Abstract
1. Ras signaling and oncogenesis depend on the dynamic interplay of Ras with distinctive plasma membrane (PM) microdomains and various intracellular compartments. Such interaction is dictated by individual elements in the carboxy-terminal domain of the Ras proteins, including a farnesyl isoprenoid group, sequences in the hypervariable region (hvr)-linker, and palmitoyl groups in H/N-Ras isoforms.
2. The farnesyl group acts as a specific recognition unit that interacts with prenyl-binding pockets in galectin-1 (Gal-1), galectin-3 (Gal-3), and cGMP phosphodiesterase δ. This interaction appears to contribute to the prolongation of Ras signals in the PM, the determination of Ras effector usage, and perhaps also the transport of cytoplasmic Ras. Gal-1 promotes H-Ras signaling to Raf at the expense of phosphoinositide 3-kinase (PI3-K) and Ral guanine nucleotide exchange factor (RalGEF), while galectin-3 promotes K-Ras signaling to both Raf and PI3-K.
3. The hvr-linker and the palmitates of H-Ras and N-Ras determine the micro- and macro-localizations of these proteins in the PM and in the Golgi, as well as in ‘rasosomes’, randomly moving nanoparticles that carry palmitoylated Ras proteins and their signal through the cytoplasm.
4. The dynamic compartmentalization of Ras proteins contributes to the spatial organization of Ras signaling, promotes redistribution of Ras, and provides an additional level of selectivity to the signal output of this regulatory GTPase.
KEY WORDS: ras, rasosomes, ras signaling, palmitoylation, galectin-1, galectin-3, prenyl-binding domains
INTRODUCTION
Ras proteins play a critical role in the control of complex and diverse networks of signaling cascades that regulate cell proliferation, differentiation, survival, and death (Barbacid, 1987; Bos, 1995; Bar-Sagi and Hall, 2000; Cox and Der, 2003; Downward, 2003; Malumbres and Barbacid, 2003). The four typical human Ras proteins, H-Ras, N-Ras, K-Ras 4A, and K-Ras 4B, share a high degree of sequence homology and a common principal mode of activation and inactivation (Shields et al., 2000). Ras is turned on by extracellular signals that activate receptors, which in turn activate Ras guanine nucleotide exchange factors (RasGEFs) (Campbell et al., 1998; Corbett and Alber, 2001) that induce exchange of GDP for GTP on Ras. Ras activation is turned off by specific Ras GTPase-activating proteins (RasGAPs), which facilitate GTP hydrolysis catalyzed by the GTPase (G) domain of Ras (Boguski and McCormick, 1993; Scheffzek et al., 1997). The active GTP-bound Ras activates multiple downstream effectors that trigger a diversity of complex intracellular signaling networks, leading to changes in cellular behavior (Shields et al., 2000; Mitin et al., 2005). These effectors include the Raf family of serine/threonine kinases (Raf-1, A-Raf, B-Raf), which phosphorylate MEK1/2 (Chong et al., 2003); the phosphoinositide 3-kinase (PI3-K) catalytic subunit family (p110α, p110β, p110γ), which catalyze the formation of phosphatidylinositol (3,4,5)-trisphosphate (Wymann et al., 2000; Vanhaesebroeck et al., 2001); the Ral guanine nucleotide exchange factor (RalGEF) family of proteins (RalGDS, Rlf, RGL2, RGL3), which act as GEFs of the RalA and RalB GTPases (Feig, 2003); the RGL and Tiam protein families, which act as GEFs of the Rho/Rac GTPases (Sierra et al., 2000; Minard et al., 2004); RIN1, which acts as a GEF of the Rab5 GTPase (Hu et al., 2005); NORE1 (RSSF5), which acts as an adaptor of the serine/threonine MST1 (Praskova et al., 2004); phospholipase Cɛ, which catalyzes the formation of diacylglycerol and inositol (1,4,5)-trisphosphate (Lopez et al., 2001); and the Ras effector protein IMP (impedes mitogenic signal propagation), which acts as an E3 ubiquitin ligase and inhibits the MEK-ERK scaffold protein KSR (Matheny et al., 2004).
How these diverse Ras signaling networks are coordinated is not yet known. It is known, however, that the strength, length, and fate of the Ras signal can be determined by different types of Ras-activating receptors (Ozaki et al., 2005), by various Ras targets that are expressed in any given cell (Ozaki et al., 2005), by Ras escort proteins such as galectins and PDEδ (Elad-Sfadia et al., 2002; Hanzal-Bayer et al., 2002; Nancy et al., 2002; Elad-Sfadia et al., 2004), and by Ras targets that can facilitate or inhibit Ras's own signal (Zimmermann and Moelling, 1999). Notably, many studies have now shown that micro- and macro-localization of various Ras isoforms at the plasma membrane (PM), endosomes (Jiang and Sorkin, 2002), the Golgi, and the endoplasmic reticulum (ER) critically determine the regulation of Ras signal output (Philips, 2005; Plowman and Hancock, 2005). It is now understood that Ras activation and signaling involve dynamic lateral segregation (Niv et al., 1999, 2002; Murakoshi et al., 2004; Rotblat et al., 2004b; Lommerse et al., 2005) and nanoclustering (Prior et al., 2003) of Ras at the PM (Roy et al., 2005), and that H-Ras and N-Ras and possibly also K-Ras 4B (Philips, 2005; Silvius et al., 2006) can signal from various cellular platforms (Philips, 2005; Plowman and Hancock, 2005). The ability of Ras proteins to act in this manner is critically dependent on their carboxy-terminal domain, and their GTPase domain (G domain) contributes to the lateral segregation of Ras in the PM as well (Hancock et al., 1989; Hancock et al., 1990; Jaumot et al., 2002; Rotblat et al., 2004b).
The carboxy-terminal domain of Ras proteins contains the farnesylcysteine carboxymethyl ester (Casey et al., 1989; Gutierrez et al., 1989; Magee et al., 1992; Zhang et al., 2002) and the upstream hypervariable region (hvr; 19–20 amino-acid residues) the latter distinguishes between various Ras isoforms. The hvr domains of H-Ras and of N-Ras contain, respectively, two palmitoylated cysteines (C181 and C184) and a single palmitoylated cysteine (C181) (Linder and Deschenes, 2003; Magee and Seabra, 2003; Smotrys and Linder, 2004; Magee and Seabra, 2005) that are important for the targeting of these isoforms to the PM and the Golgi (Rocks et al., 2005; Roy et al., 2005). Collectively, the palmitoylated cysteines and the C-terminal region of the farnesylcysteine carboxymethyl ester are termed the lipid anchor (Prior et al., 2003). The hvr upstream of the anchor is termed the hvr-linker domain (Prior et al., 2003). The hvr domain of K-Ras 4B does not contain a palmityolated cysteine; instead it contains a stretch of six lysine residues that are critical for targeting of K-Ras 4B to the PM (Hancock et al., 1990). The carboxy-terminal domains play a critical role in the regulation of signal output not only in Ras proteins but also in many Ras homologs. The hvr motifs analogous to those of Ras and modified by lipids are contained in many Ras homologs. For example, Rho proteins contain a carboxy-terminal geranylgeranylcysteine (Casey and Seabra, 1996) and some of them, such as TC10 (a Cdc42-related GTPase), are also palmitoylated (Michaelson et al., 2001). As in the case of Ras isoforms, closely related Rho isoforms, e.g. RhoA, RhoB, and RhoC, can exhibit different subcellular localizations and biological functions because of their different C-terminal sequences and modifications (Adamson et al., 1992; Du et al., 1999). Interestingly, a novel C-terminal palmitoylation motif was recently discovered in Wrch-1, a Wnt-regulated Cdc42 homolog (Berzat et al., 2005). It was shown in that study that the membrane association, subcellular localization, and biological activity of Wrch-1 are all mediated by palmitoylation of the second cysteine in the C-terminal motif CCFV of this protein (Berzat et al., 2005).
In our studies, we have focused on the C-terminal hvr domain of Ras proteins in an attempt to understand how this domain promotes modulation of Ras signaling.
MATERIALS AND METHODS
Plasmids and Immunoblotting and Biochemical Procedures
The expression vectors pcDNA-H-Ras(G12V), pcDNA-Gal-1, pcDNA-p120 RasGAP, green fluorescent protein (GFP), GFP-H-Ras(G12V), GFP-H-Ras(G12V)Δ2ala, Ras(G12V)Δ1ala, and GFP-H-Ras(G12V) 181S, 184S have been previously described (Elad-Sfadia et al., 2004; Rotblat et al., 2004a, 2006).
To determine the impact of galectin-1 (Gal-1) on p120 RasGAP activity, HEK-293 cells were transfected with pcDNA3 vector control or with a plasmid encoding Gal-1, or with a plasmid encoding p120 RasGAP, as described previously (Elad-Sfadia et al., 2004). Cells were also co-transfected with Gal-1 and p120 RasGAP vectors. After 24 h, the cells were stimulated by 100 ng/mL epidermal growth factor (EGF) for the indicated times. Ras-GTP levels were determined by pull-down assay of the GST-Ras-binding domain (RBD) of Raf-1 followed by immunoblotting with pan anti-Ras Ab, as described earlier (Elad-Sfadia et al., 2004). Immunoblots were visualized by enhanced chemiluminescence and were then subjected to densitometry. To determine the impact of Gal-1 on Ral, COS-7 cells were transfected with H-Ras(G12V), co-transfected with H-Ras(G12V) and Gal-1, and lysed 48 h later (left panel). Cells were also transfected with H-Ras, co-transfected with H-Ras and Gal-1, serum-starved for 24 h, and then stimulated by 100 ng/mL EGF (7.5 min) and lysed. Controls were transfected with the empty vector. Ral-GTP levels in the cell lysates were then determined by the GST-RalBD assay, as described (Kfir et al., 2005). Levels of Ras, Ral, Ral-GTP, and Gal-1 were determined by immunoblotting with anti-Ras, anti-Ral, and anti-Gal-1 Abs, as described (Elad-Sfadia et al., 2002; Kfir et al., 2005).
The effect of the hvr-linker peptide on membrane bound H-Ras(G12V) and Gal-1 were examined in vitro using total cell membranes of H-Ras(G12V)-transformed Rat-1 cells (Marom et al., 1995) that were incubated for 1 h with 50 μM of a synthetic (custom ordered) peptide corresponding to the hvr-linker sequence of H-Ras (residues 166–179) as described earlier (Paz et al., 2001; Elad-Sfadia et al., 2004). The membranes were then precipitated (100,000×g) and immunoblotted with anti-Ras or anti-Gal-1 Ab. To determine the distribution of Gal-1 and H-Ras(G12V) in the light and in heavy membranes fractions total membranes of Rat-1 stably expressing H-Ras(G12V) (Marom et al., 1995) were fractionated using a non-detergent sucrose gradient flotation procedure (Niv et al., 2002). Fractions were collected from top (fraction 1) to bottom (fraction 10) and the apparent amounts of H-Ras(G12V) and Gal-1 in each fraction were determined by immunoblotting with anti-Ras and anti-Gal-1 Abs (Niv et al., 2002). In this protocol, lipid raft components are enriched with caveolin-1 in the light membranes (fractions 4 and 5) and the heavy membranes are enriched in fractions 8–10 (see (Niv et al., 2002).
