Abstract
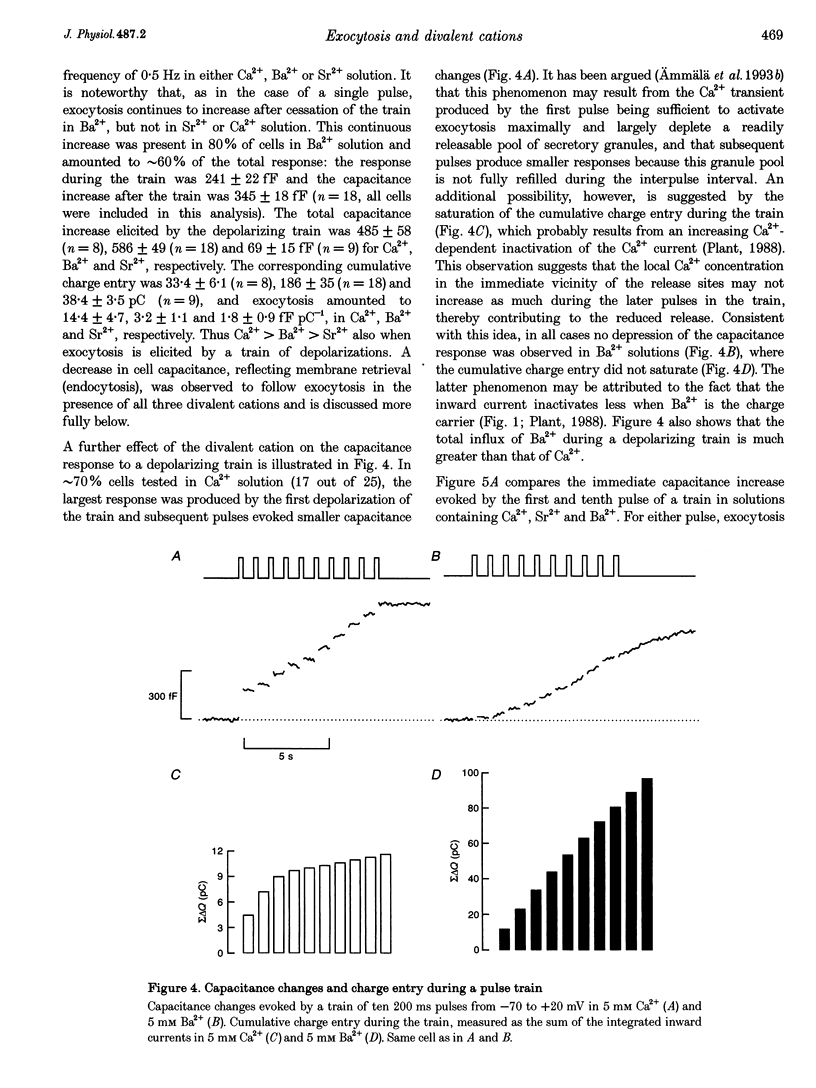
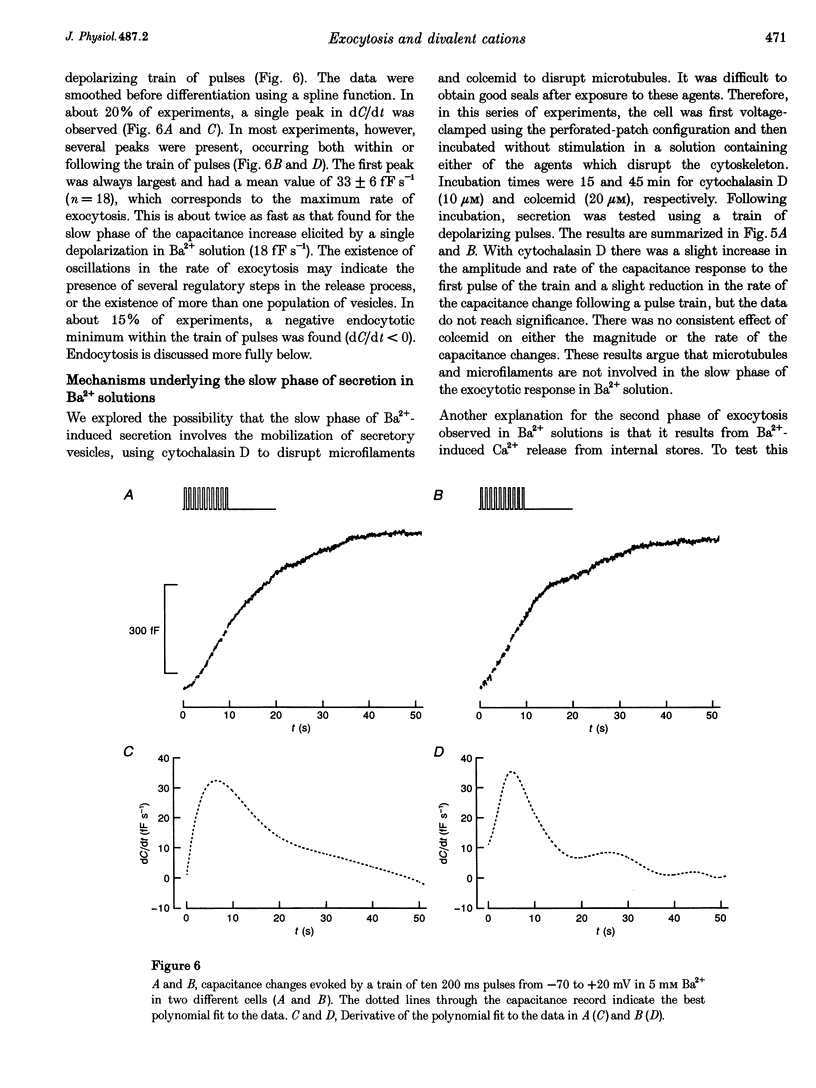
1. The effects of the divalent cations Ca2+, Ba2+ and Sr2+ on exocytosis and endocytosis from single isolated mouse pancreatic beta-cells were investigated by monitoring changes in cell capacitance. 2. The immediate increase in capacitance elicited by a single depolarization from -70 to +20 mV was dependent on the divalent cation species, with Ca2+ (8.2 +/- 1.1 fF pC-1) > Ba2+ (1.0 +/- 0.2 fF pC-1) > Sr2+ (0.7 +/- 0.2 fF pC-1) in perforated-patch recordings. 3. In Ba2+ solutions alone there was subsequently an additional slow increase in capacitance (to 4.3 +/- 1.1 fF pC-1). This second phase of exocytosis was unaffected by preincubation with colcemid (20 microM, 45 min) or cytochalasin D (10 microM, 15 min), suggesting that interaction of secretory granules with microtubules or microfilaments is not involved. 4. An increase in cell capacitance was elicited by depolarization in Ba2+ solutions when intracellular Ca2+ was buffered with 10 mM EGTA. Infusion of the beta-cell with Ba2+ also stimulated exocytosis although the rate was much slower (1.1 +/- 0.2 fF s-1; 8 microM free Ba2+) than for Ca2+ (39 +/- 5 fF s-1; 2 microM free Ca2+). These data indicate that Ba2+ does not evoke secretion by promoting Ca2+ release from internal stores. 5. The lower efficacy of Ba2+ in supporting exocytosis may be related to the fact that this cation does not activate calmodulin-dependent processes and the slow second phase of secretion may result from this ion being removed only slowly from the cytoplasm. 6. Endocytosis was faster in Sr2+ than in Ca2+ or Ba2+ solution, and the speed increased when the external concentration of all three divalent cation species was raised. The ability of Ba2+ to support endocytosis suggests calmodulin-dependent processes are not involved. These data suggest membrane retrieval is regulated differently from exocytosis in beta-cells.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Ashcroft F. M., Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993 May 27;363(6427):356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. Cations and the secretion of insulin from rabbit pancreas in vitro. J Physiol. 