Abstract
Recent evidences indicate the existence of an atypical D1 dopamine receptor other than traditional D1 dopamine receptor in the brain that mediates PI hydrolysis via activation of phospholipase Cβ (PLCβ). To further understand the basic physiological function of this receptor in brain, the effects of a selective phosphoinositide (PI)-linked D1 dopamine receptor agonist SKF83959 on cytosolic free calcium concentration ([Ca2+]i) in cultured rat prefrontal cortical astrocytes were investigated by calcium imaging. The results indicated that SKF83959 caused a transient dose-dependent increase in [Ca2+]i. Application of D1 receptor, but not D2, α1 adrenergic, 5-HT receptor, or cholinergic antagonist prevented SKF83959-induced [Ca2+]i rise, indicating that activation of the D1 dopamine receptor was essential for this response. Increase in [Ca2+]i was a two-step process characterized by an initial increase in [Ca2+]i mediated by release from intracellular stores, supplemented by influx through voltage-gated calcium channels, receptor-operated calcium channels, and capacitative Ca2+ entry. Furthermore, SKF83959-stimulated increase in [Ca2+]i was abolished following treatment with a PLC inhibitor. Overall, these results suggested that activation of D1 receptor by SKF83959 mediates a dose-dependent mobilization of [Ca2+]i via the PLC signaling pathway in cultured rat prefrontal cortical astrocytes.
Keywords: SKF83959, Dopamine receptor, Calcium, Astrocyte, Phospholipase C
Introduction
In the mammalian brain, the major catecholamine neurotransmitter dopamine (DA) plays an instrumental role in the regulation of physiological functions, including locomotion, cognition, emotion, and endocrine function. DA signaling through D1 receptors in the prefrontal cortex (PFC) is considered to play a critical role in the maintenance of higher cognitive functions, such as working memory (Goldman-Rakic and Selemon 1997). The physiological actions of DA are mediated via five distinct subtypes (D1–D5) of G protein-coupled receptors (GPCRs). Stimulation (D1, D5 subtypes) or inhibition (D2–D4 subtypes) of adenylyl cyclase (AC) through Gs/olf or Gi/o proteins by DA receptors is the traditional signaling pathway. In addition to the Gs/olf-coupled classical D1 DA receptor that increases the formation of cyclic AMP (cAMP), a D1-like DA receptor that couples to Gq protein, stimulates phospholipase Cβ (PLCβ) and results in hydrolysis of phosphoinositide (PI) has been described (Felder et al. 1989; Undie et al. 1994; Yu et al. 1996; Pacheco and Jope 1997; Panchalingam and Undie 2005). This DA receptor that activates the PLC/IP3 pathway has been named as PI-linked DA receptor (Friedman et al. 1997). SKF83959, a recently identified selective agonist for the PI-linked DA receptor (Panchalingam and Undie 2001; Jin et al. 2003; Zhen et al. 2005; Rashid et al. 2007), provided a powerful tool for exploring the function of this novel signal pathway in brain. In the recent work from our and other laboratory, it has been shown that SKF83959 mediates more effective functions of anti-Parkinsonian symptom with less dyskinesia (Gnanalingham et al. 1995; Andringa et al. 1999b; Waddington et al. 2005; Wirtshafter and Osborn 2005; Zhang et al. 2007).
Calcium signaling has profound effects on almost all aspects of neuronal and glial function. A series of studies have highlighted the ability of the D1 subtype receptors, or a D1-like receptor to elevate cytosolic free Ca2+ concentration ([Ca2+]i) both in brain slices and cultured neurons (Lezcano and Bergson 2002; Lee et al. 2004; Tang and Bezprozvanny 2004; Rashid et al. 2007). Recent evidence from our laboratory indicates that SKF83959 also elevates [Ca2+]i via the activation of PLCβ in hippocampal neurons (Ming et al. 2006).
Astrocytes, the largest population of non-excitable cells in mammalian central nervous system (CNS), were initially considered to form a substrate with supportive and metabolic roles in the CNS. However, nowadays it is known that astrocytes provided more than a merely structural and trophic support for the neurons and, in addition, were able to regulate neuronal activity and synaptic neurotransmission (Araque et al. 2001; Haydon 2001; Koizumi et al. 2003). Ca2+ excitability is an essential characteristic of astrocytes. Astrocytes have numerous receptors and ion channels that enable them to maintain a complex Ca2+-signaling machinery. The elevation of [Ca2+]i in astrocytes has many consequences, including the induction of the chemical transmitter glutamate release (Parpura et al. 1994; Innocenti et al. 2000), which can signal to adjacent neurons (Pasti et al. 1997; Bezzi et al. 1998) and modulate neuronal activity (Newman and Zahs 1998; Parpura and Haydon 2000) and synaptic transmission (Araque et al. 1998; Newman 2003; Perea and Araque 2007). The spatial and temporal regulation of Ca2+ signalling in astrocytes is therefore critical.
It has been demonstrated that astrocytes express D1- and D2-like DA receptors (Zanassi et al. 1999; Reuss et al. 2001). DA has been reported to regulate [Ca2+]i via activation of classical D1-like and D2-like receptors in astrocytes (Reuss et al. 2000, 2001). However, it is unknown about the roles of the selective agonist SKF83959 for the PI-linked atypical DA receptor upon [Ca2+]i in astrocytes. Therefore, the present study was designed to investigate whether SKF83959 modulated Ca2+ signaling in cultured prefrontal cortical astrocytes using pharmacological agents, in order to elucidate the functional implications of this PI-linked atypical DA receptor pathway in the brain.
Materials and Methods
Chemicals
6-Chloro-7,8-dihydroxy-3-methyl-1-(3-methylphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine (SKF83959), hygrobronmide((±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzapepine-7,8-diol hygrobronmide) (SKF38393), spiperone, mesulergine HCl, scopolamine, and prazosin were purchased from RBI (Natric, MA, USA). R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (R-(+)-SCH23390) was purchased from Tocris Cookson (Ellisville, MO). 1-[6-[((17β)-3-methohcyestra-1,3,5[10]-trien-17yl)amino]hexyl]-1H-pyrrole-2,5-dione (U73122), 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-2,5-pyrrolidinedione (U73343), pCPT-cAMP, verapamil, cyclopiazonic acid (CPA), DL-2-amino-5-phonovaleric acid (D-AP5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), GF109203X, 2-aminoethoxydiphenylborate (2-APB) were purchased from Sigma (St. Louis, MO, USA). Fura-2/AM was obtained from Biotium (Hayward, CA, USA). Dulbecco’s modified Eagle’s medium (DMEM)/F12 were obtained from Gibco Invitrogen Corporation (Carlsbad, CA, USA). Other general agents were purchased from commercial suppliers.
