Abstract
1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an extremely abundant glycolytic enzyme, and exemplifies the class of proteins with multiple, seemingly unrelated functions. Recent studies indicate that it is a major intracellular messenger mediating apoptotic cell death. This paper reviews the GAPDH cell death cascade and discusses its clinical relevance.
2. A wide range of apoptotic stimuli activate NO formation, which S-nitrosylates GAPDH. The S-nitrosylation abolishes catalytic activity and confers upon GAPDH the ability to bind to Siah, an E3-ubiquitin-ligase, which translocates GAPDH to the nucleus. In the nucleus, GAPDH stabilizes the rapidly turning over Siah, enabling it to degrade selected target proteins and affect apoptosis.
3. The cytotoxicity of mutant Huntingtin (mHtt) requires nuclear translocation which appears to be mediated via a ternary complex of GAPDH—Siah—mHtt. The neuroprotective actions of the monoamine oxidase inhibitor R-(—)-deprenyl (deprenyl) reflect blockade of GAPDH—Siah binding. Thus, novel cytoprotective therapies may emerge from agents that prevent GAPDH—Siah binding.
KEY WORDS: GAPDH, nitric oxide, nitric oxide synthase, S-nitrosylation, siah, apoptosis, parkinson's disease, huntington's disease
INTRODUCTION
One of Julie Axelrod's favorite aphorisms went something like, “If Nature finds a useful molecule, she will use it over and over again in different contexts.” He emphasized this to me when in 1963 I was working on my very first project in his laboratory dealing with the fate of radiolabeled histamine in the body. He noted that histamine behaved very differently as a mediator of allergic reactions than as a stimulus to acid secretion in the stomach. He pushed me to explore the possibility that histamine might, like norepinephrine and serotonin, have some sort of neurotransmitter-like role. Of course, Julie turned out to be absolutely right.
Our research on nitric oxide (NO) taught me the importance of keeping an open mind as to diverse functions of a molecule. NO was first identified as endothelial-derived relaxing factor, the primary physiologic relaxant of blood vessels. It was discovered about the same time in a completely different scenario enabling macrophages to kill tumor cells and bacteria. The remarkable properties of NO prompted my student David Bredt and me to explore the possibility that it might function as a neurotransmitter. NO is now appreciated as a major neurotransmitter/neuromodulator. The distinct functions of NO involve different pools of the molecule formed by enzymes derived from distinct genes, neuronal NO synthase (nNOS), endothelial NOS (eNOS), and the macrophage form of NOS, which can be present in all tissues of the body during inflammatory responses and is the sole form of the enzyme which is markedly and rapidly inducible, inducible NOS (iNOS).
GAPDH is generally regarded as a housekeeping glycolytic enzyme. However, over the years, there have been reports that it can display other activities including functioning as a uracil DNA glycosylase that participates in DNA repair (Meyer-Siegler et al., 1991) or as an Oct-1 coactivator OCA-S that is a key component for S-phase-dependent histone H2B transcription (Zheng et al., 2003). It also can bind to transfer RNA and to DNA (Morgenegg et al., 1986; Singh and Green, 1993; Sundararaj et al., 2004). GAPDH also binds to the inositol 1,4,5-triphosphate receptor (IP3R), modulating calcium release via NADH production (Patterson et al., 2005). While GAPDH is generally regarded as a static marker protein, its expression level can be dynamic with reports of tissue level alterations associated with hypoxia, calcium influx, and oncogenesis (Tokunaga et al., 1987; Graven et al., 1994; Yamaji et al., 2003).
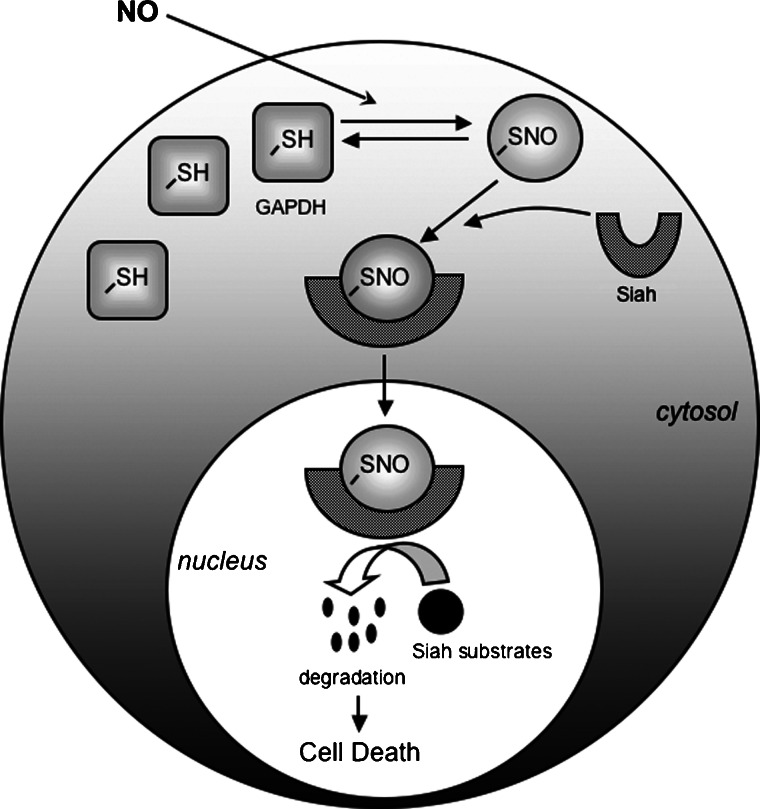
During the past several years, we have identified a novel role for GAPDH. Apoptotic stimuli augment the formation of NO either by induction of iNOS or by activation of nNOS. NO S-nitrosylates GAPDH at cysteine-150 (C150), critical to the catalytic function of the enzyme, which abolishes catalytic activity but confers upon GAPDH the ability to bind to the E3-ubiquitin-ligase Siah1, which we will hereafter designated as Siah. Siah, which possesses a nuclear localization signal, escorts GAPDH to the nucleus where GAPDH stabilizes the rapidly turning over Siah. This enables Siah to degrade selected protein targets eliciting cell death (Fig. 1). We have also shown that the cytoprotective actions of the monoamine oxidase inhibitor deprenyl and related drugs derive from their binding to GAPDH and blocking its interactions with Siah (Table I). We also have shown that the nuclear translocation of the N-terminal fragment of mutant Huntingtin (mHtt), which is required for neurotoxicity, is mediated by its binding to GAPDH and Siah which effectuate its nuclear translocation (Table I).
