Abstract
1. M-current (IM) is regulated by intracellular free Ca2+ ([Ca2+]i). Suppression and overrecovery of IM induced by muscarine and luteinizing-hormone releasing hormone (LHRH) are also regulated by [Ca2+]i. The role of the arachidonic acid (AA) pathway in the Ca(2+)-dependent modulation of IM was investigated using whole-cell voltage clamp and intracellular perfusion in dissociated bullfrog sympathetic B neurons. 2. Quinacrine (10-20 microM) and 4-bromophenacyl bromide (4-BPB; 4-10 microM), the inhibitors of phospholipase A2, blocked the enhancement of IM evoked by raising [Ca2+]i. 3. AA (6-120 microM) increased IM by about 50% of the control current in a Ca(2+)-dependent manner. 4. Enhancements of IM by Ca2+ and AA were blocked by the lipoxygenase (LO) inhibitors nordihydroguaiaretic acid (NDGA; 1-5 microM) and 5,8,11-eicosatrynoic acid (ETI; 10 microM). The cyclo-oxygenase inhibitor indomethacin (10 microM) had no effect. 5. Enhancement of IM by Ca2+ was abolished by the selective 12-LO inhibitors baicalein (1-2 microM) and 15(S)-hydroxy-5-cis-8-cis-11-cis-13-trans-eicosatetraenoic acid (15-HETE; 6.5 microM). A 12-LO product, 2(S)-hydroxy-5-cis-8-cis-10-trans-14-cis- eicosatetraenoic acid (12-HETE; 13-20 microM), increased IM without Ca2+ requirement. 6. Enhancement of IM by Ca2+ was not affected by the selective 5-LO inhibitors AA-861 (10 microM), 5,6-dehydroarachidonic acid (5,6-DAA, 10 microM) and L-651,896 (10 microM). The 5-LO metabolites leukotriene C4 (1.5-8 microM) and leukotriene B4 (1.5-5 microM) showed no obvious effect on IM. 7. NDGA alone inhibited IM with an IC50 of 0.73 microM at 120 nM Cai(2+). 8. NDGA did not affect suppression of IM by muscarine or LHRH; however, overrecovery of IM upon removing these agonists was totally eliminated by 1 microM NDGA. 9. Inhibitors of phosphatases, calyculin A (0.1 microM) and okadaic acid (1 microM), completely abolished overrecovery of IM. Calyculin A also blocked the Ca(2+)-induced IM enhancement. 10. It is suggested that Ca2+ enhances IM by stimulating the AA metabolic pathway. Dephosphorylation probably upregulates IM. Overrecovery of IM is probably a result of stimulation of the LO pathway and phosphatases by increased [Ca2+]i.
Full text
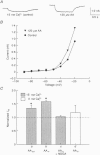
PDF














Images in this article
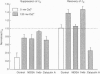
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Tokimasa T. Cellular metabolism regulating H and M currents in bullfrog sympathetic ganglia. Can J Physiol Pharmacol. 1992;70 (Suppl):S51–S55. doi: 10.1139/y92-243. [DOI] [PubMed] [Google Scholar]
- Armstrong D. L. Calcium channel regulation by calcineurin, a Ca2+-activated phosphatase in mammalian brain. Trends Neurosci. 1989 Mar;12(3):117–122. doi: 10.1016/0166-2236(89)90168-9. [DOI] [PubMed] [Google Scholar]
- Baba A., Sakuma S., Okamoto H., Inoue T., Iwata H. Calcium induces membrane translocation of 12-lipoxygenase in rat platelets. J Biol Chem. 1989 Sep 25;264(27):15790–15795. [PubMed] [Google Scholar]
- Bosma M. M., Bernheim L., Leibowitz M. D., Pfaffinger P. J., Hille B. Modulation of M current in frog sympathetic ganglion cells. Soc Gen Physiol Ser. 1990;45:43–59. [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Effects of phorbol dibutyrate on M currents and M current inhibition in bullfrog sympathetic neurons. Cell Mol Neurobiol. 1987 Sep;7(3):255–269. doi: 10.1007/BF00711303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B. J., Barchowsky A., Dolor R. J., Whorton A. R. Regulation of arachidonic acid release in vascular endothelium. Ca(2+)-dependent and -independent pathways. Biochem J. 1991 Dec 1;280(Pt 2):281–287. doi: 10.1042/bj2800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béhé P., Sandmeier K., Meves H. The effect of arachidonic acid on the M current of NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1992 Nov;422(2):120–128. doi: 10.1007/BF00370411. [DOI] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Wu P. H., Su W. G., Corey E. J., Pace-Asciak C. R. Actions of arachidonic acid and hepoxilin A3 on mammalian hippocampal CA1 neurons. Brain Res. 1989 Sep 11;497(1):171–176. doi: 10.1016/0006-8993(89)90984-0. [DOI] [PubMed] [Google Scholar]
- Caulfield M. P., Robbins J., Sim J. A., Brown D. A., Mac Neil S., Blackburn G. M. The naphthalenesulphonamide calmodulin antagonist W7 and its 5-iodo-1-C8 analogue inhibit potassium and calcium currents in NG108-15 neuroblastoma x glioma cells in a manner possibly unrelated to their antagonism of calmodulin. Neurosci Lett. 1991 Apr 15;125(1):57–61. doi: 10.1016/0304-3940(91)90130-l. [DOI] [PubMed] [Google Scholar]