All the chemicals were prepared as stock solutions. U73122, U73343, GF109203X, 2-APB, and Fura-2/AM were dissolved in dimethylsulfoxide (DMSO) and stored at −20°C. SKF83959 was dissolved in the distilled water. These stock solutions were diluted to the final concentrations with the extracellular solution before application. The final concentration of DMSO was <0.05%. No detectable effect of the vehicles was found in the experiments.
Cell Culture
Neonatal Sprague-Dawley (SD) rats (day 0–3) of both sexes were obtained from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science & Technology. The University Animal Welfare Committee approved the animal protocol used. Primary rat cortical astrocytes were isolated as described in our previous study (Chen et al. 1997) with some modifications. Briefly, PFC of newborn SD rats were dissected and rinsed in ice cold Dulbecco’s phosphate buffered saline. Blood vessels and white matter were removed and tissues were treated with 0.125% trypsin for 25 min at 37°C. Cells were collected by centrifugation and resuspended in DMEM/F12 (1:1) with 10% fetal bovine serum (heat-inactivated, Hyclone), 2 mM l-glutamine, penicillin (100 units/ml), streptomycin (100 units/ml). Cells were plated at a density of 106–107/mm2 on 50 cm2 flasks pre-coated with poly-l-lysine and kept at 37°C in a 5% CO2 incubator (SHELLAB, Oregon, USA). The medium was replaced with fresh medium every 3 days. At confluence (9–10 days), the flask was subjected to shaking for 16–18 h at 37°C. The monolayer was treated with 0.125% trypsin −0.02% EDTA for a short duration, after which the cells were dissociated and plated onto uncoated glass coverslips. The cells were maintained for 3–4 days in the culture until used for [Ca2+]i measurements.
Calcium Imaging
Digital calcium imaging was performed as described by Ming et al. (2006). Cultured prefrontal cortical astrocytes were rinsed three times in artificial cerebrospinal fluid (ACSF) containing (mM) NaCl 140, KCl 5, MgCl2 1, CaCl2 2, glucose 10, and HEPES 10 (pH 7.3). Then, cells were incubated with 1 μM Fura-2/AM for 30 min at 37°C, and subsequently washed three times with ACSF to remove the excess extracellular Fura-2/AM. In Ca2+-free solutions, CaCl2 was omitted, and 100 μM EGTA was added. Coverslips were mounted on a recording chamber and perfused with HEPES-buffered solution using a peristaltic pump at a rate of 1.5–4 ml/min. Experiments were performed at room temperature. [Ca2+]i changes were measured by a Ratio Vision digital fluorescence microscopy system (TILL Photonics GmbH, Germany). Fura-2/AM loaded cells were illuminated at 340 nm for 150 ms and 380 nm for 50 ms at 1 s intervals using a TILL Polychrome monochromator. Fura-2 fluorescence emission was imaged at 510 nm by an intensified cooled charge coupled device (TILL Photonics GmbH) through fluor oil immersion lens (Olympus IX70) and a 460 nm long-pass barrier filter. F340/F380 fluorescence ratios were generated by TILLVISION 4.0 software. Paired F340/F380 fluorescence ratio images were acquired every second for [Ca2+]i. [Ca2+]i was presented as the ratio of the fluorescence signals obtained (340/380 nm). All experiments were repeated at least three times using different batches of cells.
Statistical Analysis
The amplitude of [Ca2+]i transient represents the difference between baseline concentration and the transient peak response to the stimulation. The amplitudes of [Ca2+]i elevation upon the basal [Ca2+]i were normalized to the basal [Ca2+]i, which was taken as 100%. Data are presented as mean ± SEM. Data were analyzed using SPSS 12.0 software. Student’s t-test or one-way ANOVA was used to test for significance. A value of P < 0.05 was considered statistically significant.
Results
SKF83959 Elevated [Ca2+]i in Cultured Rat Prefrontal Cortical Astrocytes
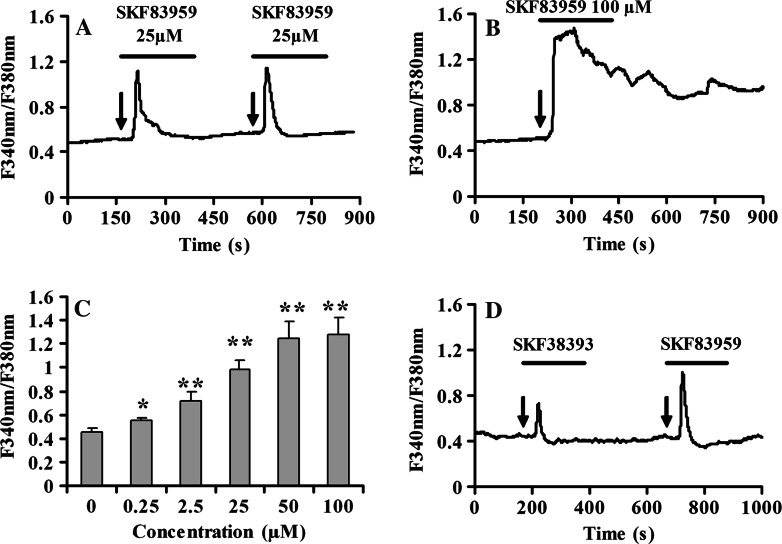
In the first set of experiments, we employed the Ca2+ imaging technique to investigate the dynamic alteration of intracellular calcium mediated by SKF83959 in cultured rat prefrontal cortical astrocytes. Application of SKF83959 (0.25–100 μM) increased [Ca2+]i in a dose-dependent manner (Fig. 1c). Stimulation was observable at 0.25 μM and reached the maximal effect at 50 μM. The EC50 for potentiation effect was 24.5 ± 1.1 μM (fitted with sigmaplot version 9.0 to the Hill equation). When cells were stimulated by concurrent administration of 25 μM SKF83959, a significant increase in [Ca2+]i was observed. Calcium mobilization was initially detected 30 s following the application of 25 μM SKF83959, and the signal peaked within 60 s of initial exposure to agonist. Although there was variability in the shape of the falling phase, typical [Ca2+]i transients rapidly reached a maximum and then slowly decayed to baseline levels. Responses were detected in 46% (54 of 117 cells tested) of astrocytes, resulting in a 118.0 ± 10.2% increase over basal levels (n = 54, Fig. 1a). However, when SKF83959 was applied for 100 μM, the 75% of the cells displayed a biphasic Ca2+ response consisting of an initial transient peak and a sustained component (n = 15, Fig. 1b). A D1 DA receptor-specific agonist SKF38393 (25 μM) also induced a 56.0 ± 8.2% increase over basal levels (n = 12, Fig. 1d). However, the magnitude of elevation was less than that in the presence of 25 μM SKF83959.