Fig. 1.
Schematic diagram of NO-S-nitrosylation—GAPDH—Siah cell death cascade. NO causes S-nitrosylation of GAPDH at C150 (–SH). S-nitrosylation (–SNO) of GAPDH augments its binding to Siah. The nuclear localization signal of Siah mediates nuclear translocation of GAPDH. GAPDH stabilizes Siah facilitating its degradation of nuclear substrates, which leads to cell death. The alteration of GAPDH's shape from square to circular following S-nitrosylation designates its presumed conformational alteration that facilitates binding to Siah. The shape change in Siah following such binding denotes the stabilization by GAPDH of Siah.
Table I.
NO–GAPDH–Siah Cascade in Huntington's Disease and Parkinson's Disease
| Disease | Links to NO–GAPDH–Siah |
|---|---|
| Huntinton's disease | Minocycline delays mortality in HD mouse model by inhibiting iNOS activation (Chen et al., 2000) |
| GAPDH binds to mHtt in a polyglutamine repeat-dependent manner (Burke et al., 1996; Koshy et al., 1996) | |
| mHtt transgenic mice show increased nuclear accumulation of GAPDH (Senatorov et al., 2003) | |
| GAPDH, Siah, and mHtt form a ternary complex in vitro and in cellular system | |
| Overexpression of GAPDH increases nuclear mHtt in cellular system | |
| GAPDH RNAi decreases nuclear mHtt in cellular system | |
| Overexpression of GAPDH increases toxicity of mHtt in cellular system | |
| Parkinson's disease | NO mediates dopaminergic neurodegeneration in MPTP model of PD (Hantraye et al., 1996; Przedborski et al., 1996; Liberatore et al., 1999) |
| Anti-Parkinsonian drug deprenyl and its derivatives bind to GAPDH (Kragten et al., 1998) | |
| GAPDH accumulates in the nucleus in PD niagra (Tatton, 2000) | |
| Siah1 facilitates ubiquitination and degradation of synphilin-1 (Nagano et al., 2003; Liani et al., 2004) | |
| Deprenyl inhibits GAPDH–Siah binding in vitro and in cellular system | |
| Deprenyl inhibits nuclear translocation of GAPDH in cellular system | |
| Deprenyl inhibits GAPDH–Siah binding in MPTP-treated mice |
EARLY STUDIES LINKING NO, GAPDH AND CELL DEATH
Our first evidence for a relationship between NO and GAPDH was serendipitous. In the early 1990s, NO was assumed to exert all its physiologic actions by binding to heme at the active site of guanylyl cyclase to augment formation of cyclic GMP (Ignarro, 1990). We obtained evidence that NO mediates the neurotoxicity of glutamate acting through N-methyl-d-aspartate (NMDA) receptors (Dawson et al., 1991). Neurotoxicity was clearly unrelated to guanylyl cyclase, as inhibitors of the enzyme did not influence neurotoxicity and 8-bromo-cyclic GMP did not elicit neurotoxicity (Dawson et al., 1993). We speculated that NO might act upon other targets. It is now widely accepted that S-nitrosylation of proteins is a major “second messenger” for NO actions, but this was not yet appreciated in early 1992. In searching for alternative targets for NO actions, Brune and Lapetina (1989) had noted that NO enhanced the ADP-ribosylation of a 37 kDa cytosolic protein. They assumed that what they were observing was ADP-ribosylation of a G-protein, as most G-proteins are 30–40 kDa and their ADP-ribosylation physiologically, as well as by agents such as cholera toxin, was well established. We developed a simple means to isolate the specific G-protein that was the postulated target of NO. To identify the NO target, presumably an ADP-ribosylated protein, we synthesized a biotinylated derivative of NAD, the substrate for ADP-ribosylation, and, utilizing avidin, we isolated the labeled protein which turned out to be GAPDH (Zhang and Snyder, 1992). We showed that the interaction with NAD involved a critical cysteine, presumably C150, which is at the catalytic site (the catalytic cysteine is C150 for rat, and C152 for the human GAPDH open reading frame).