Structural Modeling
Human galectin-3 (Gal-3), rat Gal-1, and bovine RhoGDI were sequentially aligned (Rotblat et al., 2004a) using the Pileup software (Accelrys). The putative isoprenoid insertion site in Gal-3 was identified as follows. First, the conserved elements of the structures of Gal-1 (Rotblat et al., 2004a) and the geranylgeranyl-bound RhoGDI, as detected in the Cdc42–RhoGDI complex (Hoffman et al., 2000), were superimposed as described earlier (Rotblat et al., 2004a). The structure of Gal-3 (amino-acid residues 114–250, PDB code 1A3K) was then superimposed on the structure of Gal-1. Swiss-Prot software was used for the structural comparisons, as described elsewhere (Rotblat et al., 2004a).
Confocal Microscopy and Live Cell Imaging Procedures
To determine the significance of the hvr sequences in PM and Golgi localization of H-Ras(G12V) COS-7 cells were transfected with GFP-H-Ras(G12V), GFP-H-Ras(G12V)Δ1Ala, or GFP-H-Ras(G12V)Δ2Ala and treated with 50 μM cycloheximide 2 h prior to labeling of the Golgi with anti-β-COP Ab and Cy3-labeled secondary Abs employing the procedures detailed (Elad-Sfadia et al., 2004; Rotblat et al., 2004a, 2006). Dual fluorescence images were then collected. And the mean ratio of Golgi GFP fluorescence to total cell GFP fluorescence was determined by line scanning.
Live cell imaging of GFP, GFP-H-Ras(G12V), and GFP-H-Ras(G12V) 181S, 184S expressing BHK cells were performed using an inverted Olympus IX-70 microscope equipped with a total internal reflection fluorescence (TIRF) condenser (TILL Photonics, Planegg, Germany). Excitation light at 473 and 532 nm was provided by two solid-state lasers (Laser Quantum, Stockport, UK) coupled into a single optical fiber that was connected to the TIRF condenser (TILL Photonics). The experiments were performed at room temperature 24 h after transfection under constant perfusion with fresh physiological saline (Rotblat et al., 2006). Time-lapse imaging was performed with a CoolSNAP HQ camera (Roper Scientific, Tucson, AZ) controlled by MetaMorph software (Universal Imaging, Downingtown, PA). Images were obtained every 100 ms. To obtain a single representation for the mobility observed during the entire duration of imaging, a standard deviation (STD) image was constructed in which each pixel represents the standard deviation of pixel intensities at the same coordinates across the entire time series as detailed elsewhere (Rotblat et al., 2006). The STD images depicting the degree of mobility in each image sequence are represented in pseudocolor (low STD in black, high STD in white).
RESULTS
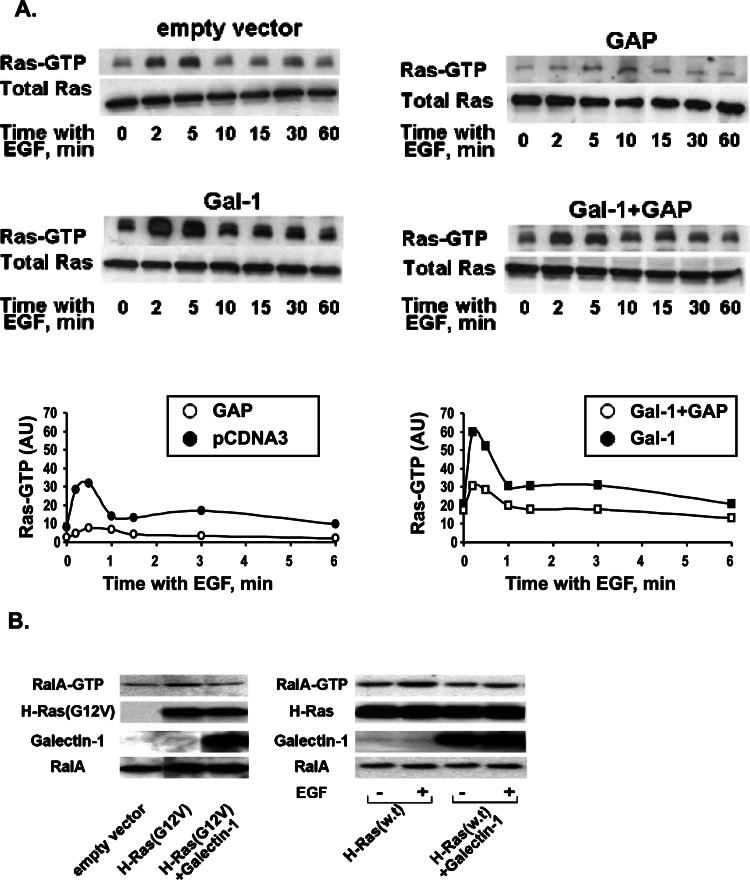
In earlier studies, we identified two Ras escort proteins, Gal-1 and Gal-3 (Paz et al., 2001; Elad-Sfadia et al., 2002, 2004; Rotblat et al., 2004a; Shalom-Feuerstein et al., 2005), which were originally discovered as β-galactoside-binding proteins. We showed that the interaction between K-Ras and Gal-3 is dependent on the farnesyl group and that Gal-3 confers on Ras a conformation that allows prolongation of the Ras signal by reducing the efficiency of RasGAP-facilitated GTP hydrolysis (Elad-Sfadia et al., 2004). To investigate whether Gal-1 reduces RasGAP-facilitated GTP hydrolysis we transfected HEK-293 cells with a plasmid encoding Gal-1, or co-transfected the cells with plasmids encoding Gal-1 and p120 RasGAP then determined the time course of the EGF-stimulated GTP loading of Ras. Empty vector and p120 RasGAP transfectants served as controls. The results of these experiments clearly showed that Gal-1 attenuates the RasGAP-facilitated GTP hydrolysis (Fig. 1A).
Fig. 1.
(A) Gal-1 reduces the efficiency of p120 RasGAP-mediated facilitation of GTP hydrolysis by Ras. HEK-293 cells were transfected with pcDNA3 vector control or with a plasmid encoding Gal-1, or with a plasmid encoding p120 RasGAP. Cells were also co-transfected with Gal-1 and p120 RasGAP vectors. After 24 h the cells were stimulated by 100 ng/mL EGF for the indicated times. Ras-GTP levels were determined by pull-down assay followed by immunoblotting with pan anti-Ras Ab as detailed in Materials and Methods section. Immunoblots were visualized by enhanced chemiluminescence and were then subjected to densitometry. Upper panels show immunoblots of the total amounts of Ras and Ras-GTP in a representative experiment. Lower panels show the results of densitometry in normalized arbitrary units (AU). Similar results were obtained in two additional experiments. (B) Gal-1 inhibits Ras activation of RalGEF. COS-7 cells were transfected with H-Ras(G12V), co-transfected with H-Ras(G12V) and Gal-1, and lysed 48 h later (left panel). Cells were also transfected with H-Ras, co-transfected with H-Ras and Gal-1, serum-starved for 24 h, and then stimulated by 100 ng/mL EGF (7.5 min) and lysed (right panel). Controls were transfected with the empty vector. Levels of Ras, Ral, Ral-GTP, and Gal-1 in the cell lysates were then determined by immunoblotting with anti-Ras, anti-Ral, and anti-Gal-1 Abs,as described in Materials and Methods section.
We showed previously that when H-Ras–GTP interacts with Gal-1 it gains a Ras(35S) effector loop mutant-like conformation that promotes activation of Raf-MEK-ERK at the expense of PI3-K (Elad-Sfadia et al., 2002). Here we examined whether H-Ras–Gal-1 interactions affect the Ras-mediated activation of RalGEF. To do so, we determined the levels of Ral-GTP in COS-7 cells transfected with H-Ras(G12V) or in cells co-transfected with H-Ras(G12V) and Gal-1 (Fig. 1B, left panel). We also determined the levels of Ral-GTP in cells transfected with H-Ras or co-transfected with H-Ras and Gal-1 and then stimulated by EGF (Fig. 1B, right panel). The results of these experiments clearly indicated that Gal-1 inhibits Ras activation of RalGEF.
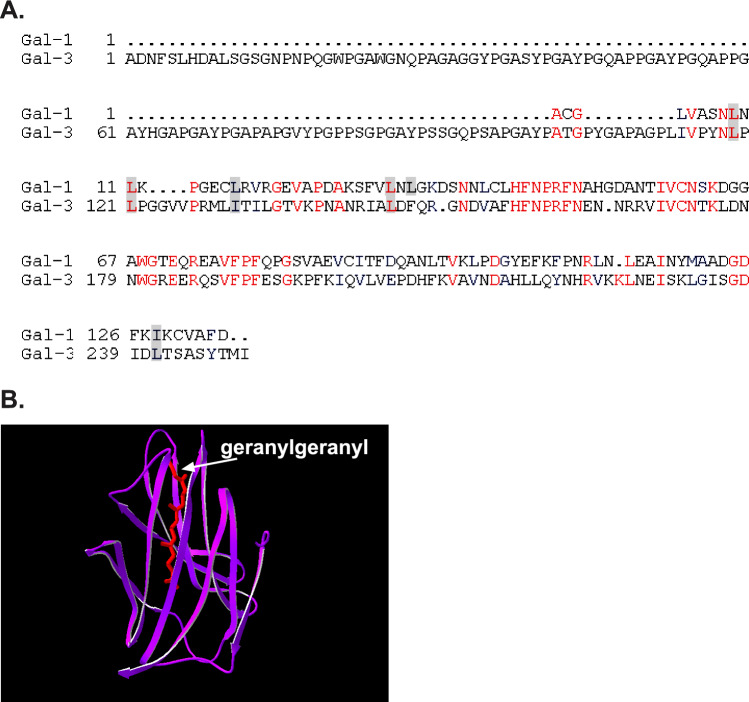
Next, we performed computational modeling in attempts to identify a putative farnesyl-binding domain in Gal-3 analogous to the geranylgeranyl-binding pocket of RhoGDI (Hoffman et al., 2000) and to the putative prenyl-binding pocket recently identified in Gal-1 (Rotblat et al., 2004a). We ran multiple sequence alignments of RhoGDI, Gal-1, and Gal-3 and found that 6 of the 13 amino-acid residues of RhoGDI that interact with the geranylgeranyl group align perfectly with identical or homologous amino acids of Gal-3 (Fig. 2A). Using computational methods (see Materials and Methods section), we identified a hydrophobic pocket in Gal-3 (Fig. 2B), analogous to the Cdc42 geranylgeranyl-binding cavity in RhoGDI (Hoffman et al., 2000) and to a hydrophobic pocket in Gal-1 (Rotblat et al., 2004a).