Fig. 1.
SKF83959 induces [Ca2+]i transient in cultured rat prefrontal cortical astrocytes. a Astrocytes were treated with 25 μM SKF83959 for designated time. The arrows indicate the time of SKF83959 application. [Ca2+]i increase was recorded as described in the methods and expressed as F340/F380 ratio. b Cultured rat prefrontal cortical astrocytes were treated with 100 μM SKF83959 for 200 s (n = 15). c Summary data of the maximal response in [Ca2+]i for each dose of SKF83959 from three independent experiments. The EC50 for potentiation effect was 24.53 ± 1.06 μM (fitted with sigmaplot version 9.0 to the Hill equation). d Cultured rat prefrontal cortical astrocytes were treated with 25 μM SKF38393 for 200 s (n = 12).*P < 0.05, **P < 0.01 vs. basal [Ca2+]i
D1-Like Dopamine Receptor Mediated SKF83959-Induced [Ca2+]i Rise in Cultured Rat Prefrontal Cortical Astrocytes
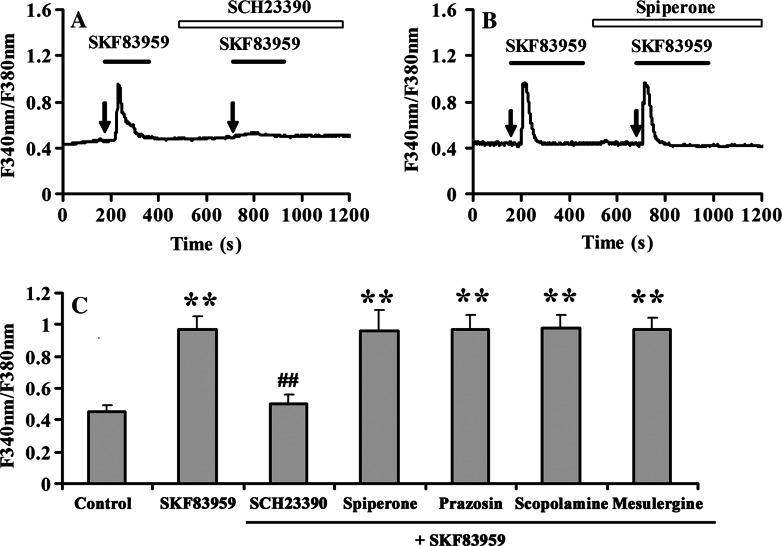
Next, we determined whether D1-like DA receptor mediated SKF83959-induced elevation of [Ca2+]i. As anticipated, pre-incubation of the D1 receptor-specific antagonist SCH23390 (10 μM) to the cultured prefrontal cortical astrocytes prior to SKF83959 (25 μM) stimulation almost completely blocked SKF83959-induced increase of [Ca2+]i (n = 30, Fig. 2a). However, application of the D2 receptor antagonist spiperone (10 μM) did not provide any significant inhibition on SKF83959-induced increase of [Ca2+]i (n = 13, Fig. 2b), demonstrating that the SKF83959-induced stimulation of [Ca2+]i increase was mainly mediated by D1-like, but not D2-like receptor. Although SKF83959 is known to have a high affinity to the D1-like receptor, it also exhibits a moderate or weak affinity to PLC-linked neurotransmitter receptors, such as D2 receptors, 5-hydroxytryptamine type 2 (5-HT2) receptors, α-adrenoceptors, and muscarinic type acetylcholine receptors. Therefore, the potential roles of these receptors in SKF83959-mediated calcium increase were investigated. Accordingly, application of other receptor antagonists such as the 5-HT1c and 5-HT2 antagonist mesulergine HCl (10 μM), the α-adrenoceptor antagonist prazosin (1 μM) or the muscarinic receptor antagonist scopolamine (10 μM) had no effect on SKF83959-stimulated [Ca2+]i elevation in cultured prefrontal cortical astrocytes (Fig. 2c). These results indicated that selective stimulation of the D1-like DA receptor was responsible for SKF83959-induced increase of [Ca2+]i.
Fig. 2.