In retrospect, we know that the dramatic augmentation of labeling of GAPDH elicited by NO reflected S-nitrosylation which permitted [32P]NAD to attach at C150, either as the intact NAD molecule, as suggested by Moss and coworkers (McDonald and Moss, 1993; Mohr et al., 1996) or via ADP-ribosylation.
A role for GAPDH in neuronal cell death was first advanced by Chuang and coworkers (Ishitani and Chuang, 1996; Ishitani et al., 1996a,b). Interested in the possibility that apoptotic stimuli augment the expression of selected apoptotic proteins, they noted that age-induced apoptosis of cerebellar granular cells leads to overexpression of a 38 kDa protein in particulate fractions which they identified as GAPDH (Ishitani et al., 1996a). Antisense oligonucleotides to GAPDH blocked this overexpression and delayed apoptosis, though basal levels of particulate GAPDH were unaffected. GAPDH is predominantly cytosolic and, though Chuang and co-workers did not directly study cytosolic GAPDH, it is likely that there would have been no effect in cytosolic fractions. Thus, these very initial studies suggested that the augmentation observed in GAPDH levels involved a small, rapidly turning over pool that was selectively influenced by antisense treatment. The selectivity for particulate fractions presaged subsequent findings about the importance of nuclear GAPDH.
We wondered whether the Chuang's findings were confined to cerebellar granular cells or reflected a general mechanism of cell death. Accordingly, we explored multiple cell types and diverse stimuli to cell death (Sawa et al., 1997). In S49 T lymphocytes, dexamethasone induction of apoptosis augmented GAPDH in a crude nuclear fraction. We obtained similar results in HEK293 cells following serum deprivation, in primary thymocytes treated with dexamethasone, PC12 neuronal cells with nerve growth factor withdrawal or cerebrocortical cultures following apoptosis elicited by prolonged culture. In all cases, antisense treatment diminished cell death.
Utilizing confocal microscopy or extensive subcellular fractionation we established that the translocation occurs selectively to nuclei (Sawa et al., 1997). Interestingly, the nuclear GAPDH appeared to be very tightly bound, as DNase and extensive washing with 5 M sodium chloride failed to liberate nuclear GAPDH. Moreover, nuclear GAPDH was devoid of catalytic activity.
NITROSYLATION OF GAPDH, BINDING TO SIAH, NUCLEAR TRANSLOCATION, AND CELL DEATH
Our initial quest was simply to understand how GAPDH can translocate to the nucleus despite its lack of a nuclear localization signal. Yeast two-hybrid search identified Siah as the sole interactor, which we confirmed by direct binding studies and co-immunoprecipitation from native tissues (Hara et al., 2005). Binding was critically dependent upon a narrow amino acid sequence such that mutating a single lysine (K225) abolished binding interactions, providing a valuable tool for probing the physiologic importance of binding.
GAPDH–Siah binding was clearly responsible for nuclear translocation. Thus, co-transfection with Siah greatly enhanced nuclear levels of GAPDH whether endogenous or exogenous and whether monitored by microscopic analysis of tissues or by subcellular fractionation. Moreover, K225 mutants of GAPDH, which cannot bind Siah, did not translocate.
We wondered how this translocation influences cell death. Siah is well known to elicit apoptosis by its E3-ubiquitin-ligase activity leading to degradation of selected proteins (Sourisseau et al., 2001; Fiucci et al., 2004). Siah is widely expressed in the brain (Nagano et al., 2003), with high expression of Siah mRNA observed in pyramidal neurons of the hippocampus as well as Purkinge cells of the cerebellum (Moriyoshi et al., 2004). Siah turns over very rapidly being itself degraded by the ubiquitin proteasome system (Hu and Fearon, 1999), so that under basal conditions, tissue levels are barely detectable. Transfecting GAPDH tripled nuclear levels of Siah, which were further augmented when we transfected GAPDH containing an exogenous nuclear localization signal. Moreover, mutating K225 prevented this augmentation of nuclear Siah. Additionally, we directly monitored Siah turnover rate and showed that GAPDH transfection markedly slows the turnover.
What is it about apoptotic stimuli that confers upon some presumably small pool of GAPDH the ability to bind Siah? We wondered whether the GAPDH that was bound to Siah in the nucleus possesses different molecular properties. Mass spectrometric analysis of nuclear GAPDH following apoptotic stimulation revealed sulphonation at the catalytic C150. Sulphonation of cysteine can arise following S-nitrosylation via hydrolysis to sulphenic acid and sequential oxidation to sulphinic acid and then sulphonic acid. This was a tantalizing possibility because our biotin switch assay for endogenous S-nitrosylated proteins revealed GAPDH as one such target whose endogenous nitrosylation was abolished in nNOS knockout brain tissue, hence was the product of physiologic actions of neuronally derived NO (Jaffrey et al., 2001). NO donors markedly augmented nuclear translocation of GAPDH and increased nuclear levels of sulphonated GAPDH detected by an antibody we developed that is selective for sulphonated GAPDH. We directly demonstrated that treatment of GAPDH with NO donors markedly enhances its binding to Siah and involves C150, whose mutation abolished such binding.
