Abstract
1. Tight seal, whole-cell recordings from auditory cortex in vivo and in vitro were obtained to investigate modification of N-methyl-D-aspartate (NMDA) receptor-mediated synaptic activity by paired-pulse afferent stimulation. 2. In recordings from urethane-anaesthetized rats (at 37 degrees C), or from cortical slices maintained in vitro (32 degrees C), afferent stimulation elicited a monosynaptic early EPSP and polysynaptic early and late IPSPs. In addition, a late EPSP could be elicited when the stimulus was preceded by an identical priming stimulus (interval approximately 200 ms). The late EPSP was attenuated by the NMDA receptor antagonist DL-2-amino-5-phosphonovalerate (APV, 50 microM). 3. Bath application of the gamma-aminobutyric acid-B (GABAB) receptor antagonist 3-amino-2-(4-chlorophenyl)-2-hydroxy-propylsulphonic acid (2-OH-saclofen; 50 microM) attenuated the late IPSP and clearly revealed a late EPSP. However, 2-OH-saclofen had lesser effects on the second late EPSP elicited during paired-pulse stimulation. Membrane depolarization in 2-OH-saclofen increased the magnitude of the early IPSP, which suppressed the late EPSP once again. Since pharmacological blockade of EPSPs revealed paired-pulse depression of monosynaptically elicited early and late IPSPs, these data indicate that (1) both early and late IPSPs were capable of suppressing the late EPSP, and (2) these effects were reduced during paired-pulse stimulation. 4. Pharmacological isolation of the late EPSP allowed testing of the direct effect of paired-pulse stimulation. Application of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 microM), picrotoxin (10 microM) and 2-OH-saclofen (50 microM) isolated the late EPSP (onset, 3 ms; peak latency, 28 ms; peak amplitude, 7 mV; duration, 240 ms), which grew in magnitude with membrane depolarization and was largely (> 90%) blocked by APV. Paired-pulse stimulation depressed the isolated late EPSP by 30%. 5. Thus, apparent paired-pulse facilitation of the late EPSP is attributable to release from GABAergic inhibition, and not to direct facilitation. Facilitation of the late EPSP is a functional consequence of IPSP depression. The results indicate the importance of inhibition in regulating synaptic activity mediated by NMDA receptors.
Full text
PDF







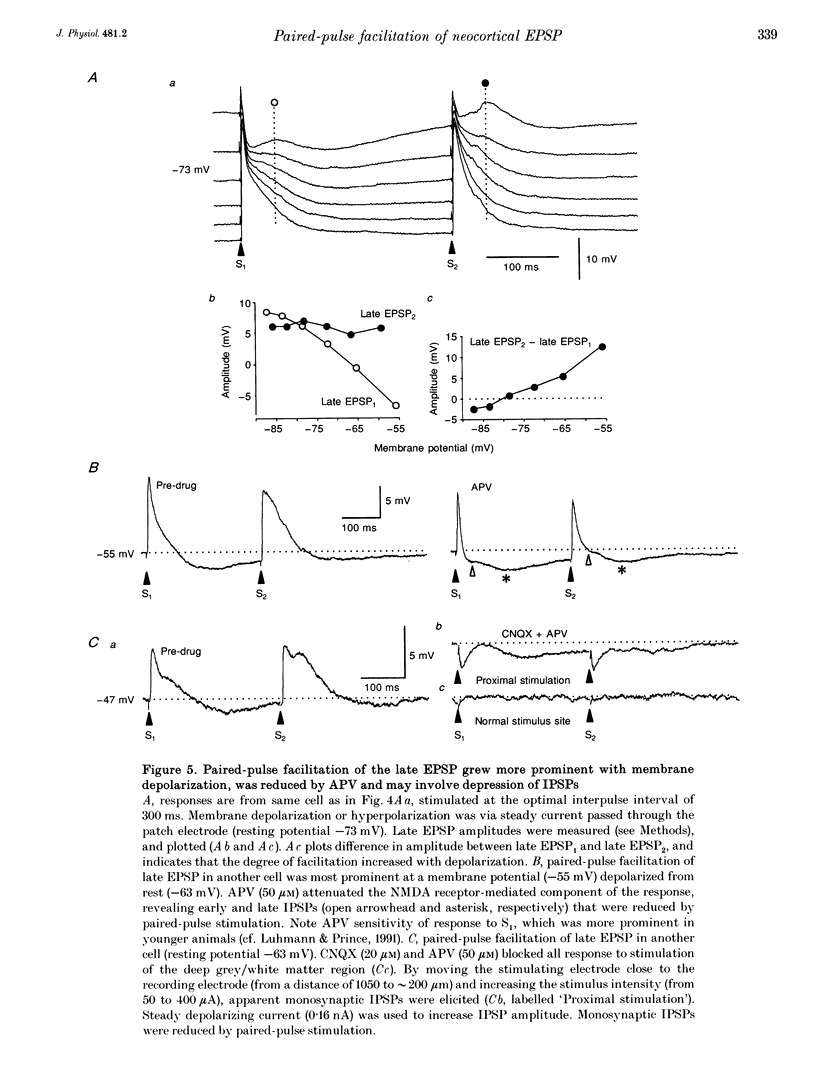