D1 dopamine receptor mediates SKF83959-stimulated [Ca2+]i elevation. a A representative trace showing pretreatment with 10 μM SCH23390 for 3 min blocked SKF83959-induced [Ca2+]i elevation (n = 30). b Effect of D2 dopamine receptor antagonist spiperone (10 μM) on SKF83959-induced [Ca2+]i (n = 13). Arrow indicates the time when SKF83959 was applied. c Summary data of pre-incubation with 10 μM SCH23390, 10 μM spiperone, 1 μM prazosin, 10 μM scopolamine, or 10 μM mesulergine HCl for 3 min on the stimulation action of SKF83959. All experiments were repeated three times using different batches of cells. **P < 0.01 vs. control, ## P < 0.01 vs. SKF83959
SKF83959-Evoked Enhancement of [Ca2+]i Depended on the Release from Intracellular Calcium Store
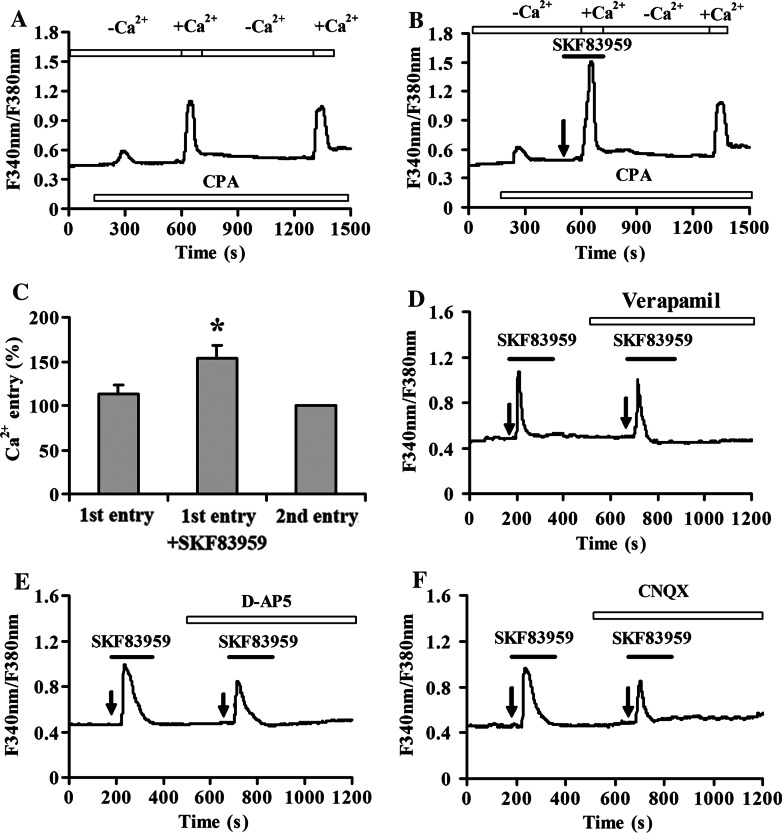
To explore the respective contribution of Ca2+ influx from extracellular compartment or the release from intracellular Ca2+ stores on SKF83959-induced [Ca2+]i elevation in astrocytes, the standard extracellular solution was replaced by Ca2+-free solution. In the absence of extracellular Ca2+, 25 μM SKF83959 was able to induce a significant increase of [Ca2+]i (66.8 ± 8.3% over basal levels, n = 25), although the magnitude of elevation was less than that in the presence of extracellular Ca2+ (Fig. 3a). It was suggested that two components of Ca2+ transit were involved. The importance of Ca2+ release from intracellular stores in triggering the elevation of [Ca2+]i was further investigated. After pre-incubation with 10 μM CPA, an inhibitor of sarcoplasmic/endoplasmic reticulum (ER) Ca2+-ATPase, to deplete the intracellular calcium store in astrocytes bathed in Ca2+-containing medium, SKF83959 failed to evoke a significant increase in [Ca2+]i (n = 32, Fig. 3b). These experiments indicated that Ca2+ release from intracellular stores was necessary and sufficient for SKF83959-induced [Ca2+]i rise.
Fig. 3.
SKF83959-mediated increase in [Ca2+]i depends on the release from intracellular calcium stores. a Representative experiment showing the amplitude of [Ca2+]i induced by SKF83959 in the absence of extracellular Ca2+ was lower than that in the presence of extracellular Ca2+ (n = 25). −Ca2+, Ca2+-free ACSF applied. +Ca2+, Ca2+-containing ACSF applied. b Application of 10 μM CPA to deplete the intracellular calcium store completely blocked SKF83959-induced [Ca2+]i increase in astrocytes (n = 32). Arrows indicate the time of SKF83959 application. The experiments were repeated at least three times
Extracellular Calcium Influx was Involved in SKF83959-induced [Ca2+]i Elevation
Although above studies suggest that Ca2+ influx from extracellular influx may contribute to SKF83959-induced [Ca2+]i elevation, the exact source of extracellular Ca2+ influx is unclear. To address this issue, the effect of SKF83959 on CPA-induced Ca2+ entry was first investigated to evaluate the role of capacitative Ca2+ entry (CCE). The following protocol was used as previously described (Jung et al. 2000). In the control group, intracellular Ca2+ stores were emptied by application of 10 μM CPA in Ca2+-free solution. In the continued presence of CPA, the amplitudes of the concomitant Ca2+ increases were compared when Ca2+ was added, removed, and returned to the extracellular solution. As shown in Fig. 4a, b, after depletion of the intracellular Ca2+ stores by CPA in Ca2+-free solution, a large [Ca2+]i increase was evoked upon Ca2+-containing solution regarded as the first Ca2+ entry phase. This increase in [Ca2+]i was abolished while calcium-free solution was applied, but recovered again after perfusion with Ca2+-containing solution regarded as the second Ca2+ entry phase. To test whether SKF83959 was able to enhance CCE, the protocol was modified slightly (Fig. 4b). Briefly, the first Ca2+ entry phase occurred in the presence of both CPA (10 μM) and SKF83959 (25 μM). Although SKF 83959 induced no further Ca2+ release from intracellular calcium stores (confirming that the stores were empty) in Ca2+-free solution, the amplitude of the first [Ca2+]i increase was significantly increased by 36.5 ± 8.7% in Ca2+-containing solution (n = 24, P < 0.01 vs. control; Fig. 4c), indicating that CCE was involved in SKF83959-induced [Ca2+]i elevation.
Fig. 4.
CCE, voltage-gated calcium channels and receptor-operated calcium channels contribute to SKF83959-induced Ca2+ influx. a, b Capacitative Ca2+ changes upon removal and readdition of external Ca2+ to cells treated with 10 μM CPA with (right panel) or without (left panel) application of SKF83959 (n = 24). −Ca2+, Ca2+-free ACSF applied. +Ca2+, Ca2+-containing ACSF applied. Arrow indicates the time of SKF83959 application. The experiments were repeated at least three times and representative data are shown for each treatment. c Statistical analysis of CCE experiment. The amplitudes of the cytosolic [Ca2+]i increases upon the second Ca2+ readdition were normalized to the second [Ca2+]i increase, which was taken as 100%. **P < 0.01 vs. control (the first entry). d Application of 10 μM verpamil for 3 min prior to the addition of 25 μM SKF83959 reduced the drug-stimulated [Ca2+]i elevation (n = 18). e Pretreatment with 50 μM D-AP5 for 3 min before the addition of 25 μM SKF83959 reduced the drug-stimulated [Ca2+]i elevation (n = 30). f SKF83959-stimulated [Ca2+]i elevation was attenuated by pre-incubation with 30 μM CNQX (n = 36)
To further determine whether voltage-gated calcium channels (VGCCs) involves SKF83959-induced [Ca2+]i elevation in these experiments, verapamil was employed to block VGCC. As shown in Fig. 4d, verapamil (10 μM) slightly reduced the SKF83959-induced [Ca2+]i increase. The maximal stimulation of [Ca2+]i by 25 μM SKF83959 in the presence of verapamil were 102.0 ± 11.2% over basal levels (n = 18).