We then showed that S-nitrosylation of GAPDH is responsible for nuclear translocation of GAPDH–Siah and cell death. Macrophages, more than any other type of cell, manifest profound augmentation of NO formation and cell death following treatment with constituents of endotoxin such as lipopolysaccharide (LPS). LPS treatment of the macrophage cell line RAW264.7 augmented GAPDH S-nitrosylation, its binding to Siah and its nuclear translocation. These events were critical for cell death, as depletion of endogenous GAPDH by RNA interference (RNAi) prevented apoptosis as did antisense to GAPDH. For a more physiologic preparation, we used peritoneal macrophages from mice treated with LPS. GAPDH–Siah binding, nuclear translocation of GAPDH, and cell death following such treatment were markedly reduced in macrophages from iNOS knockout mice.
We extended these findings to the brain showing that NO derived from nNOS as well as iNOS elicits GAPDH S-nitrosylation. Glutamate stimulation of NMDA receptors is well known to cause neuronal cell death via nNOS activation. We found that NMDA elicited neuronal death was prevented by depleting Siah or GAPDH with RNAi techniques.
Recently, Brown et al. (2004) have determined that GAPDH contains a CRM1-mediated nuclear export signal (NES). Because CRM-1 possesses a critical cysteine in the central conserved region (Kudo et al., 1999), NO may affect this cysteine and inhibit CRM1 activity. This could afford a synergistic effect on nuclear accumulation of GAPDH via Siah.
To ascertain whether the E3-ubiquitin-ligase activity of Siah is responsible for cell death in the NO–GAPDH–Siah cascade, we took advantage of the importance of the RING finger domain of Siah for such activity. In transfected HEK293 cells, cytotoxicity was abolished by deleting Siah's RING finger domain.
Both human and mouse contain multiple Siah family members. Siah2 is expressed widely at a low level in embryonic and adult mouse tissues (Della et al., 1993). To test the effect of a different isoform of Siah, we transfected HEK293 cells with Siah2, and observed translocation of GAPDH to the nucleus. However, Siah2 RNAi was not as effective as Siah1 RNAi in protecting neurons from cell death (Hara, Snyder, and Sawa, unpublished observations).
Siah can degrade a variety of nuclear proteins, which might mediate cell death. We examined one of these, nuclear receptor co-repressor (N-CoR). Transfection of Siah led to degradation of N-CoR and such degradation was enhanced by co-transfection with GAPDH. Of course, other proteins critical for cell life are also degraded by Siah and might participate as executioners in the NO–GAPDH–Siah pathway. For instance, phosphorylation-independent degradation of β-catenin requires Siah (Matsuzawa and Reed, 2001; Fukushima et al., 2006).
CLINICAL RELEVANCE
Multiple signaling systems participate in apoptosis. How does the NO–GAPDH–Siah cascade interface with other mechanisms? Caspase-dependent apoptosis has been well characterized. The intrinsic pathway involves mitochondrial release of cytochrome c and activation of caspase-9, whereas the extrinsic pathway, which responds to stimulation of cell surface death receptors such as Fas, activates caspase-8. Mitochondrial initiation of the intrinsic pathway can be triggered by nuclear events. For instance, genomic DNA damage activates p53, which translocates from the nucleus to mitochondria. Thus, nuclear effects of Siah could impact mitochondria and the intrinsic caspase pathway.
Excess intracellular release by IP3 of calcium stores in the endoplasmic reticulum may also influence mitochondria. We discovered that cytochrome c physiologically binds IP3R (Boehning et al., 2003). In the early phases of apoptosis, cytochrome c translocates to the endoplasmic reticulum where it binds to IP3R and very potently prevents the physiologic feedback whereby released calcium inhibits further release by IP3R of calcium. Inhibition of this feedback mechanism enhances the release of calcium, which passes to the closely apposed mitochondria to trigger further release of cytochrome c and a vicious cycle with uncontrolled calcium and cytochrome c release initiating cell-wide apoptotic events.
Another instance of communication between nuclei and mitochondria in apoptosis involves the inositol pyrophosphate IP7 (diphosphoinositol pentakisphosphate) and one of its biosynthetic enzymes IP6 kinase-2 (IP6K2) (Nagata et al., 2005). Transfection of IP6K2 in multiple cell lines augments the apoptotic actions of several cell stressors and is accompanied by major increases in IP7 formation. Deleting IP6K2, but not IP6K1 or IP6K3, prevents apoptosis. Interestingly, apoptotic stimuli cause IP6K2 to translocate from nuclei selectively to damaged mitochondria, whereas no translocation of IP6K1 or IP6K3 is observed. The selective association of IP6K2 with damaged mitochondria suggests that IP6K2 and its product IP7 are responsible for this damage.