Fig. 2.
(A) Amino-acid residues of Gal-1 that align perfectly with geranylgeranyl-interacting residues in RhoGDI are conserved in the Gal-3. Human Gal-3, rat Gal-1, and bovine RhoGDI were sequentially aligned (Rotblat et al., 2004a) using the Pileup software (Accelrys). Identical amino-acid residues are shown in red and homologous residues in blue. (B) Identification of a putative isoprenoid insertion site in Gal-3. First, the conserved elements of the structures of Gal-1 (Rotblat et al., 2004a) and the geranylgeranyl-bound RhoGDI, as detected in the Cdc42–RhoGDI complex (Hoffman et al., 2000), were superimposed as described earlier (Rotblat et al., 2004a). The structure of Gal-3 (amino-acid residues 114-250, PDB code 1A3K) was then superimposed on the structure of Gal-1. Results of the analysis illustrate the position of the geranylgeranyl group of the Cdc42 (red) within the Gal-3 structure (purple). Swiss-Prot software was used for the structural comparisons, as described elsewhere (Rotblat et al., 2004a).
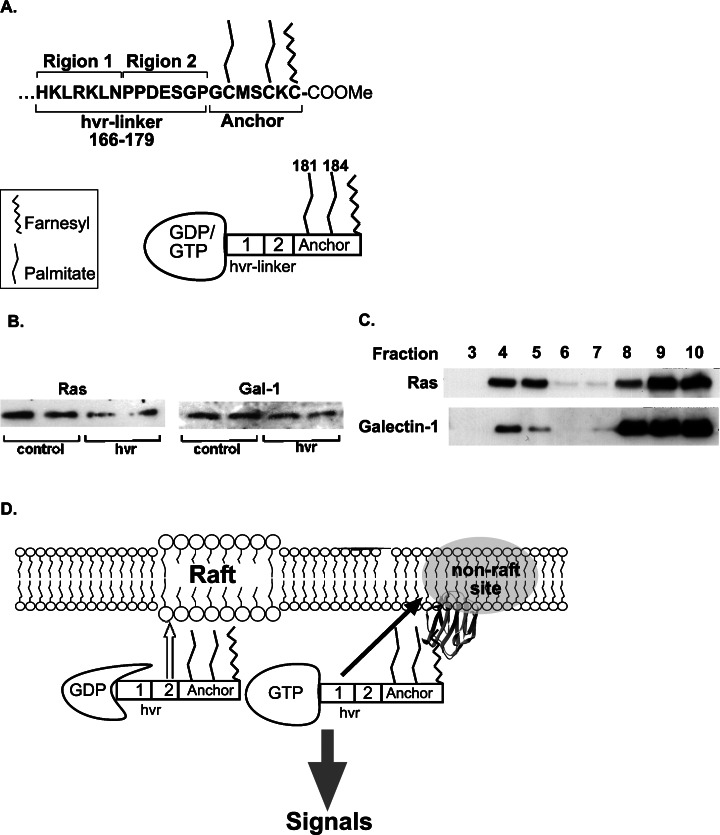
Next, we asked whether the hvr-linker domain of H-Ras may play a role in H-Ras–Gal-1 interactions. To test this possibility, we examined whether a synthetic peptide corresponding to the hvr-linker domain of H-Ras (amino-acid residues 166–179; see scheme in Fig. 3A) can compete with the H-Ras(G12V)-dependent Gal-1 membrane association (Paz et al., 2001; Rotblat et al., 2004a). We found that incubation of membranes of H-Ras(G12V)-transformed Rat-1 cells with the hvr-linker peptide significantly reduced the amount of membrane-bound Gal-1 and H-Ras(G12V) (Fig. 3B). This experiment suggested that association of Gal-1 and H-Ras(G12V) with the membrane depends on the hvr domain of H-Ras, possibly owing to direct interaction of the hvr with Gal-1.
Fig. 3.
(A) Schematic representation of the C-terminal domain of H-Ras. The domain consists of the hvr-linker sequence and the lipid anchor with its two palmitoylated cysteines and the farnesylcysteine carboxymethyl ester (upper panel). Lower panel: A graphic depiction of the H-Ras G domain (GDP- or GTP-bound) and the C-terminal domain. Region 1 and region 2 of the hvr-linker and C181 and C184 palmitoyl moieties are denoted. (B) The hvr-linker peptide of H-Ras dislodges H-Ras(G12V) and Gal-1 from the membrane. Total cell membranes of H-Ras(G12V)-transformed Rat-1 cells were prepared and incubated for 1 h with 50 μM of a synthetic peptide corresponding to the hvr-linker sequence of H-Ras, as described earlier. The membranes were then precipitated (100,000 × g) and immunoblotted with anti-Ras or anti-Gal-1 Ab. Results of a typical experiment performed in duplicate are shown. (C) Gal-1 and H-Ras(G12V) are enriched in the heavy membrane fractions of H-Ras-transformed Rat-1 cells. Total membranes of Rat-1 stably expressing H-Ras(G12V) were fractionated using a non-detergent sucrose gradient flotation procedure as described in Materials and Methods section. The apparent amounts of H-Ras(G12V) and Gal-1 in each fraction were determined by immunoblotting with anti-Ras and anti-Gal-1 Abs. In this protocol, lipid raft components are enriched with caveolin-1 in the light membranes (fractions 4 and 5) and the heavy membranes are enriched in fractions 8–10 (see Niv et al., 2002). Results of a typical experiment are shown. (D) Scheme depicting forces that govern H-Ras membrane association and micro-localization. The scheme is based on the results of FRAP and immunogold EM experiments using cells expressing GFP-H-Ras mutants (Rotblat et al., 2004b) and of experiments which demonstrated that Gal-1 enhances the association of H-Ras-GTP with the PM (Paz et al., 2001). The graphic depiction of H-Ras is as described in (A). Notably, region 2 targets H-Ras to membrane rafts (white arrow) and region 1 to non-raft domains (black arrow); both region provide membrane anchorage forces. The scheme also depicts the interaction of Gal-1 with the farnesyl group of H-Ras-GTP in non-raft sites (part (C) and Rotblat et al., 2004b).
Recent experiments showed that the hvr sequence is required for segregation of H-Ras in non-raft microdomains (Prior et al., 2003; Rotblat et al., 2004b). We therefore postulated that Gal-1 and H-Ras-GTP are co-localized in non-raft microdomains. To examine this possibility we used a non-detergent sucrose gradient flotation procedure (Song et al., 1996) to fractionate the membranes of Rat-1 cells that stably express H-Ras(G12V) and exhibit high levels of Gal-1 (Paz et al., 2001). In agreement with earlier reports (Jaumot et al., 2002), we found that H-Ras(G12V) was enriched in the non-raft heavy membrane fractions (Fig. 3C). Gal-1 exhibited similar enrichment in the same fractions (Fig. 3C). Relatively smaller amounts of the two proteins were found in the light membrane fractions (rafts, Fig. 3C). Thus, Gal-1 and H-Ras-GTP are co-localized in non-raft microdomains (see scheme in Fig. 3D).
Specific sequences of the hvr-linker domain (see scheme in Fig. 3D) were recently found to have distinctive roles in the interactions of H-Ras with the PM (Jaumot et al., 2002; Prior et al., 2003; Rotblat et al., 2004b). This was demonstrated by the use of H-Ras(G12V) mutants with alanine substitutions in the hvr-linker domain (Jaumot et al., 2002): H-Ras(G12V)Δ1ala, in which alanine was substituted for amino-acid residues 166–172, and H-Ras(G12V)Δ2ala, in which alanine was substituted for amino acids 173–179 (see regions 1 and 2 in Fig. 3A). Here we used these mutants to investigate the function of specific regions of the hvr domain in macrolocalization of H-Ras(G12V). We used COS-7 cells transfected with GFP-H-Ras(G12V), GFP-H-Ras(G12V)Δ1Ala, or GFP-H-Ras(G12V)Δ2Ala. The cells were then treated with 50 μM cycloheximide for 2 h to inhibit protein synthesis enabling determination of Ras localization at steady state. The cells were than fixed and the Golgi was labeled with anti-β-COP Ab and subjected to dual fluorescence microscopy imaging analysis. In agreement with earlier studies (Choy et al., 1999; Chiu et al., 2002), we found that GFP-H-Ras(G12V) is localized both in the PM and in the Golgi (Fig. 4A). However, H-Ras(G12V)Δ1ala is localized only in the PM, while H-Ras(G12V)Δ2ala is localized mainly in the Golgi (Fig. 4A). Thus, region 1 promotes Golgi and non-raft PM localization of H-Ras(G12V), whereas region 2 promotes its localization to the PM (mainly to membrane rafts; see scheme in Fig. 4B).
Fig. 4.
(A) Region 1 of the hvr sequence promotes Golgi localization and region 2 promotes plasma membrane localization of H-Ras(G12V). Shown are dual fluorescence images of COS-7 cells transfected with GFP-H-Ras(G12V), GFP-H-Ras(G12V)Δ1Ala, or GFP-H-Ras(G12V)Δ2Ala and treated with 50 μM cycloheximide 2 h prior to labeling of Golgi with anti-β-COP Ab. Green, GFP-H-Ras(G12V) variants; red, β-COP. Scale bar, 10 μm. Right panel: The mean ratio of Golgi GFP fluorescence to total cell GFP fluorescence is given (n=10 cells; p<0.01). (B) Schematic representation of the results shown in (A). The graphic depiction of H-Ras and the hvr domain are as described in Fig. 3A. Notably, region 2 targets H-Ras to rafts (white arrow) and region 1 to non-raft microdomains and to the Golgi (black arrows). (C) Schematic representation of the distinct roles of individual palmitoyl residues in micro- and macro-localization of H-Ras, based on Roy et al. (2005). The graphic depiction of the hvr domain and the palmitoyl groups are as described in Fig. 3A. Notably, palmitate at C181 (white arrow) targets H-Ras to rafts and palmitate at C184 (black arrows) to non-raft microdomains and to the Golgi.