Next, the role of receptor-operated calcium channels (ROCC) in this process was checked. 50 μM D-AP5 (Fig. 4e) and 30 μM CNQX (Fig. 4f) attenuated the SKF83959-induced elevation of [Ca2+]i. The maximal increases in [Ca2+]i over basal levels were 73.3 ± 8.3% (n = 30) and 70.4 ± 7.6% (n = 36) for D-AP5 and CNQX, respectively. This result indicated that activation of AMPA receptor and NMDA receptor also contributed to the calcium influx stimulated by SKF83959 in astrocytes.
Activation of Phospholipase C (PLC) Signaling Pathways was Essential for SKF83959-Stimulated [Ca2+]i Elevation
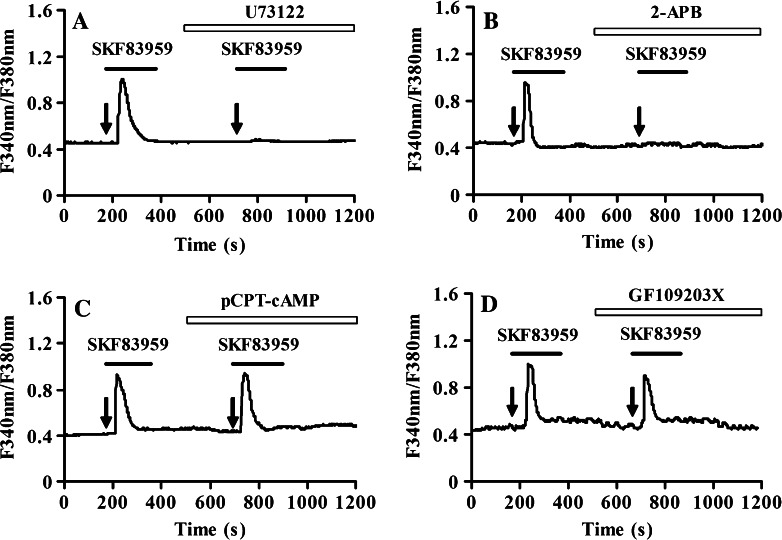
To ascertain which intracellular signaling pathway is activated in SKF83959-induced Ca2+ release from intracellular calcium stores, cells were treated with 10 μM U73122, a PLC inhibitor. Notably, the increase in intracellular calcium levels by SKF83959 was prevented by pre-treatment with U73122 (n = 30, Fig. 5a), whereas U73343, an inactive analog of U73122 (without inhibitory activity), did not alter SKF83959-stimulated [Ca2+]i elevation (data not shown). 100 μM 2-APB, a blocker of IP3-sensitive Ca2+ channels and TRP channels, including store-operated calcium channels (SOCC), also effectively abolished SKF83959-elicited [Ca2+]i elevation (n = 28, Fig. 5b). To determine whether coupling to the AC signaling pathway was affected by SKF83959, cells treated with pCPT-cAMP, a membrane permeable cAMP analog was examined. In contrast, pretreated with 200 μM pCPT-cAMP for 3 min had no effect on the increase in SKF83959-mediated intracellular calcium levels (n = 22, Fig. 5c). Meanwhile, H-89, a permeable PKA inhibitor did not alter SKF83959-stimulated [Ca2+]i elevation (data not shown). When cells were treated with PKC inhibitor GF109203X (2 μM) and SKF83959, SKF83959-evoked responses were attenuated to 93.1 ± 11.4% over basal levels (n = 18, Fig. 5d), which suggested that the potentiation effect of SKF83959 was partially dependent on the activation of PKC. These data suggested that SKF83959-mediated calcium signaling was not dependent on PKA pathway, but a PLC dependent pathway.
Fig. 5.
Activation of PLC/IP3 pathway is essential for SKF83959-stimulated [Ca2+]i increase. Cultured rat prefrontal cortical astrocytes were pretreated with various drugs for 3 min prior to application of 25 μM SKF83959. a Pretreatment with 10 μM U73122 blocked SKF83959-induced [Ca2+]i increase in astrocytes (n = 30). The experiments were repeated at least three times and representative data are shown for each treatment. b Pretreatment with 100 μM 2-APB blocked SKF83959-induced [Ca2+]i increase in astrocytes (n = 28). c Pretreatment with 200 μM pCPT-cAMP had no effect on SKF83959-induced [Ca2+]i increase in astrocytes (n = 22). d Pretreatment with 2 μM GF109203X reduced SKF83959-induced [Ca2+]i increase in astrocytes (n = 18)
Discussion
In the present study, we found that SKF83959 enhanced [Ca2+]i in a dose-dependent manner in cultured prefrontal cortical astrocytes, which was mediated by intracellular Ca2+ release via PLC activation, but not PKA activation. Although the increase in [Ca2+]i initially driven by Ca2+ release from intracellular stores, influx through VGCC, ROCC, and CCE contributed to this response.
The PI-linked D1 receptors are enriched in brain tissue, including frontal cortical, the striatum, and hippocampal areas (Jin et al. 2003). A previous study showed that application of D1 receptor-specific agonist SKF38393 increased [Ca2+]i immediately in fetal human astrocyte cell line (SVG cells) (Kinor et al. 2001). In our study, SKF83959 and SKF38393 elicited Ca2+ transients in cultured rat prefrontal cortical astrocytes, however, the magnitude of [Ca2+]i elevation evoked by SKF83959 was larger than that evoked by SKF38393. Moreover, the time course of SKF83959 activation of [Ca2+]i transients in the present experiments was similar to that of fetal human astrocyte cell line.
