Abstract
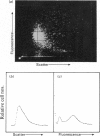
Flow-cytometric analysis of complement-mediated lysis of antibody-coated pigeon erythrocyte ghosts containing fluorescein was carried out to determine whether lysis involved a gradual release of fluorescein or a 'threshold' release from individual cells. Antibody-coated ghosts were comprised of three subpopulations identified by fluorescence and scatter (size). These were: (a) highly fluorescent, medium scatter, (b) medium fluorescence, high scatter, and (c) low (or zero) fluorescence, low scatter. Lysed ghosts and isolated nuclei were identified by fluorescence microscopy and scanning electron microscopy. Fluorescence distributions analysed by flow cytometry indicated that, after complement attack, those ghosts remaining intact retained all their fluorescent label. A time course of changes in ratios of the three subpopulations indicated that once lysis of an individual ghost was initiated, release of label was complete within 1 min; no stages of intermediary fluorescence appeared, and those ghosts remaining at the end of the experiment retained the same fluorescence intensity as control ghosts. The results supported the hypothesis that complement-mediated cell lysis is a 'threshold' phenomenon; a submaximal response by a cell population representing a complete response by only some of the cells rather than a partial response by all of the cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer J., Valet G. Cell volume and osmotic properties of erythrocytes after complement lysis measured by flow cytometry. J Immunol. 1983 Feb;130(2):839–844. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular weight of the membrane C5b-9 complex of human complement: characterization of the terminal complex as a C5b-9 monomer. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1818–1822. doi: 10.1073/pnas.78.3.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Podack E. R., Halverson C. A., Müller-Eberhard H. J. C5b-9 dimer: isolation from complement lysed cells and ultrastructural identification with complement-dependent membrane lesions. J Exp Med. 1979 Feb 1;149(2):448–458. doi: 10.1084/jem.149.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Borsos T. The terminal stages of immune hemolysis--a brief review. Mol Immunol. 1980 Mar;17(3):425–432. doi: 10.1016/0161-5890(80)90064-4. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Daw R. A., Luzio J. P. Rapid increase in intracellular free Ca2+ induced by antibody plus complement. FEBS Lett. 1979 Nov 1;107(1):55–60. doi: 10.1016/0014-5793(79)80462-7. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Luzio J. P. Intracellular free calcium as a pathogen in cell damage initiated by the immune system. Experientia. 1981 Oct 15;37(10):1110–1112. doi: 10.1007/BF02085041. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Kolb W. P., Podack E. R., Müller-Eberhard H. J. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. doi: 10.1073/pnas.76.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Dourmashkin R. R., Humphrey J. H. Observations on the mechanism of immune hemolysis: importance of immunoglobulin class and source of complement on the extent of damage. J Immunol. 1970 Jun;104(6):1502–1510. [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Measurement of changes in cytoplasmic free CA2+ in fused cell hybrids. Nature. 1982 Jan 14;295(5845):155–158. doi: 10.1038/295155a0. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Itakura N., Inai S. A new theoretical model of immune hemolysis: application to the reaction between EAC1-8 and C9. Immunochemistry. 1976 Sep;13(9):771–777. doi: 10.1016/0019-2791(76)90199-3. [DOI] [PubMed] [Google Scholar]
- Kruth H. S. Flow cytometry: rapid biochemical analysis of single cells. Anal Biochem. 1982 Sep 15;125(2):225–242. doi: 10.1016/0003-2697(82)90001-x. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. The complement system. Sci Am. 1973 Nov;229(5):54–66. doi: 10.1038/scientificamerican1173-54. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. C. Uptake of proteins by red blood cells. Exp Cell Res. 1975 Jul;93(2):487–492. doi: 10.1016/0014-4827(75)90478-4. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Rosse W. F., Bell S., Shelburne J. Differences in the terminal steps of complement lysis of normal and paroxysmal nocturnal hemoglobinuria red cells. Blood. 1978 Feb;51(2):325–330. [PubMed] [Google Scholar]
- Shapiro H. M. Flow cytometric probes of early events in cell activation. Cytometry. 1981 Mar;1(5):301–312. doi: 10.1002/cyto.990010502. [DOI] [PubMed] [Google Scholar]
- Watson J. V. Enzyme kinetic studies in cell populations using fluorogenic substrates and flow cytometric techniques. Cytometry. 1980 Sep;1(2):143–151. doi: 10.1002/cyto.990010209. [DOI] [PubMed] [Google Scholar]