To investigate the spatial and temporal organization of Ras proteins localization, we used GFP-Ras transfectants and employed TIRF microscopy to record fluorescent objects near and at the PM, together with epifluorescence microscopy to record fluorescent objects in the cytoplasm, and combined both with fast time-lapse imaging (Rotblat et al., 2006). Results of a typical experiment, in line with our earlier observations, demonstrate the presence of nanometric GFP-H-Ras particles (Fig. 5A) that were termed rasosomes (Rotblat et al., 2006). We next examined the role of palmitoylation in the association of Ras with the rasosomes. In these experiments, we used cells transfected with GFP-H-Ras(G12V) 181S, 184S. This non-palmitoylated H-Ras mutant fails to associate with rasosomes (Fig. 5A).
Fig. 5.
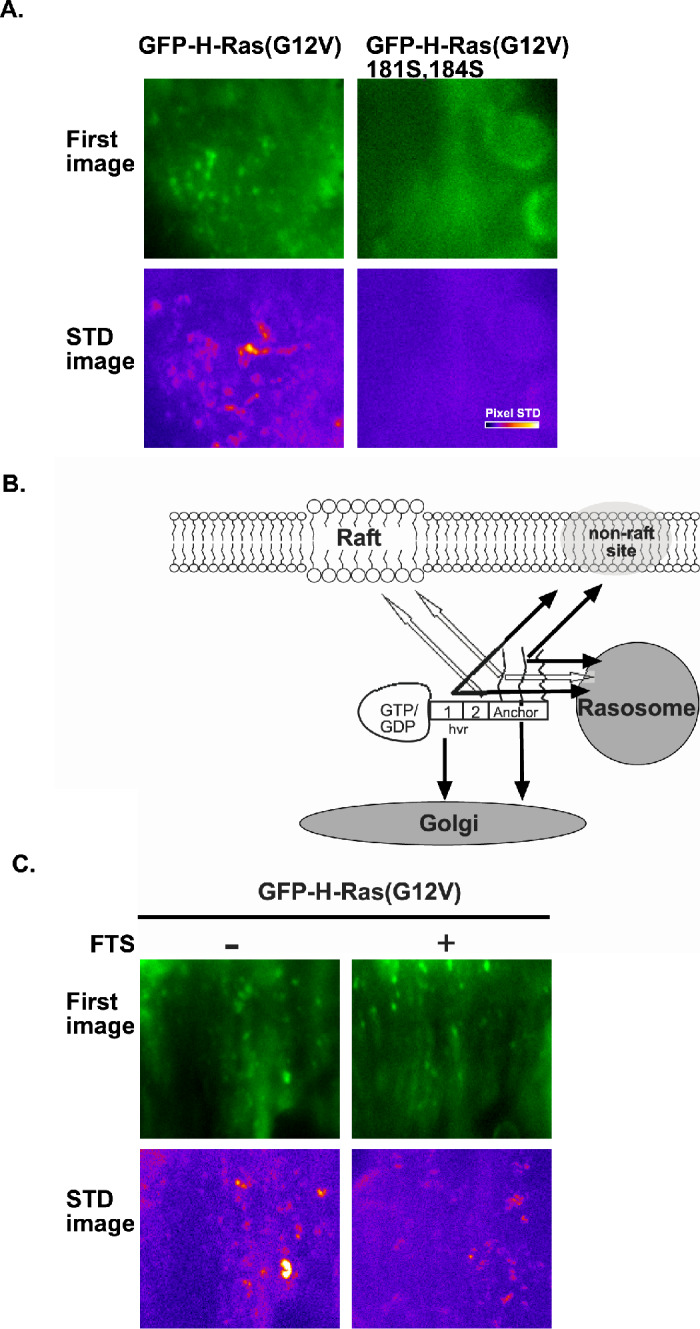
(A) The association of GFP-H-Ras(G12V) with rasosomes depends on its palmitoylation. Shown are the first image of each sequence of live-cell TIRF imaging of BHK cells expressing GFP-H-Ras(G12V) or BHK cells expressing the non-palmitoylated GFP-H-Ras(G12V) 181S, 184S (upper panels). The corresponding STD images depicting the degree of rasosome mobility in each image sequence are represented in pseudocolor (low STD in black, high STD in white; lower panels). (B) Scheme depicting the targeting roles of the hvr-linker sequences and the palmitates in targeting H-Ras to the rasosomes. The scheme is based on results obtained by TIRF combined with time-lapse imaging in cells expressing GFP-H-Ras mutants (Rotblat et al., 2006, and results shown in (A)). The graphic depiction of H-Ras is as described in Fig. 3A. Notably, region 2 targets H-Ras to membrane rafts, but not to rasosomes (white arrow), and region 1 targets H-Ras to rasosomes, to non-raft sites, and the Golgi (black arrows). The palmitoyl groups target H-Ras to rasosomes. (C) The Ras inhibitor FTS does not disrupt the association of GFP-H-Ras(G12V) with the rasosome. Shown are the first image of each sequence of live-cell epifluorescence imaging of BHK cells expressing GFP-H-Ras(G12V), with or without FTS treatment (20 μM, 30 min) to inhibit Ras membrane interactions (Marom et al., 1995). The corresponding STD images depicting the degree of rasosome mobility in each image sequence are represented in pseudocolor (lower panels). Typical results of one of three experiments are shown.
In a similar set of experiments, we investigated the role of the farnesyl group in H-Ras–rasosome interactions. We used the farnesyl derivative FTS, shown to be a useful pharmacological tool in studies of the farnesyl-dependent interaction of Ras with the PM and galectins (Paz et al., 2001; Rotblat et al., 2004a). We performed time-lapse imaging under epifluorescence illumination in GFP-H-Ras(G12V)-expressing cells before and 30 min after the addition of 20 μM S-farnesylthiosalicylic acid (FTS), a treatment that affects Ras membrane interaction and Ras biology (Kloog et al., 1999; Kloog and Cox, 2000). In agreement with early studies (Marom et al., 1995; Egozi et al., 1999; Weisz et al., 1999; Gana-Weisz et al., 2002), the FTS treatment induced a rapid change in morphology of the GFP-H-Ras(G12V) transfectants (not shown). The time-lapse imaging nevertheless showed that FTS does not block the interactions of GFP-H-Ras(G12V) with the rasosomes (Fig. 5B).
DISCUSSION
New Aspects of the Galectin Switch in Ras Signaling
Early studies showed that the farnesylcysteine carboxymethyl ester of Ras is an absolute requirement for normal and oncogenic Ras functions (Casey et al., 1989; Kato et al., 1992; Shields et al., 2000). The farnesyl group appeared to act as a specific recognition unit that would interact with prenyl-binding domains (Marshall, 1993; Kloog et al., 1999; Magee and Seabra, 2003; Kloog and Cox, 2004; Plowman and Hancock, 2005). Consistent with this concept, synthetic farnesylcysteine analogs such as FTS (Kloog et al., 1999; Kloog and Cox, 2004) and N-acetyl-S-farnesyl-l-cysteine (AFC) (Chiu et al., 2004) were found to compete with the binding of Ras to saturable sites in the cell membrane (Niv et al., 2002) and to inhibit Ras transformation (Kloog et al., 1999; Kloog and Cox, 2004). The presence of prenyl-binding domains in proteins has been well documented in a number of guanine nucleotide-dissociation inhibitors (GDIs) that bind prenylated Ras homologs, including Rho (Hoffman et al., 2000) and Rab (Martincic et al., 1997; Pfeffer, 2001) proteins. The geranylgeranyl moieties of these GTPases were shown to be recognized specifically by the corresponding GDIs. The prenyl-dependent interactions between Rho/Rab and their corresponding GDIs lead to conformational changes in the interacting binding partners and are critical for regulation of the GTPases that they bind (Hoffman et al., 2000; An et al., 2003).
By analogy, we identified two Ras escort/regulator proteins, Gal-1 and Gal-3 (Paz et al., 2001; Elad-Sfadia et al., 2002, 2004; Rotblat et al., 2004a; Shalom-Feuerstein et al., 2005), which were originally discovered as β-galactoside-binding proteins. Gal-1 binds active H-Ras-GTP and active K-Ras-GTP, preferentially with the former. Gal-3 binds K-Ras-GTP but not H-Ras-GTP (Elad-Sfadia et al., 2002, 2004). Gal-1 and Gal-3 bind Ras in a farnesyl-dependent manner and the binding is inhibited by FTS (Paz et al., 2001; Elad-Sfadia et al., 2004). Expression of Gal-1 enhances association of H-Ras(G12V) with the cell membrane and downregulation of Gal-1 causes mislocalization of H-Ras(G12V) (Paz et al., 2001; Rotblat et al., 2004a; Hancock and Parton, 2005). Interaction between Ras and Gal-1 and between Ras and Gal-3 confers on Ras a conformation that allows prolongation of the Ras signal (reducing the efficiency of RasGAP-facilitated GTP hydrolysis; Fig. 1A) and association of Ras with distinctive effectors (Elad-Sfadia et al., 2002, 2004). Thus, when H-Ras-GTP interacts with Gal-1 it gains a Ras(35S) effector loop mutant-like conformation that promotes activation of Raf-MEK-ERK at the expense of PI3-K (Elad-Sfadia et al., 2002) and RalGEF (Fig. 1B). In contrast, when K-Ras-GTP interacts with Gal-3 it gains a conformation that promotes activation of Raf, PI3-K, and a third signal that attenuates ERK activation (Elad-Sfadia et al., 2004). These studies thus provided evidence that a new class of Ras-interacting proteins that includes Gal-1 and Gal-3, act as regulators of Ras signal duration and effector usage.
The biological activity of Ras necessitates membrane anchorage that depends on the Ras farnesyl moiety (Casey et al., 1989; Kato et al., 1992; Shields et al., 2000). Similarly interactions between Ras and Gal-1 and between Ras and Gal-3 depend on the farnesyl moiety (Paz et al., 2001; Elad-Sfadia et al., 2004). We postulated that Gal-1 and Gal-3 might possess prenyl-binding domains analogous to the geranylgeranyl-binding pocket of RhoGDI (Hoffman et al., 2000). Using computational methods, we identified a hydrophobic pocket in Gal-1, analogous to the Cdc42 geranylgeranyl-binding cavity in RhoGDI (Rotblat et al., 2004a). This pocket possesses amino-acid residues that are homologous with the isoprenoid-binding residues of RhoGDI, including the critical L11 in Gal-1, whose RhoGDI L77 homolog changes dramatically upon binding with Cdc42 (Hoffman et al., 2000). Use of the same procedure enabled us to identify a similar hydrophobic pocket in Gal-3 (Fig. 2B).