Two major intracellular sources contribute to [Ca2+]i mobilization: an intracellular influx through plasma membrane channels, and an internal reservoir in the ER and mitochondria (Bootman et al. 2001). Our data showed that SKF83959 still induce an increase in [Ca2+]i in the absence of extracellular Ca2+ and was abolished in cells that were depleted of intracellular calcium stores by CPA, which suggested that the mobilization of Ca2+ in the ER was necessary and sufficient for this process.
It has been well characterized that the binding of IP3, the product of a phosphatidylinositol-specific phospholipase C, to its receptor (IP3R) on the ER is the main pathway to initiate Ca2+ release from intracellular stores in many cell types. Our data showed SKF83959-induced [Ca2+]i response was dependent on the activation of the PLC pathway and opening of IP3-sensitive Ca2+ channels on the ER based on the inhibition of the SKF83959-induced [Ca2+]i elevation with U73122 and 2-APB. These results are consistent with the idea that SKF83959 in astrocytes activates a PLC signaling cascade that generates IP3. SKF83959 produces a rapid stereospecific rise in [Ca2+]i from intracellular stores upon stimulation of the PLC/IP3 cascade, which probably represents the first physiological cellular response defining further down-stream signaling events such as PKC. PKC inhibitor treatment attenuated [Ca2+]i increase by SKF83959, suggesting that PKC could be activated by DAG originating from PIP2 cleavage as a potential mechanism of calcium activation. In contrast, pCPT-cAMP, a membrane permeable cAMP analog, did not induce a detectable change, suggesting that SKF83959-induced increase of [Ca2+]i was mediated by a cAMP-independent pathway. Previous reports showed the similar results that SKF83959 did not stimulate AC in rat striatal membranes or in human or monkey glial cell membranes (Arnt et al. 1992; Andringa et al. 1999a; Jin et al. 2003), indicating that activation of cAMP pathway was not responsible for the behavioral responses to SKF83959. Unlike other D1 DA receptor agonists, which stimulate both cAMP and IP3 production in the brain, SKF83959 elicits no stimulation of cAMP production, exerting its IP3 stimulation via a putative PI-linked D1 DA receptor (Deveney and Waddington 1995; Jin et al. 2003). Indeed, our previous study showed that the application of cell-permeant cAMP also did not elicit any alteration of SKF83959 induced [Ca2+]i increase in primary cultured hippocampal neurons (Ming et al. 2006). Our present study further demonstrated that Gq-activated PLC isoforms were a prerequisite for Ca2+ regulation evoked by SKF83959 in cultured prefrontal cortical astrocytes.
In addition to the central role of intracellular Ca2+ for the SKF83959 response, Ca2+ release from intracellular stores is typically accompanied by a substantial Ca2+ influx. It is widely accepted that three categories of Ca2+-permeable channels mediate Ca2+ entry from extracellular space (Barritt 1999; Bootman et al. 2001): VGCCs, ROCCs, and store-operated calcium channel (SOCCs). The presence of VGCCs in cultured astrocytes, although initially subjected to great controversy (Carmignoto et al. 1998), is now widely accepted (Sontheimer 1994; Latour et al. 2003; D’Ascenzo et al. 2004; Burgos et al. 2007), suggesting that Ca2+ influx through VGCCs may play a fundamental role in producing Ca2+ signals in these cells. In our present study, a large proportion of the SKF83959-induced [Ca2+]i was still apparent in the presence of VGCC blockers again suggesting that VGCC entry does not represent in principal mode of Ca2+ entry in cultured rat prefrontal cortical astrocytes. However, verapamil slightly reduced SKF83959-induced increase in [Ca2+]i in cultured rat prefrontal cortical astrocytes. PKC had the ability to regulate VGCC and facilitate calcium influx in astrocytes (Burgos et al. 2007). Therefore, VGCCs may be mediated by PLC and further influence [Ca2+]i in cultured rat prefrontal cortical astrocytes.
It is well known that ROCCs include the nicotinic acetylcholine receptor, NMDA receptor, and AMPA receptor. The expression of NMDA receptor and AMPA receptor mRNA were detected in cultured astrocytes by immunohistochemistry, in situ hybridization and PCR (Van Bockstaele and Colago 1996; Conti 1997; Fan et al. 1999; Schipke et al. 2001). An interaction between D1 DA receptor and NMDA receptor has been established (Lee et al. 2002; Yang and Chen 2005). Activation of NMDA and AMPA receptors in astrocytes caused increase in [Ca2+]i with an associated release of Ca2+ from intracellular stores (Hu et al. 2004). Activation of PLC and of PKC can positively modulate the function of NMDA receptors and AMPA receptors through phosphorylation-mediated events (Tingley et al. 1993; Logan et al. 1999; Lee et al. 2007). In our study, NMDA and AMPA antagonists attenuated the SKF83959-induced elevation of [Ca2+]i in cultured astrocytes, indicating the involvement of activation of NMDA and AMPA receptors. Meanwhile, NMDA receptor also possessed Ca2+-permeability and contributes to calcium influx, so we could not exclude the possibility that the Ca2+-permeability of NMDA receptor also contributes to calcium influx in this study.
In a non-excitable cell system, CCE via SOCCs is considered to be the major mechanism for Ca2+ influx. In the CNS, SOCC has been found in astrocytes (Lo et al. 2002; Golovina 2005). The level of ER Ca2+ store is the primary regulatory signal for SOCC in the plasma membrane. IP3 can lead to activation of CCE (Taylor 2002). If ER [Ca2+]i decreases, the SOCC will be activated that contributes to the magnitude of Ca2+ responses triggered by IP3-linked receptor activation (Grimaldi et al. 1999). Our data showed that when cytosolic Ca2+ stores were emptied by CPA in the absence of extracellular Ca2+, a pronounced [Ca2+]i elevation could be observed upon addition of Ca2+ to the extracellular solution, which suggested the existence of capacitative entry mechanisms in cultured rat prefrontal cortical astrocytes. In the presence of SKF83959, the [Ca2+]i increase upon readdition of external Ca2+ was enlarged even beyond the level observed when the CCE pathway was activated by CPA alone. Hence, it can be concluded that in cultured rat prefrontal cortical astrocytes, SKF83959 induced calcium entry can be mimicked by store depletion. It should be noted that the increases in [Ca2+]i induced by SKF83959 in astrocytes were mediated by CCE in a manner different from that in the hippocampal neurons (Ming et al. 2006).