Huntington's disease (HD) provides an example whereby pathophysiology involves both nuclear and mitochondrial events and in which we have found a critical role for the NO–GAPDH–Siah system. One difficulty in elucidating the pathophysiology of a disease such as HD which causes profound brain damage is that observed abnormalities in patient brains might reflect secondary degeneration. One solution to this dilemma is to seek disturbances in peripheral tissues in which mHtt is presumably expressed but in which no gross abnormalities are evident. Thus, lymphoblasts from HD patients display abnormal mitochondrial membrane depolarization closely correlated with the length of expanded polyglutamine repeats in mHtt which account for disease pathology (Sawa et al., 1999).
Nuclear disturbances are also implicated in HD. In transgenic animal models of the disease, as well as in cell lines containing mHtt, nuclear accumulation of an N-terminal fragment of mHtt (N-mHtt) is critical for cytotoxicity. A link between nuclear and mitochondrial abnormalities in HD is suggested by our findings that p53 mediates both cellular dysfunction and behavioral abnormalities in HD (Bae et al., 2005). mHtt binds to p53 and upregulates its nuclear levels as well as its transcriptional activity. p53 levels are increased in HD patients as well as in brains of mHtt transgenic mice. Depletion of p53 by RNAi, genetic deletion or the p53 antagonist pifithrin-α prevents cytotoxicity in HD cells. Genetic deletion of p53 suppresses neurodegeneration in mHtt transgenic flies and the behavioral abnormalities of mHtt transgenic mice. p53 may link nuclei and mitochondria in HD pathophysiology in multiple ways. p53 induces the expression of Bax and Puma which elicit mitochondrial membrane depolarization (Vogelstein et al., 2000). p53 also influences levels of reactive oxygen species via p66SHC (Sharpless and DePinho, 2002).
As the previous summary indicates, the well established nuclear translocation of N-mHtt can readily explain both nuclear and mitochondrial pathology as well as cell death. One open question was how N-mHtt, lacking a nuclear localization signal, enters the nucleus. Since an interaction of GAPDH and mHtt was reported previously (Burke et al., 1996), we wondered whether the NO–GAPDH–Siah cascade plays a role. We directly demonstrated that mHtt, GAPDH and Siah form a ternary complex in intact tissues (Bae et al., 2006) (Table I). Moreover, overexpression of GAPDH increases both the nuclear targeting and cytotoxicity of mHtt. Transfection of a form of GAPDH, which is trapped by microtubules and cannot enter the nucleus (GAPDH–P234S), fails to augment cell death. Moreover, depletion of GAPDH by RNAi prevents nuclear targeting of mHtt. Consistent with these observations, mHtt transgenic mice display increased nuclear accumulation of GAPDH, which is associated with cellular toxicity (Senatorov et al., 2003). Other findings supporting clinical relevance for the NO–GAPDH–Siah cascade in HD are summarized in Table I.
Assuming that nuclear targeting of N-mHtt is critical for neurotoxicity, agents that prevent such nuclear targeting may well be therapeutic in HD. Obvious candidates would be drugs that block the binding of GAPDH to Siah. Of course, such drugs would be predicted to prevent cell death in all sorts of diseases, not just neurological disabilities. The prevention of cytotoxicity by dominant-negative forms of GAPDH that cannot bind to Siah supports such a therapeutic strategy (Hara et al., 2005).
The possibility of small molecule inhibitors of GAPDH–Siah binding emerged from an unlikely source, studies of neuroprotective actions of the monoamine oxidase (MAO) inhibitor deprenyl. MAO inhibitors were first developed as antidepressants but are used only sparingly because inhibition of one form of the enzyme, MAO-A, markedly augments tissue levels of tyramine, a ubiquitous food constituent which potently releases norepinephrine from sympathetic nerve terminals and can elicit lethal hypertension. MAO-B does not metabolize tyramine so that deprenyl, a selective MAO-B inhibitor, is widely used to treat Parkinson's disease (PD) presumably by elevating brain levels of dopamine. Abundant animal studies indicated a neuroprotective action of deprenyl (Jenner, 2004). For instance, it prevents the degradation of dopamine neurons elicited by the neurotoxin MPTP. Moreover, clinical investigations have suggested a slowing of neuronal loss in patients with PD (Birkmayer et al., 1985), though definitive clinical evidence awaits imaging of dopamine neurons in patients.
To ascertain if deprenyl's neuroprotective actions are independent of its MAO inhibitory activity, Novartis chemists developed a series of derivatives that do not inhibit MAO. One of these, TCH346, aka CGP3466 (dibenzo[b,f]oxepin-10-ylmethyl-methyl-prop-2-ynyl-amine), is an extremely potent neuroprotectant both in culture models and in intact animals (Waldmeier et al., 2000). Because it acts at low nM concentrations, Waldmeier and associates (Kragten et al., 1998) sought potential “receptor” targets of TCH346 by synthesizing a derivative that could covalently link to such targets. They observed binding to a single protein, GAPDH. Since GAPDH is such an abundant constituent of all tissues, one might assume that these investigators were only observing non-specific binding associated with the high chemical reactivity of the alkylating agent employed.