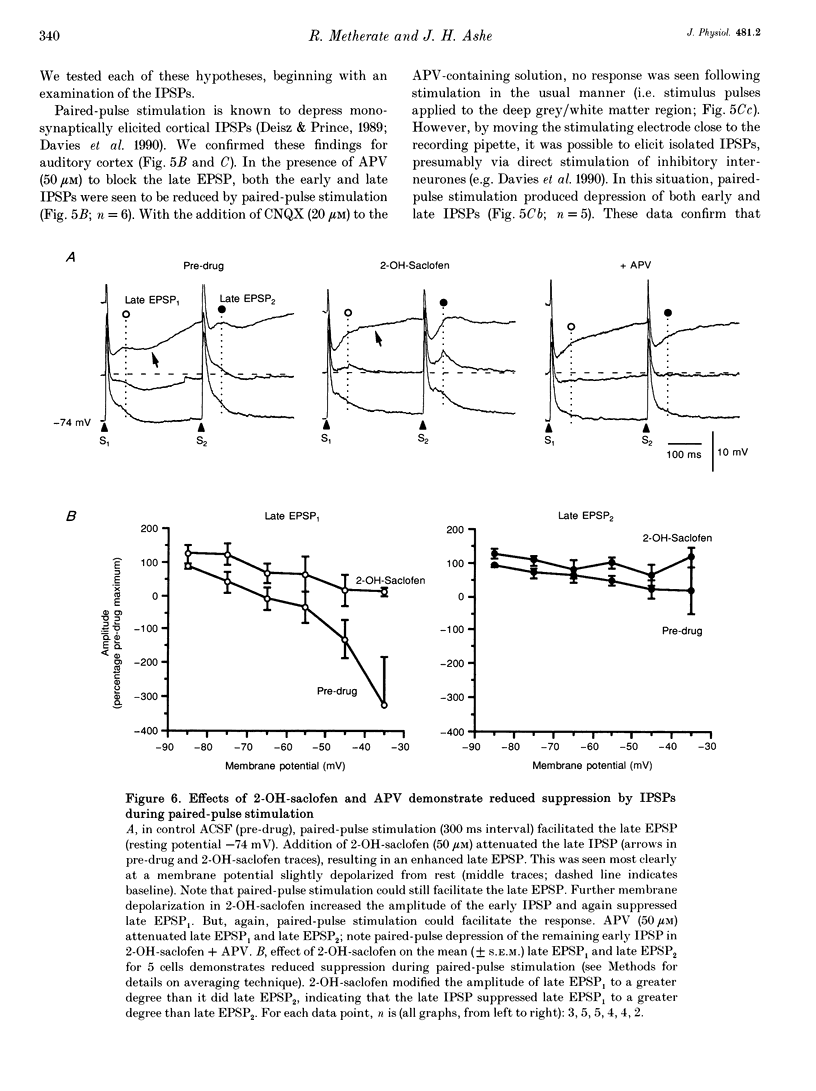








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addae J. I., Stone T. W. Involvement of N-methyl-D-aspartate receptors in the augmenting response in rat neocortex. Neurosci Lett. 1987 Aug 5;78(3):323–327. doi: 10.1016/0304-3940(87)90381-8. [DOI] [PubMed] [Google Scholar]
- Andreasen M., Lambert J. D., Jensen M. S. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989 Jul;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A., Bröcher S., Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990 Sep 6;347(6288):69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Avoli M. Inhibitory potentials in neurons of the deep layers of the in vitro neocortical slice. Brain Res. 1986 Apr 2;370(1):165–170. doi: 10.1016/0006-8993(86)91118-2. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. L., Metherate R., Weinberger N. M., Ashe J. H. Synaptic potentials and effects of amino acid antagonists in the auditory cortex. Brain Res Bull. 1992 Mar;28(3):401–410. doi: 10.1016/0361-9230(92)90039-z. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D., Jagadeesh B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. J Neurosci. 1992 Apr;12(4):1262–1274. doi: 10.1523/JNEUROSCI.12-04-01262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum S. R., Miller R. J. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J Neurosci. 1993 Apr;13(4):1636–1641. doi: 10.1523/JNEUROSCI.13-04-01636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J., Sutor B. Excitatory postsynaptic potentials in rat neocortical neurons in vitro. III. Effects of a quinoxalinedione non-NMDA receptor antagonist. J Neurophysiol. 1990 Oct;64(4):1282–1290. doi: 10.1152/jn.1990.64.4.1282. [DOI] [PubMed] [Google Scholar]
- Higashi H., Tanaka E., Nishi S. Synaptic responses of guinea pig cingulate cortical neurons in vitro. J Neurophysiol. 1991 Apr;65(4):822–833. doi: 10.1152/jn.1991.65.4.822. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Hwa G. G., Avoli M. Excitatory postsynaptic potentials recorded from regular-spiking cells in layers II/III of rat sensorimotor cortex. J Neurophysiol. 1992 Mar;67(3):728–737. doi: 10.1152/jn.1992.67.3.728. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Baughman R. W. NMDA- and non-NMDA-receptor components of excitatory synaptic potentials recorded from cells in layer V of rat visual cortex. J Neurosci. 