In summary, our finding demonstrated that the main source of the initial SKF83959 induced [Ca2+]i increase was intracellular and mediated through the PLC signaling pathway in cultured prefrontal cortical astrocytes. Calcium influx through VGCC, ROCC, and CCE contributed to this response. Although the physiological and pathological roles of the [Ca2+]i increase in astrocytes have not been determined, dynamic changes in the [Ca2+]i in astrocytes have received attention as a possible important mechanism for information transferring from astrocytes to neurons. Our results provide further understanding for the regulation mechanism in the activation of DA receptor mediated calcium signaling in astrocytes that may mediate important physiological or pathological challenges in the brain.
Acknowledgments
This work was supported by grants from the National Science Foundation for the Distinguished Young Scientists in China (No. 30425024), the National Basic Research Program of China (973 Program) (No. 2007CB507404), the National Natural Science Foundation of China (No.30570556) to Dr. Jian-Guo Chen, and the Joint Research Fund for Overseas Chinese Young Scholars to Dr. Yong Xia and Dr. Jian-Guo Chen (No. 30728010).
References
- Andringa G, Drukarch B, Leysen JE, Cools AR, Stoof JC (1999a) The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur J Pharmacol 364:33–41. doi:10.1016/S0014-2999(98)00825-5 [DOI] [PubMed] [Google Scholar]
- Andringa G, Stoof JC, Cools AR (1999b) Sub-chronic administration of the dopamine D(1) antagonist SKF 83959 in bilaterally MPTP-treated rhesus monkeys: stable therapeutic effects and wearing-off dyskinesia. Psychopharmacology (Berl) 146:328–334. doi:10.1007/s002130051124 [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG (2001) Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63:795–813. doi:10.1146/annurev.physiol.63.1.795 [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG (1998) Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci 18:6822–6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Sanchez C (1992) Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol 213:259–267. doi:10.1016/0014-2999(92)90690-6 [DOI] [PubMed] [Google Scholar]
- Barritt GJ (1999) Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J 337:153–169. doi:10.1042/0264-6021:3370153 [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL et al (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285. doi:10.1038/34651 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P et al (2001) Calcium signaling—an overview. Semin Cell Dev Biol 12:3–10. doi:10.1006/scdb.2000.0211 [DOI] [PubMed] [Google Scholar]
- Burgos M, Pastor MD, Gonzalez JC, Martinez-Galan JR, Vaquero CF, Fradejas N et al (2007) PKCepsilon upregulates voltage-dependent calcium channels in cultured astrocytes. Glia 55:1437–1448. doi:10.1002/glia.20555 [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Pasti L, Pozzan T (1998) On the role of voltage-dependent calcium channels incalcium signaling of astrocytes in situ. J Neurosci 18:4637–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Backus KH, Deitmer JW (1997) Intracellular calcium transients and potassium current oscillations evoked by glutamate in cultured rat astrocytes. J Neurosci 17:7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F (1997) Localization of NMDA receptors in the cerebral cortex: a schematic overview. Braz J Med Biol Res 30:555–560. doi:10.1590/S0100-879X1997000500001 [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C (2004) Electrophysiological and molecular evidence of L-(Cav1), N-(Cav2.2), and R-(Cav2.3) type Ca2+ channels in rat cortical astrocytes. Glia 45:354–363. doi:10.1002/glia.10336 [DOI] [PubMed] [Google Scholar]
- Deveney AM, Waddington JL (1995) Pharmacological characterization of behavioural responses to SK&F 83959 in relation to‚ D1-like’ dopamine receptors not linked to adenylyl cyclase. Br J Pharmacol 116:2120–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Grooms SY, Araneda RC, Johnson AB, Dobrenis K, Kessler JA et al (1999) AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J Neurosci Res 57:557–571. doi:10.1002/(SICI)1097-4547(19990815)57:4<557::AID-JNR16>3.0.CO;2-I [PubMed] [Google Scholar]
- Felder CC, Jose PA, Axelrod J (1989) The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther 248:171–175 [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR et al (1997) D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol 51:6–11 [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Hunter AJ, Jenner P, Marsden CD (1995) The differential behavioural effects of benzazepine D1 dopamine agonists with varying efficacies, co-administered with quinpirole in primate and rodent models of Parkinson’s disease. Psychopharmacology (Berl) 117:287–297. doi:10.1007/BF02246103 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD (1997) Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 23:437–458 [DOI] [PubMed] [Google Scholar]
- Golovina VA (2005) Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol 564:737–749. doi:10.1113/jphysiol.2005.085035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M, Favit A, Alkon DL (1999) cAMP-induced cytoskeleton rearrangement increases calcium transients through the enhancement of capacitative calcium entry. J Biol Chem 274:33557–33564. doi:10.1074/jbc.274.47.33557 [DOI] [PubMed] [Google Scholar]
- Haydon PG (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2:185–193. doi:10.1038/35058528 [DOI] [PubMed] [Google Scholar]
- Hu B, Sun SG, Tong ET (2004) NMDA and AMPA receptors mediated intracellular calcium increase in rat cortical astrocytes. Acta Pharmacol Sin 25:714–720 [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG (2000) Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci 20:1800–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E (2003) SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem 85:378–386. doi:10.1046/j.1471-4159.2003.01698.x [DOI] [PubMed] [Google Scholar]
- Jung S, Pfeiffer F, Deitmer JW (2000) Histamine-induced calcium entry in rat cerebellar astrocytes: evidence for capacitative and non-capacitative mechanisms. J Physiol 527:549–561. doi:10.1111/j.1469-7793.2000.00549.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinor N, Geffen R, Golomb E, Zinman T, Yadid G (2001) Dopamine increases glial cell line-derived neurotrophic factor in human fetal astrocytes. Glia 33:143–150. doi:10.1002/1098-1136(200102)33:2<143::AID-GLIA1013>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci USA 100:11023–11028. doi:10.1073/pnas.1834448100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA (2003) Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 41:347–353. doi:10.1002/glia.10162 [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y et al (2002) Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 111:219–230. doi:10.1016/S0092-8674(02)00962-5 [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L et al (2007) Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci 36:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ et al (2004) Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem 279:35671–35678. doi:10.1074/jbc.M401923200 [DOI] [PubMed] [Google Scholar]