By substituting L11 for A in Gal-1 we obtained a dominant interfering Gal-1 that possessed normal carbohydrate-binding capacity but inhibited H-Ras-GTP loading and ERK activation, dislodged H-Ras(G12V) from the cell membrane, and attenuated H-Ras(G12V) fibroblast transformation and PC12-cell neurite outgrowth (Rotblat et al., 2004a). It thus appeared that, independently of carbohydrate binding, Gal-1 cooperates with Ras, whereas Gal-1(L11A) inhibits it (Rotblat et al., 2004a). Altogether these studies suggest that prenyl-binding domains in Gal-1 and Gal-3 are functionally important elements in the control of Ras functions.
Micro-Localization of H-Ras-GTP and Gal-1 in Non-Raft Sites Require the hvr Domain of H-Ras
The Ras C-terminal hvr domain differentiates H-Ras from K-Ras. The apparent selectivities of Gal-1 and Gal-3 toward the two distinct Ras isoforms seem to be related to structural differences between their hvr C-terminal domains. This suggested that not only the farnesylcysteine but also the hvr domains of H-Ras and K-Ras proteins participate in Ras–galectin interactions. Our results showed that a synthetic peptide corresponding to the hvr-linker domain of H-Ras can compete with the H-Ras(G12V)-dependent Gal-1 membrane association (Fig. 3A). Thus, association of Gal-1 and H-Ras(G12V) with the membrane depends on the hvr domain of H-Ras, possibly owing to direct interaction of the hvr with Gal-1.
It is important to note that Gal-1 exhibits preferential interaction with the activated GTP-bound form of H-Ras (Paz et al., 2001; Rotblat et al., 2004a). H-Ras-GTP and H-Ras-GDP cluster in distinct membrane microdomains, the former in non-raft microdomains and the latter in membrane rafts (Song et al., 1996; Niv et al., 2002; Prior et al., 2003). In addition, recent experiments showed that the hvr sequence is required for segregation of H-Ras in non-raft microdomains (Prior et al., 2003; Rotblat et al., 2004b). Interestingly, we found that Gal-1 and H-Ras-GTP are co-localized in non-raft microdomains (Fig. 3C). These results are in line with a recent immunogold electron microscopic (EM) analysis of the localization of Gal-1 and H-Ras(G12V) in the inner surface of the PM, which demonstrated that Gal-1 and H-Ras(G12V) are clustered in non-raft microdomains (Hancock and Parton, 2005). Downregulation of Gal-1 by antisense Gal-1 caused disappearance of the Gal-1-H-Ras(G12V) clusters (Hancock and Parton, 2005). Taken together, these studies demonstrated that active H-Ras-GTP and Gal-1 are co-localized in non-raft microdomains, and that this micro-localization depends on the presence of the hvr domain of H-Ras.
Spatial Organization of H-Ras Signaling Depends on the hvr-Linker and on Individual Palmitates
In related experiments, we studied the role of the hvr-linker domain and of the palmitates in the cellular localization of H-Ras. Earlier studies indicated that association of H-Ras with the cell membrane is determined by the palmitate groups (Choy et al., 1999) and the hvr-linker sequence (Hancock, 2003; Prior et al., 2003). Our own fluorescence recovery after photobleaching (FRAP) experiments showed that the hvr-linker sequence is absolutely required for stable association of H-Ras with the cell membrane (Rotblat et al., 2004b). Earlier experiments showed that GFP-tagged H-Ras and GFP-H-Ras(G12V) are stably associated with the PM and exhibit pure lateral diffusion (Niv et al., 2002). More recently we found that the GFP-H-Ras(G12V)Δhvr mutant, which lacks the hvr-linker sequence (amino acids 166–179; see Fig. 3A), undergoes rapid exchange between the PM and the cytosol, resulting in impaired Ras functions (Jaumot et al., 2002; Rotblat et al., 2004b). Moreover, the FRAP experiments showed that the dynamic processes of PM association and lateral segregation of H-Ras from raft to non-raft microdomains depend on the GTP-loading state of Ras; whereas the lipid moieties and the hvr-linker domain provide a positive force for association with the PM, the G domain provides a negative force that is stronger in H-Ras-GTP (mainly non-raft-resident) than in H-Ras-GDP (mainly raft-resident) (Rotblat et al., 2004b). Recent studies of the dynamics of Ras interactions with the PM, recorded by single-molecule tracking, demonstrated activation-dependent transient immobilization of H-Ras (Murakoshi et al., 2004; Lommerse et al., 2005). Together these experiments indicated that interaction of H-Ras with the PM is a highly dynamic process that depends on the hvr-linker domain, the lipid anchor, and Ras activation.
Previous studies showed that H-Ras(G12V)Δ1ala is localized to lipid rafts and is biologically inactive (for example, it cannot induce neurite outgrowth or disrupt actin stress fibers), whereas H-Ras(G12V)Δ2ala is localized to non-raft sites and is biologically active (Jaumot et al., 2002). Thus, region 1 is important for targeting H-Ras to non-raft sites, whereas region 2 is important for targeting H-Ras to membrane rafts (see scheme in Fig. 4B). Here we show that these two regions of the hvr-linker domain also have distinctive roles in the macro-localization of H-Ras. In agreement with earlier studies (Choy et al., 1999; Chiu et al., 2002), we found that GFP-H-Ras(G12V) is localized both in the PM and in the Golgi (Fig. 4A). However, H-Ras(G12V)Δ1ala is localized only in the PM, while H-Ras(G12V)Δ2ala is localized mainly in the Golgi (Fig. 4A). Thus, region 1 promotes Golgi and non-raft PM localization of H-Ras(G12V), whereas region 2 promotes its localization to the PM (mainly to membrane rafts; see scheme in Fig. 4B). Together these experiments suggested that specific sequences of the hvr domain are critical for the dynamic processes involved in the spatial organization of activated H-Ras, both in the PM (micro-localization) and in the cell (macro-localization). In this context, it is important to emphasize that H-Ras and N-Ras transmit signals not only from the PM but also from the Golgi and the ER (Chiu et al., 2002).
The spatial organization of H-Ras in the cell is also determined by the palmitoyl groups in the C-terminal lipid anchor. It was shown, firstly, that H-Ras(G12V)181S,184S, a non-palmitoylated mutant, is localized to the ER and is not targeted to the PM (Choy et al., 1999; Goodwin et al., 2005). Second, the two palmitates of H-Ras were recently shown to have different roles in the micro-localization of H-Ras in the PM: palmitate at C181 targets H-Ras to membrane rafts, whereas palmitate at C184 targets H-Ras to non-raft mirodomains (Rocks et al., 2005; Roy et al., 2005) (see scheme in Fig. 4C). Third, it was also shown that the two palmitates of H-Ras have distinctive roles in H-Ras macro-localization: palmitate at C181 targets H-Ras to the PM, whereas palmitate at C184 targets H-Ras to the Golgi (Roy et al., 2005) (see scheme in Fig. 4C). Fourth, depalmitoylation of H-Ras was shown to be a dynamic process that depends on Ras-GTP loading: H-Ras-GTP is depalmitoylated at a significantly faster rate than H-Ras-GDP (Baker et al., 2003). In addition, recent studies showed that H-Ras recycles between the cell membrane and the Golgi, and that the recycling is promoted by depalmitoylation/repalmitoylation (Rocks et al., 2005). Enzymes that catalyze protein depalmitoylation (protein acyl thioesterase) and protein palmitoylation (protein:S-acyltransferases, PATs; reviewed in (Smotrys and Linder, 2004; Magee and Seabra, 2005) are likely to be involved in the recycling of H-Ras and N-Ras. Interestingly, a human PAT for Ras proteins was recently identified (Swarthout et al., 2005); this heterodimeric protein complex (DHHC9/hErff4) is the human ortholog of the yeast Erf2/Erf4 complex that S-acylates the yeast Ras2p (Lobo et al., 2002). All of these studies pointed to specific roles for the palmitoyl groups in targeting and mobilization of palmitoylated Ras proteins to various cellular localities.
Rasosomes: Rapidly Diffusing Nanometric Particles Carrying H-Ras and Its Signal
The dynamic nature of the micro-localization, macro-localization, and signaling of palmitoylated Ras protein from different localities (Bivona and Philips, 2003; Goodwin et al., 2005; Hancock and Parton, 2005; Philips, 2005) suggested that specific mechanisms allow the rapid transfer of these lipid-modified GTPases through the cell. We postulated that they might be carried on mobile cellular platforms. To investigate this possibility, we used GFP-Ras transfectants and employed TIRF microscopy to record fluorescent objects near and at the PM, together with epifluorescence microscopy to record fluorescent objects in the cytoplasm, and combined both with fast time-lapse imaging. Utilizing these methods, we were able to demonstrate the presence of nanometric particles (see for example Fig. 5A) that carry multiple GFP-H-Ras molecules and diffuse rapidly (with diffusion coefficients (D) of 10−2 to 10−1 μm2/s) through the cytoplasm in a random motion, independent of ATP (Rotblat et al., 2006). We termed these particles ‘rasosomes.’ Rasosomes do not co-localize with endocytic or exocytic markers and are independent of protein synthesis and the Golgi (Rotblat et al., 2006). Rasosomes forced to diffuse out of live cells and trapped by Ras-antibody beads appear as round structures 100 nm in diameter (Rotblat et al., 2006). We found that EGF induces a rapid increase in active H-Ras-GTP and phosphorylated ERK on rasosomes (Rotblat et al., 2006). Similarly, rasosomes carrying H-Ras(G12V), but not H-Ras, are loaded with active ERK. Thus, the rasosome represents a hitherto unknown particle that enables Ras-signal information to spread rapidly across cells.
Rasosomes Carry Palmitoylated Ras Proteins but not Non-Palmitoylated K-Ras
We next examined whether the rasosomes can carry Ras isoforms other than H-Ras. We performed TIRF microscopy combined with fast time-lapse imaging in cells expressing the palmitoylated GFP-H-Ras and GFP-N-Ras isoforms and the non-palmitoylated GFP-K-Ras isoform. This comparative analysis required a method to quantify the movement of the entire population of rasosomes appearing transiently in an image sequence (Rotblat et al., 2006) above the background fluorescence (indicated by diffuse membrane staining; see Fig. 5A). We therefore developed a time-lapse image-processing procedure (Rotblat et al., 2006), which enabled us to calculate the overall mobility of fluorescent objects in image sequences. We produced a single image from a movie (a whole stack of images) in which each pixel represents the standard deviation (STD) of pixel intensities at the same coordinates across the entire time series (STD image; for an STD image see Fig. 5A). The STD image was then quantified to yield a single parameter of the overall fluorescence variability, defined as the mobility factor (MF; Rotblat et al., 2006). We validated the MF as a quantitative measure of the mobility of fluorescent objects by recording the movement of mobile Golgi-derived vesicles in untreated cells and of immobile Golgi-derived vesicles in ATP-depleted cells (Rotblat et al., 2006). The MF was 3.5 bits/pixel for the mobile vesicles and 2.9 bits/pixel for the immobile vesicles. These experiments also defined the range of MF values under the conditions used (Rotblat et al., 2006). By employing this method, we found that the palmitoylated H-Ras and N-Ras isoforms (MF=3.5 and 3.3 bits/pixel, respectively), but not the non-palmitoylated K-Ras protein (MF=2.9 bits/pixel), were associated with rasosomes. It therefore appears that palmitoylation or a distinctive hvr domain or both are required for association of Ras with the rasosome.