1988 Sep;8(9):3522–3534. doi: 10.1523/JNEUROSCI.08-09-03522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H. J., Prince D. A. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991 Feb;65(2):247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Metherate R., Ashe J. H. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J Neurosci. 1993 Dec;13(12):5312–5323. doi: 10.1523/JNEUROSCI.13-12-05312.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R., Ashe J. H. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993 Jun;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R., Cox C. L., Ashe J. H. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992 Dec;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G., Davis S., Butcher S. P. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos Trans R Soc Lond B Biol Sci. 1990 Aug 29;329(1253):187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Morrisett R. A., Mott D. D., Lewis D. V., Swartzwelder H. S., Wilson W. A. GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J Neurosci. 1991 Jan;11(1):203–209. doi: 10.1523/JNEUROSCI.11-01-00203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. B., Sur M. NMDA receptors in sensory information processing. Curr Opin Neurobiol. 1992 Aug;2(4):484–488. doi: 10.1016/0959-4388(92)90184-m. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Nuñez A., Amzica F., Steriade M. Electrophysiology of cat association cortical cells in vivo: intrinsic properties and synaptic responses. J Neurophysiol. 1993 Jul;70(1):418–430. doi: 10.1152/jn.1993.70.1.418. [DOI] [PubMed] [Google Scholar]
- Pfrieger F. W., Gottmann K., Lux H. D. Kinetics of GABAB receptor-mediated inhibition of calcium currents and excitatory synaptic transmission in hippocampal neurons in vitro. Neuron. 1994 Jan;12(1):97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. EPSPs in rat neocortical neurons in vitro. I. Electrophysiological evidence for two distinct EPSPs. J Neurophysiol. 1989 Mar;61(3):607–620. doi: 10.1152/jn.1989.61.3.607. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. EPSPs in rat neocortical neurons in vitro. II. Involvement of N-methyl-D-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989 Mar;61(3):621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]


