the lsmeans Package. The R project. http://cran.r-project.org/web/packages/lsmeans/vignettes/using-lsmeans.pdf. Accessed 23 Feb 2014
- MacLaren EJ, Charlesworth P, Coba MP, Grant SG (2011) Knockdown of mental disorder susceptibility genes disrupts neuronal network physiology in vitro. Mol Cell Neurosci 47(2):93–99. doi:10.1016/j.mcn.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melom JE, Littleton JT (2011) Synapse development in health and disease. Curr Opin Genet Dev 21(3):256–261. doi:10.1016/j.gde.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Misztal M, Frankiewicz T, Parsons CG, Danysz W (1996) Learning deficits induced by chronic intraventricular infusion of quinolinic acid—protection by MK-801 and memantine. Eur J Pharmacol 296(1):1–8 [DOI] [PubMed] [Google Scholar]
- Mohajerani MH, Sivakumaran S, Zacchi P, Aguilera P, Cherubini E (2007) Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1 connections in the hippocampus. Proc Natl Acad Sci USA 104(32):13176–13181. doi:10.1073/pnas.0704533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A, Duan J, Mo-Boquist LL, Benedikz E, Sundstrom E (2007) Characterisation of the human NMDA receptor subunit NR3A glycine binding site. Neuropharmacology 52(4):1151–1159. doi:10.1016/j.neuropharm.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Otal R, Martinez A, Soriano E (2005) Lack of TrkB and TrkC signaling alters the synaptogenesis and maturation of mossy fiber terminals in the hippocampus. Cell Tissue Res 319(3):349–358. doi:10.1007/s00441-004-1020-5 [DOI] [PubMed] [Google Scholar]
- Pachernegg S, Strutz-Seebohm N, Hollmann M (2012) GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci 35(4):240–249. doi:10.1016/j.tins.2011.11.010 [DOI] [PubMed] [Google Scholar]
- Paoletti P (2011) Molecular basis of NMDA receptor functional diversity. Eur J Neurosci 33(8):1351–1365. doi:10.1111/j.1460-9568.2011.07628.x [DOI] [PubMed] [Google Scholar]
- Papa M, Bundman MC, Greenberger V, Segal M (1995) Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci 15(1 Pt 1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14(3):285–293. doi:10.1038/nn.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32(8):421–431. doi:10.1016/j.tins.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Pickering M, Pickering BW, Murphy KJ, O’Connor JJ (2008) Discrimination of cell types in mixed cortical culture using calcium imaging: a comparison to immunocytochemical labeling. J Neurosci Methods 173(1):27–33. doi:10.1016/j.jneumeth.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Plangar I, Zadori D, Klivenyi P, Toldi J, Vecsei L (2011) Targeting the kynurenine pathway-related alterations in Alzheimer’s disease: a future therapeutic strategy. J Alzheimer’s Dis 24(Suppl 2):199–209. doi:10.3233/JAD-2011-110131 [DOI] [PubMed] [Google Scholar]
- Pyka M, Busse C, Seidenbecher C, Gundelfinger ED, Faissner A (2011a) Astrocytes are crucial for survival and maturation of embryonic hippocampal neurons in a neuron-glia cell-insert coculture assay. Synapse 65(1):41–53. doi:10.1002/syn.20816 [DOI] [PubMed] [Google Scholar]
- Pyka M, Wetzel C, Aguado A, Geissler M, Hatt H, Faissner A (2011b) Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci 33(12):2187–2202. doi:10.1111/j.1460-9568.2011.07690.x [DOI] [PubMed] [Google Scholar]
- Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ (2009) The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One 4(7):e6344. doi:10.1371/journal.pone.0006344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC (2001) Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50(7):521–530 [DOI] [PubMed] [Google Scholar]
- Segal M (2010) Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci 31(12):2178–2184. doi:10.1111/j.1460-9568.2010.07270.x [DOI] [PubMed] [Google Scholar]
- Sivanesan S, Tan A, Rajadas J (2013) Pathogenesis of Abeta oligomers in synaptic failure. Curr Alzheimer Res 10(3):316–323 [DOI] [PubMed] [Google Scholar]
- Smetters D, Majewska A, Yuste R (1999) Detecting action potentials in neuronal populations with calcium imaging. Methods 18(2):215–221. doi:10.1006/meth.1999.0774 [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ (2006) Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry 59(10):929–939. doi:10.1016/j.biopsych.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Stipek S, Stastny F, Platenik J, Crkovska J, Zima T (1997) The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochem Int 30(2):233–237 [PubMed] [Google Scholar]
- Stone TW, Perkins MN (1981) Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol 72(4):411–412 [DOI] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG (2013) An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 34(2):136–143. doi:10.1016/j.tips.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA (1995) Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci 15(10):6509–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB (1993) Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron 10(2):267–278 [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM (2001) Electrical activity and development of neural circuits. Nat Neurosci 4(Suppl):1207–1214. doi:10.1038/nn753 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.