Because of our interest in GAPDH as an apoptotic signal, we investigated a potential role for GAPDH in the neuroprotective actions of TCH346 and deprenyl. Concentrations of deprenyl or TCH346 as low as 1 nM prevented the binding of GAPDH to Siah (Hara et al., 2006) (Table I). The concentration–response properties of deprenyl in blocking GAPDH–Siah binding and in preventing neurotoxicity were closely parallel. Moreover, in intact rodents MPTP augmented the binding of GAPDH to Siah in dopamine-rich brain areas. This binding was prevented by treatment with deprenyl. Thus, the neuroprotective actions of deprenyl and related drugs can be readily explained by influences on GAPDH–Siah binding. Conceivably novel types of cytoprotective agents can be developed by systematic screening for inhibition of this protein–protein interaction.
ACKNOWLEDGMENTS
This work was supported by USPHS grants DA-00266 and Research Scientist AwardDA00074 (SHS). We thank Dr. Akira Sawa, Dr. Byoung-Il Bae, and Matthew B. Cascio for their helpful comments. We thank Dr. Peter Waldmeier for providing us TCH346.
REFERENCES
- Bae, B. I., Xu, H., Igarashi, S., Fujimuro, M., Agrawal, N., Taya, Y., Hayward, S. D., Moran, T. H., Montell, C., Ross, C. A., Snyder, S. H., and Sawa, A. (2005). p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron47:29–41. [DOI] [PubMed] [Google Scholar]
- Bae, B. I., Hara, M. R., Cascio, M. B., Wellington, C. L., Hayden, M. R., Ross, C. A., Ha, H. C., Li, X. J., Snyder, S. H., and Sawa, A. (2006). Mutant Huntingtin: Nuclear translocation and cytotoxicity mediated by GAPDH. Proc. Natl. Acad. Sci. U.S.A.103:3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer, W., Knoll, J., Riederer, P., Youdim, M. B., Hars, V., and Marton, J. (1985). Increased life expectancy resulting from addition of l-deprenyl to Madopar treatment in Parkinson's disease: A longterm study. J. Neural. Transm.64:113–127. [DOI] [PubMed] [Google Scholar]
- Boehning, D., Patterson, R. L., Sedaghat, L., Glebova, N. O., Kurosaki, T., and Snyder, S. H. (2003). Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell. Biol.5:1051–1061. [DOI] [PubMed] [Google Scholar]
- Brown, V. M., Krynetski, E. Y., Krynetskaia, N. F., Grieger, D., Mukatira, S. T., Murti, K. G., Slaughter, C. A., Park, H. W., and Evans, W. E. (2004). A novel CRM1-mediated nuclear export signal governs nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase following genotoxic stress. J. Biol. Chem.279:5984–5992. [DOI] [PubMed] [Google Scholar]
- Brune, B., and Lapetina, E. G. (1989). Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J. Biol. Chem.264:8455–8458. [PubMed] [Google Scholar]
- Burke, J. R., Enghild, J. J., Martin, M. E., Jou, Y. S., Myers, R. M., Roses, A. D., Vance, J. M., and Strittmatter, W. J. (1996). Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nat. Med.2:347–350. [DOI] [PubMed] [Google Scholar]
- Chen, M., Ona, V. O., Li, M., Ferrante, R. J., Fink, K. B., Zhu, S., Bian, J., Guo, L., Farrell, L. A., Hersch, S. M., Hobbs, W., Vonsattel, J. P., Cha, J. H., and Friedlander, R. M. (2000). Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med.6:797–801. [DOI] [PubMed] [Google Scholar]
- Dawson, V. L., Dawson, T. M., Bartley, D. A., Uhl, G. R., and Snyder, S. H. (1993). Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J. Neurosci.13:2651–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, V. L., Dawson, T. M., London, E. D., Bredt, D. S., and Snyder, S. H. (1991). Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. U.S.A.88:6368–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della, N. G., Senior, P. V., and Bowtell, D. D. (1993). Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina). Development117:1333–1343. [DOI] [PubMed] [Google Scholar]
- Fiucci, G., Beaucourt, S., Duflaut, D., Lespagnol, A., Stumptner-Cuvelette, P., Geant, A., Buchwalter, G., Tuynder, M., Susini, L., Lassalle, J. M., Wasylyk, C., Wasylyk, B., Oren, M., Amson, R., and Telerman, A. (2004). Siah-1b is a direct transcriptional target of p53: Identification of the functional p53 responsive element in the siah-1b promoter. Proc. Natl. Acad. Sci. U.S.A.101:3510–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima, T., Zapata, J. M., Singha, N. C., Thomas, M., Kress, C. L., Krajewska, M., Krajewski, S., Ronai, Z., Reed, J. C., and Matsuzawa, S. (2006). Critical function for SIP, a ubiquitin E3 ligase component of the beta-catenin degradation pathway, for thymocyte development and G1 checkpoint. Immunity24:29–39. [DOI] [PubMed] [Google Scholar]