Palmitates and the hvr-Linker Sequence are Essential for H-Ras Association with Rasosomes
To examine the role of palmitoylation in the association of Ras with the rasosomes, we used the general palmitoylation inhibitor, 2-bromopalmitate (2BP) (Coleman et al., 1992). Time-lapse imaging under TIRF illumination demonstrated a strong decrease in the MF of GFP-H-Ras(G12V), from 3.6 bits/pixel in control GFP-H-Ras transfectants to 2.9 bits/pixel in the 2BP-treated cells (Rotblat et al., 2006). These experiments indicated that palmitoylation is required for interactions of H-Ras with rasosomes. It did not show, however, whether palmitoylation of the H-Ras protein itself is required for its association with the rasosome. We therefore conducted a similar set of experiments using cells transfected with GFP-H-Ras(G12V) 181S, 184S. This non-palmitoylated H-Ras mutant failed to associate with rasosomes (MF=2.9; Fig. 5A). Thus, association with the rasosome evidently requires the palmitoyl moieties of H-Ras itself. To examine the role of the hvr-linker sequence in interactions of GFP-H-Ras(G12V) with the rasosome, we performed time-lapse imaging under TIRF illumination in cells expressing the GFP-H-Ras(G12V)Δhvr mutant. No rasosomes were detectable in GFP-H-Ras(G12V)Δhvr transfectants (MF=3.1; Rotblat et al., 2006), indicating that the hvr-linker domain is important for such interactions. Interestingly, region 2 of the hvr-linker domain was not required for association of GFP-H-Ras(G12V) with rasosomes, whereas region 1 was found to be necessary and sufficient (Rotblat et al., 2006; see scheme in Fig. 5B). This was evident from the low MF of GFP-H-Ras(G12V)Δ1ala and the high MF of GFP-H-Ras(G12V)Δ2ala (MF=3.0 and 4.0, respectively; Rotblat et al., 2006).
Because the non-rasosomal GFP-H-Ras(G12V) 181S, 184S mutant (Fig. 5A) undergoes farnesylation and contains the hvr-linker domain, it appears that the farnesyl group and the hvr-linker sequence, in the absence of palmitates, are not sufficient for the association of H-Ras with the rasosome. Similarly, because the GFP-H-Ras(G12V)Δhvr mutant undergoes both farnesylation and palmitoylation but does not associate with the rasosome (Rotblat et al., 2006), it appears that the farnesyl moiety and the palmitates, in the absence of the hvr-linker sequence, are also not sufficient for such association. Thus, the combinations of palmitates and a farnesyl group only, or of the hvr sequence and a farnesyl moiety only, cannot support association of H-Ras with the rasosome. We could not tell, however, whether the combination of palmitates and the hvr-linker (in the absence of a farnesyl group) would support the association. Interaction of the palmitoylated non-farnesylated H-Ras with rasosomes would indicate that the farnesyl group is not essential for the association of H-Ras with the rasosome. In principle, this possibility could have been tested by the use of H-Ras 186S, which is not farnesylated. However, H-Ras 186S is not a useful tool for these experiments because without farnesylation H-Ras is not processed and remains unpalmitoylated. We therefore employed the farnesyl derivative FTS, shown to be a useful pharmacological tool in studies of the farnesyl-dependent interaction of Ras with the PM and galectins (Paz et al., 2001; Rotblat et al., 2004a). We found that FTS fails to block the interactions of GFP-H-Ras(G12V) with the rasosomes (Fig. 5C). These results strongly suggested that interaction of H-Ras with the rasosome is not coordinated by the farnesyl group. Thus, unlike the essential role of the farnesyl group in the association of H-Ras(G12V) with the PM or with Gal-1 (Hancock et al., 1989; Kato et al., 1992; Marshall, 1993; Paz et al., 2001; Rotblat et al., 2004a), the prenyl group might play a limited role in H-Ras–rasosome interactions.
SUMMARY AND CONCLUSIONS
The C-terminal domain of Ras proteins plays a critical role in normal and oncogenic Ras functions. Recent experiments have provided new insights into the significance of individual elements of the C-terminal domain by demonstrating their functional roles in the interplay of Ras proteins with distinctive PM microdomains and various intracellular compartments (Philips, 2005; Plowman and Hancock, 2005). The significance of these studies is heightened by the knowledge that signal outputs of H-Ras and N-Ras, and possibly of K-Ras 4B, are determined not only in the PM but also in various cellular localities (Philips, 2005; Plowman and Hancock, 2005). The examples discussed here show that the farnesyl group, the specific sequences in region 1 and region 2 of the hvr-linker domain, and the palmitates of H-Ras critically determine selectivity of the Ras signal and its micro- and macro-localization in different cellular localities. The compartmentalization of Ras proteins appears to provide an additional level of selectivity to the signal output of these regulatory GTPases.
The farnesyl group acts as a specific recognition unit. Through its interactions with prenyl-binding pockets in Gal-1 and Gal-3, the farnesyl group contributes to prolongation of the Ras signal in the PM and determination of Ras effector usage. Gal-1 promotes H-Ras signaling to Raf at the expense of PI3-K and RalGEF, while Gal-3 promotes K-Ras signaling to both Raf and PI3-K. Interaction of the farnesyl group of H-Ras-GTP with Gal-1 strengthens the association of active Ras with the PM in non-raft microdomains. The clustering of H-Ras-GTP and Gal-1 observed in these microdomains (Hancock and Parton, 2005) suggests that they can operate as robust platforms for signaling from the PM. Compartmentalization of H-Ras is governed by the G domain with its different GDP/GTP binding-state conformations, the hvr-linker domain, the palmitoyl moieties, and the farnesyl group. These elements act in concert to facilitate a dynamic process through which H-Ras is translocated within the PM from lipid rafts to non-raft microdomains and from the cell membrane to intracellular compartments. Each of these elements contributes forces and specificity to the interactions of H-Ras with PM rafts or non-raft domains, Golgi and rasosomes, as summarized in the following:
PM rafts and non-raft microdomains. The GDP-loaded H-Ras is localized mainly in raft microdomains. Forces that specify the interaction of H-Ras-GDP with membrane rafts, and either strengthen or weaken it, are provided by the G domain (week repulsive forces), region 2 of the hvr-linker domain, and the adjacent palmitoyl group at C181 (Fig. 5B). The GTP-loaded H-Ras is mainly localized in non-raft microdomains. Forces that specify and either strengthen or weaken the interactions of H-Ras-GTP with non-raft microdomains are provided by the G domain (strong repulsive forces), region 1 of the hvr-linker, the palmitoyl group at C184, and the farnesyl group associated with Gal-1 (Fig. 5B).
Golgi localization of H-Ras. H-Ras localization in the Golgi is governed by the same elements as those specifying and strengthening the non-raft localization of H-Ras-GTP, namely region 1 of the hvr-linker and palmitate at C184 (Fig. 5B). This finding, as well as the observed rapid translocation of active H-Ras from the PM to the Golgi by a depalmitoylation/repalmitoylation mechanism (Rocks et al., 2005), would suggest that at least C184 must be repalmitoylated in the Golgi or in other compartments from which the active H-Ras is transferred to the Golgi. The depalmitoylation/repalmitoylation mechanism suggests that loss of both palmitates at C181 and C184 would promote detachment of active H-Ras from the PM, allowing it subsequently to diffuse freely through the cytoplasm to the ER and then be trapped at the Golgi. Alternatively, perhaps only C181 is depalmitoylated during the translocation of H-Ras-GTP from non-raft microdomains to the Golgi. Consistent with such a possibility is the observation that the kinetics of H-Ras-GTP depalmitoylation proceed in two steps (Baker et al., 2003). In this case, H-Ras-GTP with two lipid moieties (the palmitate at C184 and the stably attached farnesyl group) is not likely to diffuse freely in the cytoplasm and might instead move on rasosomes. Even if H-Ras-GTP loses its two palmitates upon detachment from the PM it is likely that the farnesyl group of the detached protein will be shielded by a protein that contains a prenyl-binding domain, as proposed earlier (Hancock and Parton, 2005; Meder and Simons, 2005). Gal-1 is not a likely candidate in this case, however, because its interaction with H-Ras-GTP provides a force acting against detachment of H-Ras from the PM (Paz et al., 2001). Perhaps PDEδ, which contains a prenyl-binding pocket and resembles the structure of RhoGDI (Hanzal-Bayer et al., 2002; Nancy et al., 2002), can bind a depalmitoylated H-Ras-GTP in the cytoplasm. An interesting question is whether the farnesyl group plays a role in H-Ras–Golgi interactions and signaling. It is not unlikely that once H-Ras-GTP is trapped in the Golgi it will recruit Gal-1 to the Golgi, as it does in non-raft microdomains in the PM.
Rasosomes carrying H-Ras. Forces that specify and strengthen the association of H-Ras with rasosomes are less discriminatory than those promoting raft versus non-raft or Golgi localization. First, unlike the stronger association of H-Ras-GDP with the PM relative to that of H-Ras-GTP, no such preference is observed in interactions of H-Ras with the rasosomes. Rasosomes carry H-Ras-GDP and H-Ras-GTP equally well (unpublished data). Second, we found that a single palmitate, either at C181 or at C184, promotes association of H-Ras-GTP with rasosomes (unpublished data). This is in contrast to the specific role of palmitate at C181 in driving the association of H-Ras with membrane rafts, or of palmitate at C184 in driving the association of H-Ras with non-raft membrane microdomains and with the Golgi. In spite of this promiscuity, it appears that region 1 of the hvr-linker specifies interactions of H-Ras-GTP with rasosomes and, as pointed out above, with non-raft microdomains and the Golgi. Finally, it is interesting that region 2 of the hvr-linker and the farnesyl group, both of which are critical for association of H-Ras with the PM, are not important for interactions of H-Ras with the rasosomes.