1968 Nov;199(1):177–187. doi: 10.1113/jphysiol.1968.sp008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. Specificity of divalent cation requirement for insulin release. Effects of strontium. Pflugers Arch. 1980 Jan;383(2):123–129. doi: 10.1007/BF00581872. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Fyles J. M., Howell S. L. Regulation of insulin secretion by cAMP in rat islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1986 Sep 15;205(2):205–209. doi: 10.1016/0014-5793(86)80898-5. [DOI] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. A., Reuter H., Wendland B., Schweizer F. E., Tsien R. W., Smith S. J. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993 Oct;11(4):713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Smith P. A., Aschroft F. M., Fewtrell C. M. Permeation and gating properties of the L-type calcium channel in mouse pancreatic beta cells. J Gen Physiol. 1993 May;101(5):767–797. doi: 10.1085/jgp.101.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers G., Devis G., van Obberghen E., Malaisse W. J. Calcium-antagonists and islet function. VI. Effects of barium. Pflugers Arch. 1976 Sep 3;365(1):21–28. doi: 10.1007/BF00583624. [DOI] [PubMed] [Google Scholar]
- Thomas P., Lee A. K., Wong J. G., Almers W. A triggered mechanism retrieves membrane in seconds after Ca(2+)-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994 Mar;124(5):667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Hu Q., Sturek M. Multiple effects of ryanodine on intracellular free Ca2+ in smooth muscle cells from bovine and porcine coronary artery: modulation of sarcoplasmic reticulum function. Br J Pharmacol. 1992 Apr;105(4):903–911. doi: 10.1111/j.1476-5381.1992.tb09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Blondel B., Trueheart P. A., Renold A. E., Sharp G. W. Calcium-induced insulin release in monolayer culture of the endocrine pancreas. Studies with ionophore A23187. J Biol Chem. 1975 Feb 25;250(4):1354–1360. [PubMed] [Google Scholar]
- von Grafenstein H., Knight D. E. Triggered exocytosis and endocytosis have different requirements for calcium and nucleotides in permeabilized bovine chromaffin cells. J Membr Biol. 1993 May;134(1):1–13. doi: 10.1007/BF00233471. [DOI] [PubMed] [Google Scholar]
- von Rüden L., García A. G., López M. G. The mechanism of Ba(2+)-induced exocytosis from single chromaffin cells. FEBS Lett. 1993 Dec 20;336(1):48–52. doi: 10.1016/0014-5793(93)81606-z. [DOI] [PubMed] [Google Scholar]