- Graven, K. K., Troxler, R. F., Kornfeld, H., Panchenko, M. V., and Farber, H. W. (1994). Regulation of endothelial cell glyceraldehyde-3-phosphate dehydrogenase expression by hypoxia. J. Biol. Chem.269:24446–24453. [PubMed] [Google Scholar]
- Hantraye, P., Brouillet, E., Ferrante, R., Palfi, S., Dolan, R., Matthews, R. T., and Beal, M. F. (1996). Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat. Med.2:1017–1021. [DOI] [PubMed] [Google Scholar]
- Hara, M. R., Agrawal, N., Kim, S. F., Cascio, M. B., Fujimuro, M., Ozeki, Y., Takahashi, M., Cheah, J. H., Tankou, S. K., Hester, L. D., Ferris, C. D., Hayward, S. D., Snyder, S. H., and Sawa, A. (2005). S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell. Biol.7:665–674. [DOI] [PubMed] [Google Scholar]
- Hara, M. R., Thomas, B., Cascio, M. B., Bae, B. I., Hester, L. D., Dawson, V. L., Dawson, T. M., Sawa, A., and Snyder, S. H. (2006). Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc. Natl. Acad. Sci. U.S.A.103:3887–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, G., and Fearon, E. R. (1999). Siah-1 N-terminal RING domain is required for proteolysis function, and C-terminal sequences regulate oligomerization and binding to target proteins. Mol. Cell. Biol.19:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro, L. J. (1990). Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: A unique transduction mechanism for transcellular signaling. Pharmacol. Toxicol.67:1–7. [DOI] [PubMed] [Google Scholar]
- Ishitani, R., and Chuang, D. M. (1996). Glyceraldehyde-3-phosphate dehydrogenase antisense oligodeoxynucleotides protect against cytosine arabinonucleoside-induced apoptosis in cultured cerebellar neurons. Proc. Natl. Acad. Sci. U.S.A.93:9937–9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani, R., Kimura, M., Sunaga, K., Katsube, N., Tanaka, M., and Chuang, D. M. (1996a). An antisense oligodeoxynucleotide to glyceraldehyde-3-phosphate dehydrogenase blocks age-induced apoptosis of mature cerebrocortical neurons in culture. J. Pharmacol. Exp. Ther.278:447–454. [PubMed] [Google Scholar]
- Ishitani, R., Sunaga, K., Hirano, A., Saunders, P., Katsube, N., and Chuang, D. M. (1996b). Evidence that glyceraldehyde-3-phosphate dehydrogenase is involved in age-induced apoptosis in mature cerebellar neurons in culture. J. Neurochem.66:928–935. [DOI] [PubMed] [Google Scholar]
- Jaffrey, S. R., Erdjument-Bromage, H., Ferris, C. D., Tempst, P., and Snyder, S. H. (2001). Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell. Biol.3:193–197. [DOI] [PubMed] [Google Scholar]
- Jenner, P. (2004). Preclinical evidence for neuroprotection with monoamine oxidase-B inhibitors in Parkinson's disease. Neurology63:S13– S22. [DOI] [PubMed] [Google Scholar]
- Koshy, B., Matilla, T., Burright, E. N., Merry, D. E., Fischbeck, K. H., Orr, H. T., and Zoghbi, H. Y. (1996). Spinocerebellar ataxia type-1 and spinobulbar muscular atrophy gene products interact with glyceraldehyde-3-phosphate dehydrogenase. Hum. Mol. Genet.5:1311–1318. [DOI] [PubMed] [Google Scholar]
- Kragten, E., Lalande, I., Zimmermann, K., Roggo, S., Schindler, P., Muller, D., van Oostrum, J., Waldmeier, P., and Furst, P. (1998). Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the antiapoptotic compounds CGP 3466 and R-(−)-deprenyl. J. Biol. Chem.273:5821–5828. [DOI] [PubMed] [Google Scholar]
- Kudo, N., Matsumori, N., Taoka, H., Fujiwara, D., Schreiner, E. P., Wolff, B., Yoshida, M., and Horinouchi, S. (1999). Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. U.S.A.96:9112–9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liani, E., Eyal, A., Avraham, E., Shemer, R., Szargel, R., Berg, D., Bornemann, A., Riess, O., Ross, C. A., Rott, R., and Engelender, S. (2004). Ubiquitylation of synphilin-1 and alpha-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A.101:5500–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore, G. T., Jackson-Lewis, V., Vukosavic, S., Mandir, A. S., Vila, M., McAuliffe, W. G., Dawson, V. L., Dawson, T. M., and Przedborski, S. (1999). Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med.5:1403–1409. [DOI] [PubMed] [Google Scholar]
- Matsuzawa, S. I., and Reed, J. C. (2001). Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol. Cell.7:915–926. [DOI] [PubMed] [Google Scholar]
- McDonald, L. J., and Moss, J. (1993). Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. U.S.A.90:6238–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Siegler, K., Mauro, D. J., Seal, G., Wurzer, J., deRiel, J. K., and Sirover, M. A. (1991). A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. U.S.A.88:8460–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, S., Stamler, J. S., and Brune, B. (1996). Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J. Biol. Chem.271:4209–4214. [DOI] [PubMed] [Google Scholar]