- Lezcano N, Bergson C (2002) D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol 87:2167–2175 [DOI] [PubMed] [Google Scholar]
- Lo KJ, Luk HN, Chin TY, Chueh SH (2002) Store depletion-induced calcium influx in rat cerebellar astrocytes. Br J Pharmacol 135:1383–1392. doi:10.1038/sj.bjp.0704594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Rivera FE, Leonard JP (1999) Protein kinase C modulation of recombinant NMDA receptor currents: roles for the C-terminal C1 exon and calcium ions. J Neurosci 19:974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Y, Zhang H, Long L, Wang F, Chen J, Zhen X (2006) Modulation of Ca2+ signals by phosphatidylinositol-linked novel D1 dopamine receptor in hippocampal neurons. J Neurochem 98:1316–1323. doi:10.1111/j.1471-4159.2006.03961.x [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR (1998) Modulation of neuronal activity by glial cells in the retina. J Neurosci 18:4022–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA (2003) New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci 26:536–542. doi:10.1016/S0166-2236(03)00237-6 [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Jope RS (1997) Comparison of [3H]phosphatidylinositol and [3H]phosphatidylinositol 4, 5-bisphosphate hydrolysis in postmortem human brain membranes and characterization of stimulation by dopamine D1 receptors. J Neurochem 69:639–644 [DOI] [PubMed] [Google Scholar]
- Panchalingam S, Undie AS (2001) SKF83959 exhibits biochemical agonism by stimulating [(35)S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology 40:826–837. doi:10.1016/S0028-3908(01)00011-9 [DOI] [PubMed] [Google Scholar]
- Panchalingam S, Undie AS (2005) Physicochemical modulation of agonist-induced [35 s]GTPgammaS binding: implications for coexistence of multiple functional conformations of dopamine D1-like receptors. J Recept Signal Transduct Res 25:125–146. doi:10.1080/10799890500184948 [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747. doi:10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA 97:8629–8634. doi:10.1073/pnas.97.15.8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G (1997) Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17:7817–7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317:1083–1086. doi:10.1126/science.1144640 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R et al (2007) D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation fo Gq/11on the striatum. Proc Natl Acad Sci USA 104:654–659. doi:10.1073/pnas.0604049104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss B, Leung DS, Ohlemeyer C, Kettenmann H, Unsicker K (2000) Regionally distinct regulation of astroglial neurotransmitter receptors by fibroblast growth factor-2. Mol Cell Neurosci 16:42–58. doi:10.1006/mcne.2000.0857 [DOI] [PubMed] [Google Scholar]
- Reuss B, Lorenzen A, Unsicker K (2001) Dopamine and epinephrine, but not serotonin, downregulate dopamine sensitivity in cultured cortical and striatal astroglial cells. Receptors Channels 7:441–451 [PubMed] [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F (2001) Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. FASEB J 15:1270–1272 [DOI] [PubMed] [Google Scholar]
- Sontheimer H (1994) Voltage-dependent ion channels in glial cells. Glia 11:156–172. doi:10.1002/glia.440110210 [DOI] [PubMed] [Google Scholar]
- Tang TS, Bezprozvanny I (2004) Dopamine receptor- mediated Ca2+ signaling in striatal medium spiny neurons. J Biol Chem 279:42082–42094. doi:10.1074/jbc.M407389200 [DOI] [PubMed] [Google Scholar]
- Taylor CW (2002) Controlling calcium entry. Cell 111:767–769. doi:10.1016/S0092-8674(02)01197-2 [DOI] [PubMed] [Google Scholar]
- Tingley WG, Roche KW, Thompson AK, Huganir RL (1993) Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature 364:70–73. doi:10.1038/364070a0 [DOI] [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E (1994) Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem 62:2045–2048 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE (1996) Selective distribution of the NMDA-R1 glutamate receptor in astrocytes and presynaptic axon terminals in the nucleus locus coeruleus of the rat brain: an immunoelectron microscopic study. J Comp Neurol 369:483–496. doi:10.1002/(SICI)1096-9861(19960610)369:4<483::AID-CNE1>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Waddington JL, O’Tuathaigh C, O’Sullivan G, Tomiyama K, Koshikawa N, Croke DT (2005) Phenotypic studies on dopamine receptor subtype and associated signal transduction mutants: insights and challenges from 10 years at the psychopharmacology-molecular biology interface. Psychopharmacology (Berl) 181:611–638. doi:10.1007/s00213-005-0058-8 [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Osborn CV (2005) The atypical dopamine D1 receptor agonist SKF 83959 induces striatal Fos expression in rats. Eur J Pharmacol 528:88–94. doi:10.1016/j.ejphar.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Yang CR, Chen L (2005) Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist 11:452–470. doi:10.1177/1073858405279692 [DOI] [PubMed] [Google Scholar]
- Yu PY, Eisner GM, Yamaguchi I, Mouradian MM, Felder RA, Jose PA (1996) Dopamine D1A receptor regulation of phospholipase C isoform. J Biol Chem 271:19503–19508. doi:10.1074/jbc.271.32.19503 [DOI] [PubMed] [Google Scholar]
- Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S (1999) Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture. J Neurosci Res 58:544–552. doi:10.1002/(SICI)1097-4547(19991115)58:4<544::AID-JNR7>3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ma L, Wang F, Chen J, Zhen X (2007) Chronic SKF83959 induced less severe dyskinesia and attenuated L-DOPA-induced dyskinesia in 6-OHDA-lesioned rat model of Parkinson’s disease. Neuropharmacology 53:125–133. doi:10.1016/j.neuropharm.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Friedman E (2005) The role of the phosphatidyinositol-linked D1 dopamine receptor in the pharmacology of SKF83959. Pharmacol Biochem Behav 80:597–601. doi:10.1016/j.pbb.2005.01.016 [DOI] [PubMed] [Google Scholar]