- Chen H., Smith P. A. M-currents in frog sympathetic ganglion cells: manipulation of membrane phosphorylation. Br J Pharmacol. 1992 Feb;105(2):329–334. doi: 10.1111/j.1476-5381.1992.tb14254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Sims S. M., Singer J. J., Walsh J. V., Jr Role for diacylglycerol in mediating the actions of ACh on M-current in gastric smooth muscle cells. Am J Physiol. 1992 Dec;263(6 Pt 1):C1274–C1281. doi: 10.1152/ajpcell.1992.263.6.C1274. [DOI] [PubMed] [Google Scholar]
- Dragan Y. P., Ellis E. F. Effect of adenine nucleotides on cyclooxygenase and lipoxygenase enzyme products of arachidonic acid in human platelets. Biochem Pharmacol. 1990 Jan 1;39(1):27–32. doi: 10.1016/0006-2952(90)90644-z. [DOI] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Purification of a high-molecular-mass form of phospholipase A2 from rat kidney activated at physiological calcium concentrations. Biochem J. 1990 Oct 1;271(1):37–43. doi: 10.1042/bj2710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Abe S., Sawanobori T., Hiraoka M. Arachidonic acid induced increase in intracellular free calcium in guinea-pig hepatocytes. Jpn J Physiol. 1991;41(2):327–332. doi: 10.2170/jjphysiol.41.327. [DOI] [PubMed] [Google Scholar]
- Hua S. Y., Nohmi M., Kuba K. Characteristics of Ca2+ release induced by Ca2+ influx in cultured bullfrog sympathetic neurones. J Physiol. 1993 May;464:245–272. doi: 10.1113/jphysiol.1993.sp019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of neuronal function by calcium. Trends Neurosci. 1989 Nov;12(11):417–420. doi: 10.1016/0166-2236(89)90089-1. [DOI] [PubMed] [Google Scholar]
- Keyser D. O., Alger B. E. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990 Oct;5(4):545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Nordihydroguaiaretic acid inhibits voltage-activated Ca2+ currents independently of lipoxygenase inhibition. Mol Pharmacol. 1990 Oct;38(4):524–530. [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Adams P. R. Release of intracellular calcium and modulation of membrane currents by caffeine in bull-frog sympathetic neurones. J Physiol. 1992 Jan;445:515–535. doi: 10.1113/jphysiol.1992.sp018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion N. V. M-current suppression by agonist and phorbol ester in bullfrog sympathetic neurons. Pflugers Arch. 1994 Feb;426(3-4):296–303. doi: 10.1007/BF00374785. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Zucker R. S., Marsh S. J., Adams P. R. Modulation of M-current by intracellular Ca2+. Neuron. 1991 Apr;6(4):533–545. doi: 10.1016/0896-6273(91)90056-6. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., den Hertog J., de Laat S. W. Epidermal growth factor activates calcium channels by phospholipase A2/5-lipoxygenase-mediated leukotriene C4 production. Cell. 1992 Apr 17;69(2):295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Shapiro E., Feinmark S. J., Schwartz J. H. Metabolites of arachidonic acid in the nervous system of Aplysia: possible mediators of synaptic modulation. J Neurosci. 1987 Nov;7(11):3675–3686. doi: 10.1523/JNEUROSCI.07-11-03675.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueringer R. J., Bahns C. C., Monick M. M., Hunninghake G. W. A23187 stimulates translocation of 5-lipoxygenase from cytosol to membrane in human alveolar macrophages. Am J Physiol. 1992 Apr;262(4 Pt 1):L454–L458. doi: 10.1152/ajplung.1992.262.4.L454. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. Arachidonic acid activates Ca2+ extrusion in macrophages. J Biol Chem. 1990 Oct 25;265(30):18059–18062. [PubMed] [Google Scholar]
- Schmitt H., Meves H. Protein kinase C as mediator of arachidonic acid-induced decrease of neuronal M current. Pflugers Arch. 1993 Oct;425(1-2):134–139. doi: 10.1007/BF00374513. [DOI] [PubMed] [Google Scholar]
- Schweitzer P., Madamba S., Champagnat J., Siggins G. R. Somatostatin inhibition of hippocampal CA1 pyramidal neurons: mediation by arachidonic acid and its metabolites. J Neurosci. 1993 May;13(5):2033–2049. doi: 10.1523/JNEUROSCI.13-05-02033.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya F., Takagi J., Sasaki K., Kawajiri K., Kobayashi Y., Sato F., Saito Y. Feedback regulation of platelet function by 12S-hydroxyeicosatetraenoic acid: inhibition of arachidonic acid liberation from phospholipids. Biochim Biophys Acta. 1990 May 1;1044(1):165–168. doi: 10.1016/0005-2760(90)90232-m. [DOI] [PubMed] [Google Scholar]
- Van der Donk E. M., Dubois G. R., Verhagen J., Veldink G. A., Vliegenthart J. F. Improved purification of 12-lipoxygenase from rat basophilic leukemia cells and conditions for optimal enzyme activity. Biochim Biophys Acta. 1991 Aug 6;1074(3):443–447. doi: 10.1016/0304-4165(91)90098-2. [DOI] [PubMed] [Google Scholar]
- Waite M. Approaches to the study of mammalian cellular phospholipases. J Lipid Res. 1985 Dec;26(12):1379–1388. [PubMed] [Google Scholar]
- Yu S. P., O'Malley D. M., Adams P. R. Regulation of M current by intracellular calcium in bullfrog sympathetic ganglion neurons. J Neurosci. 1994 Jun;14(6):3487–3499. doi: 10.1523/JNEUROSCI.14-06-03487.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]