- Morgenegg, G., Winkler, G. C., Hubscher, U., Heizmann, C. W., Mous, J., and Kuenzle, C. C. (1986). Glyceraldehyde-3-phosphate dehydrogenase is a nonhistone protein and a possible activator of transcription in neurons. J. Neurochem.47:54–62. [DOI] [PubMed] [Google Scholar]
- Moriyoshi, K., Iijima, K., Fujii, H., Ito, H., Cho, Y., and Nakanishi, S. (2004). Seven in absentia homolog 1A mediates ubiquitination and degradation of group 1 metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U.S.A.101:8614–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano, Y., Yamashita, H., Takahashi, T., Kishida, S., Nakamura, T., Iseki, E., Hattori, N., Mizuno, Y., Kikuchi, A., and Matsumoto, M. (2003). Siah-1 facilitates ubiquitination and degradation of synphilin-1. J. Biol. Chem.278:51504–51514. [DOI] [PubMed] [Google Scholar]
- Nagata, E., Luo, H. R., Saiardi, A., Bae, B, I., Suzuki, N., and Snyder, S. H. (2005). Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J. Biol. Chem.280:1634–1640. [DOI] [PubMed] [Google Scholar]
- Patterson, R. L., van Rossum, D. B., Kaplin, A. I., Barrow, R. K., and Snyder, S. H. (2005). Inositol 1,4,5-trisphosphate receptor/GAPDH complex augments Ca2+ release via locally derived NADH. Proc. Natl. Acad. Sci. U.S.A.102:1357–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski, S., Jackson-Lewis, V., Yokoyama, R., Shibata, T., Dawson, V. L., and Dawson, T. M. (1996). Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc. Natl. Acad. Sci. U.S.A.93:4565–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa, A., Khan, A. A., Hester, L. D., and Snyder, S. H. (1997). Glyceraldehyde-3-phosphate dehydrogenase: Nuclear translocation participates in neuronal and nonneuronal cell death. Proc. Natl. Acad. Sci. U.S.A.94:11669–11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa, A., Wiegand, G. W., Cooper, J., Margolis, R. L., Sharp, A. H., Lawler, J. F.Jr., Greenamyre, J. T., Snyder, S. H., and Ross, C. A. (1999). Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med.5:1194–1198. [DOI] [PubMed] [Google Scholar]
- Senatorov, V. V., Charles, V., Reddy, P. H., Tagle, D. A., and Chuang, D. M. (2003). Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington's disease. Mol. Cell. Neurosci.22:285–297. [DOI] [PubMed] [Google Scholar]
- Sharpless, N. E., and DePinho, R. A. (2002). p53: Good cop/bad cop. Cell110:9–12. [DOI] [PubMed] [Google Scholar]
- Singh, R., and Green, M. R. (1993). Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science259:365–368. [DOI] [PubMed] [Google Scholar]
- Sourisseau, T., Desbois, C., Debure, L., Bowtell, D. D., Cato, A. C., Schneikert, J., Moyse, E., and Michel, D. (2001). Alteration of the stability of Bag-1 protein in the control of olfactory neuronal apoptosis. J. Cell. Sci.114:1409–1416. [DOI] [PubMed] [Google Scholar]
- Sundararaj, K. P., Wood, R. E., Ponnusamy, S., Salas, A. M., Szulc, Z., Bielawska, A., Obeid, L. M., Hannun, Y. A., and Ogretmen, B. (2004). Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem.279:6152–6162. [DOI] [PubMed] [Google Scholar]
- Tatton, N. A. (2000). Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp. Neurol.166:29–43. [DOI] [PubMed] [Google Scholar]
- Tokunaga, K., Nakamura. Y., Sakata, K., Fujimori, K., Ohkubo, M., Sawada, K., and Sakiyama, S. (1987). Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res.47:5616–5619. [PubMed] [Google Scholar]
- Vogelstein, B., Lane, D., and Levine, A. J. (2000). Surfing the p53 network. Nature408:307–310. [DOI] [PubMed] [Google Scholar]
- Waldmeier, P. C., Boulton, A. A., Cools, A. R., Kato, A. C., and Tatton, W. G. (2000). Neurorescuing effects of the GAPDH ligand CGP 3466B. J. Neural. Transm. Suppl.60:197–214. [DOI] [PubMed] [Google Scholar]
- Yamaji, R., Fujita, K., Takahashi, S., Yoneda, H., Nagao, K., Masuda, W., Naito, M., Tsuruo, T., Miyatake, K., Inui, H., and Nakano, Y. (2003). Hypoxia up-regulates glyceraldehyde-3-phosphate dehydrogenase in mouse brain capillary endothelial cells: involvement of Na+/Ca2+ exchanger. Biochim. Biophys. Acta1593:269–276. [DOI] [PubMed] [Google Scholar]
- Zhang, J., and Snyder, S. H. (1992). Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. U.S.A.89:9382–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L., Roeder, R. G., and Luo, Y. (2003). S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell114:255–266. [DOI] [PubMed] [Google Scholar]