In conclusion, some of the structural elements in H-Ras that strengthen H-Ras–PM association are unimportant for H-Ras–rasosome interactions, suggesting that these elements would allow rasosomal H-Ras to be uploaded in the PM. Moreover, some other elements that specify the association of H-Ras with the rasosome are identical to elements that target H-Ras to rafts, while other elements are identical to those that target H-Ras to non-raft and Golgi. This, and the more restricted structural elements that specify H-Ras compartmentalization in the PM and in the Golgi, suggest the following: (i) Rasosomes exhibit properties of universal carriers of palmitoylated GDP/GTP-bound Ras proteins. A single rasosome that carries multiple copies of H-Ras (Rotblat et al., 2006) might contain both H-Ras-GDP and H-Ras-GTP, and perhaps other palmitoylated proteins as well. (ii) Rasosomes can potentially unload and upload H-Ras in various cellular compartments.
These suggestions raise an important question: How can a randomly moving universal carrier gain selectivity towards its target? We propose a model (the ‘sampling’ model) that offers a possible explanation. In our model, the rasosome acts as a ‘sampler’, meaning that it samples various cellular localities at which it will either unload or upload H-Ras. The model takes into account the observation that the rasosomes move rapidly and randomly and collide frequently with the PM and with internal compartments (Rotblat et al., 2006). Rasosomal H-Ras will be unloaded only in localities for which H-Ras has a higher affinity than its affinity for the rasosome. H-Ras localized in a sampled microdomain (a rasosome collision spot) will be uploaded onto the rasosome only if it has a higher affinity for the rasosome than for the collision spot. Accordingly, collision of a rasosome with a domain characterized by a force that favorably attracts H-Ras-GDP or H-Ras-GTP will result in selective unloading of one of the molecules of the H-Ras species, or uploading of the other, or both. As an example, consider a rasosome colliding with rafts or with non-raft microdomains. ‘Sampling’ of the rafts will result in unloading of rasosomal H-Ras-GDP and uploading of H-Ras-GTP, whereas sampling of the non-raft microdomains will result in unloading of H-Ras-GTP and uploading of H-Ras-GDP.
ACKNOWLEDGEMENTS
We thank S.R. Smith for editorial assistance. Yoel Kloog is an incumbent of The Jack H. Skirball Chair in Applied Neurobiology. This work was supported in part by grants from The Israel Science Foundation Grants 339/02-3(YK) 424/02-16.6 (UA), the Wolfson Family Foundation Trust (YK), and the Minerva Junior Research Group (UA).
Abbreviations:
- FRAP
fluorescence recovery after photobleaching
- G domain
GTPase domain
- Gal-1
galectin-1
- Gal-3
galectin-3
- GDIs
guanine nucleotide-dissociation inhibitors
- GFP
green fluorescent protein
- hvr
hypervariable region
- PI3-K
phosphoinositide 3-kinase
- PM
plasma membrane
- RalBD
Ral-binding domain of Ral-binding protein 1
- RasGAPs
Ras GTPase-activating proteins
- RalGEFs
Ral guanine nucleotide exchange factors
- RasGEFs
Ras guanine nucleotide exchange factors
- RBD
Ras-binding domain of Raf-1
- TIRF
Total internal reflection fluorescence.
REFERENCES
- Adamson, P., Paterson, H. F., and Hall, A. (1992). Intracellular localization of the P21rho proteins. J. Cell Biol.119:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Y., Shao, Y., Alory, C., Matteson, J., Sakisaka, T., Chen, W., Gibbs, R. A., Wilson, I. A., and Balch, W. E. (2003). Geranylgeranyl switching regulates GDI-Rab GTPase recycling. Structure (Camb.) 11:347–357. [DOI] [PubMed] [Google Scholar]
- Baker, T. L., Zheng, H., Walker, J., Coloff, J. L., and Buss, J. E. (2003). Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic H-ras. J. Biol. Chem.278:19292–19300. [DOI] [PubMed] [Google Scholar]
- Barbacid, M. (1987). ras genes. Annu. Rev. Biochem.56:779–827. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi, D., and Hall, A. (2000). Ras and Rho GTPases: A family reunion. Cell103:227–238. [DOI] [PubMed] [Google Scholar]
- Berzat, A. C., Buss, J. E., Chenette, E. J., Weinbaum, C. A., Shutes, A., Der, C. J., Minden, A., and Cox, A. D. (2005). Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J. Biol. Chem.280:33055–33065. [DOI] [PubMed] [Google Scholar]
- Bivona, T. G., and Philips, M. R. (2003). Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol.15:136–142. [DOI] [PubMed] [Google Scholar]
- Boguski, M. S., and McCormick, F. (1993). Proteins regulating Ras and its relatives. Nature366:643–654. [DOI] [PubMed] [Google Scholar]
- Bos, J. L. (1995). p21ras: An oncoprotein functioning in growth factor-induced signal transduction. Eur. J. Cancer31:1051–1054. [DOI] [PubMed] [Google Scholar]
- Campbell, S. L., Khosravi-Far, R., Rossman, K. L., Clark, G. J., and Der, C. J. (1998). Increasing complexity of Ras signaling. Oncogene17:1395–1413. [DOI] [PubMed] [Google Scholar]
- Casey, P. J., and Seabra, M. C. (1996). Protein prenyltransferases. J. Biol. Chem.271:5289–5292. [DOI] [PubMed] [Google Scholar]
- Casey, P. J., Solski, P. A., Der, C. J., and Buss, J. E. (1989). p21ras is modified by a farnesyl isoprenoid. Proc. Natl. Acad. Sci. U.S.A.86:8323–8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, V. K., Bivona, T., Hach, A., Sajous, J. B., Silletti, J., Wiener, H., Johnson, R. L., 2nd, Cox, A. D., and Philips, M. R. (2002). Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell. Biol.4:343–350. [DOI] [PubMed] [Google Scholar]
- Chiu, V. K., Silletti, J., Dinsell, V., Wiener, H., Loukeris, K., Ou, G., Philips, M. R., and Pillinger, M. H. (2004). Carboxyl methylation of Ras regulates membrane targeting and effector engagement. J. Biol. Chem.279:7346–7352. [DOI] [PubMed] [Google Scholar]
- Chong, H., Vikis, H. G., and Guan, K. L. (2003). Mechanisms of regulating the Raf kinase family. Cell. Signal.15:463–469. [DOI] [PubMed] [Google Scholar]
- Choy, E., Chiu, V. K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Ivanov, I. E., and Philips, M. R. (1999). Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell98:69–80. [DOI] [PubMed] [Google Scholar]
- Coleman, R. A., Rao, P., Fogelsong, R. J., and Bardes, E. S. (1992). 2-Bromopalmitoyl-CoA and 2-bromopalmitate: Promiscuous inhibitors of membrane-bound enzymes. Biochim. Biophys. Acta1125:203–209. [DOI] [PubMed] [Google Scholar]
- Corbett, K. D., and Alber, T. (2001). The many faces of Ras: Recognition of small GTP-binding proteins. Trends Biochem. Sci.26:710–716. [DOI] [PubMed] [Google Scholar]
- Cox, A. D., and Der, C. J. (2003). The dark side of Ras: Regulation of apoptosis. Oncogene22:8999–9006. [DOI] [PubMed] [Google Scholar]
- Downward, J. (2003). Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer3:11–22. [DOI] [PubMed] [Google Scholar]
- Du, W., Lebowitz, P. F., and Prendergast, G. C. (1999). Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol. Cell. Biol.19:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egozi, Y., Weisz, B., Gana-Weisz, M., Ben-Baruch, G., and Kloog, Y. (1999). Growth inhibition of ras-dependent tumors in nude mice by a potent ras-dislodging antagonist. Int. J. Cancer80:911–918. [DOI] [PubMed] [Google Scholar]
- Elad-Sfadia, G., Haklai, R., Ballan, E., Gabius, H. J., and Kloog, Y. (2002). Galectin-1 augments Ras activation and diverts Ras signals to Raf-1 at the expense of phosphoinositide 3-kinase. J. Biol. Chem.277:37169–37175. [DOI] [PubMed] [Google Scholar]
- Elad-Sfadia, G., Haklai, R., Balan, E., and Kloog, Y. (2004). Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem.279:34922–34930. [DOI] [PubMed] [Google Scholar]
- Feig, L. A. (2003). Ral-GTPases: Approaching their 15 minutes of fame. Trends Cell Biol.13:419–425. [DOI] [PubMed] [Google Scholar]
- Gana-Weisz, M., Halaschek-Wiener, J., Jansen, B., Elad, G., Haklai, R., and Kloog, Y. (2002). The Ras inhibitor S-trans,trans-farnesylthiosalicylic acid chemosensitizes human tumor cells without causing resistance. Clin. Cancer Res.8:555–565. [PubMed] [Google Scholar]
- Goodwin, J. S., Drake, K. R., Rogers, C., Wright, L., Lippincott-Schwartz, J., Philips, M. R., and Kenworthy, A. K. (2005). Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol.170:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, L., Magee, A. I., Marshall, C. J., and Hancock, J. F. (1989). Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J.8:1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, J. F. (2003). Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol.4:373–384. [DOI] [PubMed] [Google Scholar]
- Hancock, J. F., Magee, A. I., Childs, J. E., and Marshall, C. J. (1989). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 57:1167–1177. [DOI] [PubMed] [Google Scholar]
- Hancock, J. F., and Parton, R. G. (2005). Ras plasma membrane signalling platforms. Biochem. J.389:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, J. F., Paterson, H., and Marshall, C. J. (1990). A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell63:133–139. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer, M., Renault, L., Roversi, P., Wittinghofer, A., and Hillig, R. C. (2002). The complex of Arl2-GTP and PDE delta: From structure to function. EMBO J.21:2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, G. R., Nassar, N., and Cerione, R. A. (2000). Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell100:345–356. [DOI] [PubMed] [Google Scholar]
- Hu, H., Bliss, J. M., Wang, Y., and Colicelli, J. (2005). RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr. Biol.15:815–823. [DOI] [PubMed] [Google Scholar]
- Jaumot, M., Yan, J., Clyde-Smith, J., Sluimer, J., and Hancock, J. F. (2002). The linker domain of the Ha-Ras hypervariable region regulates interactions with exchange factors, Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem.277:272–278. [DOI] [PubMed] [Google Scholar]
- Jiang, X., and Sorkin, A. (2002). Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol. Biol. Cell.13:1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, K., Cox, A. D., Hisaka, M. M., Graham, S. M., Buss, J. E., and Der, C. J. (1992). Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc. Natl. Acad. Sci. U.S.A.89:6403–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kfir, S., Ehrlich, M., Goldshmid, A., Liu, X., Kloog, Y., and Henis, Y. I. (2005). Pathway- and expression level-dependent effects of oncogenic N-Ras: p27(Kip1) mislocalization by the Ral-GEF pathway and Erk-mediated interference with Smad signaling. Mol. Cell. Biol.25:8239–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog, Y., and Cox, A. D. (2000). RAS inhibitors: Potential for cancer therapeutics. Mol. Med. Today6:398–402. [DOI] [PubMed] [Google Scholar]
- Kloog, Y., and Cox, A. D. (2004). Prenyl-binding domains: Potential targets for Ras inhibitors and anti-cancer drugs. Semin. Cancer Biol. 14:253–261. [DOI] [PubMed] [Google Scholar]
- Kloog, Y., Cox, A. D., and Sinensky, M. (1999). Concepts in Ras-directed therapy. Expert Opin. Invest. Drugs8:2121–2140. [DOI] [PubMed] [Google Scholar]
- Linder, M. E., and Deschenes, R. J. (2003). New insights into the mechanisms of protein palmitoylation. Biochemistry42:4311–4320. [DOI] [PubMed] [Google Scholar]
- Lobo, S., Greentree, W. K., Linder, M. E., and Deschenes, R. J. (2002). Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem.277:41268–41273. [DOI] [PubMed] [Google Scholar]
- Lommerse, P. H., Snaar-Jagalska, B. E., Spaink, H. P., and Schmidt, T. (2005). Single-molecule diffusion measurements of H-Ras at the plasma membrane of live cells reveal microdomain localization upon activation. J. Cell Sci.118:1799–1809. [DOI] [PubMed] [Google Scholar]
- Lopez, I., Mak, E. C., Ding, J., Hamm, H. E., and Lomasney, J. W. (2001). A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J. Biol. Chem.276:2758–2765. [DOI] [PubMed] [Google Scholar]
- Magee, A. I., Newman, C. M., Giannakouros, T., Hancock, J. F., Fawell, E., and Armstrong, J. (1992). Lipid modifications and function of the ras superfamily of proteins. Biochem. Soc. Trans.20:497–499. [DOI] [PubMed] [Google Scholar]
- Magee, A. I., and Seabra, M. C. (2003). Are prenyl groups on proteins sticky fingers or greasy handles? Biochem. J.376:e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee, T., and Seabra, M. C. (2005). Fatty acylation and prenylation of proteins: What's hot in fat. Curr. Opin. Cell Biol.17:190–196. [DOI] [PubMed] [Google Scholar]
- Malumbres, M., and Barbacid, M. (2003). RAS oncogenes: The first 30 years. Nat. Rev. Cancer3:459–465. [DOI] [PubMed] [Google Scholar]
- Marom, M., Haklai, R., Ben-Baruch, G., Marciano, D., Egozi, Y., and Kloog, Y. (1995). Selective inhibition of Ras-dependent cell growth by farnesylthiosalisylic acid. J. Biol. Chem.270:22263–22270. [DOI] [PubMed] [Google Scholar]
- Marshall, C. J. (1993). Protein prenylation: A mediator of protein–protein interactions. Science259:1865–1866. [DOI] [PubMed] [Google Scholar]
- Martincic, I., Peralta, M. E., and Ngsee, J. K. (1997). Isolation and characterization of a dual prenylated Rab and VAMP2 receptor. J. Biol. Chem.272:26991–26998. [DOI] [PubMed] [Google Scholar]
- Matheny, S. A., Chen, C., Kortum, R. L., Razidlo, G. L., Lewis, R. E., and White, M. A. (2004). Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature427:256–260. [DOI] [PubMed] [Google Scholar]
- Meder, D., and Simons, K. (2005). Cell biology. Ras on the roundabout. Science307:1731–1733. [DOI] [PubMed] [Google Scholar]
- Michaelson, D., Silletti, J., Murphy, G., D'Eustachio, P., Rush, M., and Philips, M. R. (2001). Differential localization of Rho GTPases in live cells: Regulation by hypervariable regions and RhoGDI binding. J. Cell Biol.152:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard, M. E., Kim, L. S., Price, J. E., and Gallick, G. E. (2004). The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res. Treat.84:21–32. [DOI] [PubMed] [Google Scholar]
- Mitin, N., Rossman, K. L., and Der, C. J. (2005). Signaling interplay in Ras superfamily function. Curr. Biol.15:R563–R574. [DOI] [PubMed] [Google Scholar]
- Murakoshi, H., Iino, R., Kobayashi, T., Fujiwara, T., Ohshima, C., Yoshimura, A., and Kusumi, A. (2004). Single-molecule imaging analysis of Ras activation in living cells. Proc. Natl. Acad. Sci. U.S.A.101:7317–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancy, V., Callebaut, I., El Marjou, A., and de Gunzburg, J. (2002). The delta subunit of retinal rod cGMP phosphodiesterase regulates the membrane association of Ras and Rap GTPases. J. Biol. Chem.277:15076–15084. [DOI] [PubMed] [Google Scholar]
- Niv, H., Gutman, O., Henis, Y. I., and Kloog, Y. (1999). Membrane interactions of a constitutively active GFP-Ki-Ras 4B and their role in signaling. Evidence from lateral mobility studies. J. Biol. Chem.274:1606–1613. [DOI] [PubMed] [Google Scholar]
- Niv, H., Gutman, O., Kloog, Y., and Henis, Y. I. (2002). Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol.157:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki, Y., Sasagawa, S., and Kuroda, S. (2005). Dynamic characteristics of transient responses. J. Biochem. (Tokyo)137:659–663. [DOI] [PubMed] [Google Scholar]
- Paz, A., Haklai, R., Elad-Sfadia, G., Ballan, E., and Kloog, Y. (2001). Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene20:7486–7493. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R. (2001). Rab GTPases: Specifying and deciphering organelle identity and function. Trends Cell Biol.11:487–491. [DOI] [PubMed] [Google Scholar]
- Philips, M. R. (2005). Compartmentalized signalling of Ras. Biochem. Soc. Trans.33:657–661. [DOI] [PubMed] [Google Scholar]
- Plowman, S. J., and Hancock, J. F. (2005). Ras signaling from plasma membrane and endomembrane microdomains. Biochim. Biophys. Acta1746:274–283. [DOI] [PubMed] [Google Scholar]
- Praskova, M., Khoklatchev, A., Ortiz-Vega, S., and Avruch, J. (2004). Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J.381:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior, I. A., Muncke, C., Parton, R. G., and Hancock, J. F. (2003). Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol.160:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks, O., Peyker, A., Kahms, M., Verveer, P. J., Koerner, C., Lumbierres, M., Kuhlmann, J., Waldmann, H., Wittinghofer, A., and Bastiaens, P. I. (2005). An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science307:1746–1752. [DOI] [PubMed] [Google Scholar]
- Rotblat, B., Niv, H., Andre, S., Kaltner, H., Gabius, H. J., and Kloog, Y. (2004a). Galectin-1(L11A) predicted from a computed galectin-1 farnesyl-binding pocket selectively inhibits Ras-GTP. Cancer Res.64:3112–3118. [DOI] [PubMed] [Google Scholar]
- Rotblat, B., Prior, I. A., Muncke, C., Parton, R. G., Kloog, Y., Henis, Y. I., and Hancock, J. F. (2004b). Three separable domains regulate GTP-dependent association of H-ras with the plasma membrane. Mol. Cell. Biol.24:6799–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblat, B., Yizhar, O., Haklai, R., Ashery, U., and Kloog, Y. (2006). Ras and its signals traverse the cell on randomly moving nanoparticles. Cancer Res.66:1974–1981. [DOI] [PubMed] [Google Scholar]
- Roy, S., Plowman, S., Rotblat, B., Prior, I. A., Muncke, C., Grainger, S., Parton, R. G., Henis, Y. I., Kloog, Y., and Hancock, J. F. (2005). Individual palmitoyl residues serve distinct roles in h-ras trafficking, microlocalization, and signaling. Mol. Cell. Biol.25:6722–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek, K., Ahmadian, M. R., Kabsch, W., Wiesmuller, L., Lautwein, A., Schmitz, F., and Wittinghofer, A. (1997). The Ras–RasGAP complex: Structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science277:333–338. [DOI] [PubMed] [Google Scholar]
- Shalom-Feuerstein, R., Cooks, T., Raz, A., and Kloog, Y. (2005). Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res.65:7292–7300. [DOI] [PubMed] [Google Scholar]
- Shields, J. M., Pruitt, K., McFall, A., Shaub, A., and Der, C. J. (2000). Understanding Ras: ‘it ain't over ‘til it's over’. Trends Cell Biol.10:147–154. [DOI] [PubMed] [Google Scholar]
- Sierra, D. A., Popov, S., and Wilkie, T. M. (2000). Regulators of G-protein signaling in receptor complexes. Trends Cardiovasc. Med. 10:263–268. [DOI] [PubMed] [Google Scholar]
- Silvius, J. R., Bhagatji, P., Leventis, R., and Terrone, D. (2006). K-ras4B and prenylated proteins lacking “second signals” associate dynamically with cellular membranes. Mol. Biol. Cell. 17:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotrys, J. E., and Linder, M. E. (2004). Palmitoylation of intracellular signaling proteins: Regulation and function. Annu. Rev. Biochem.73:559–587. [DOI] [PubMed] [Google Scholar]
- Song, S. K., Li, S., Okamoto, T., Quilliam, L. A., Sargiacomo, M., and Lisanti, M. P. (1996). Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem.271:9690–9697. [DOI] [PubMed] [Google Scholar]
- Swarthout, J. T., Lobo, S., Farh, L., Croke, M. R., Greentree, W. K., Deschenes, R. J., and Linder, M. E. (2005). DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem.280:31141–31148. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S. J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P. C., Woscholski, R., Parker, P. J., and Waterfield, M. D. (2001). Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem.70:535–602. [DOI] [PubMed] [Google Scholar]
- Weisz, B., Giehl, K., Gana-Weisz, M., Egozi, Y., Ben-Baruch, G., Marciano, D., Gierschik, P., and Kloog, Y. (1999). A new functional Ras antagonist inhibits human pancreatic tumor growth in nude mice. Oncogene18:2579–2588. [DOI] [PubMed] [Google Scholar]
- Wymann, M. P., Sozzani, S., Altruda, F., Mantovani, A., and Hirsch, E. (2000). Lipids on the move: Phosphoinositide 3-kinases in leukocyte function. Immunol. Today21:260–264. [DOI] [PubMed] [Google Scholar]
- Zhang, B., Prendergast, G. C., and Fenton, R. G. (2002). Farnesyltransferase inhibitors reverse Ras-mediated inhibition of Fas gene expression. Cancer Res.62:450–458. [PubMed] [Google Scholar]
- Zimmermann, S., and Moelling, K. (1999). Phosphorylation and regulation of Raf by Akt (protein kinase B). Science286:1741–1744. [DOI] [PubMed] [Google Scholar]