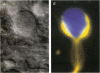
Abstract
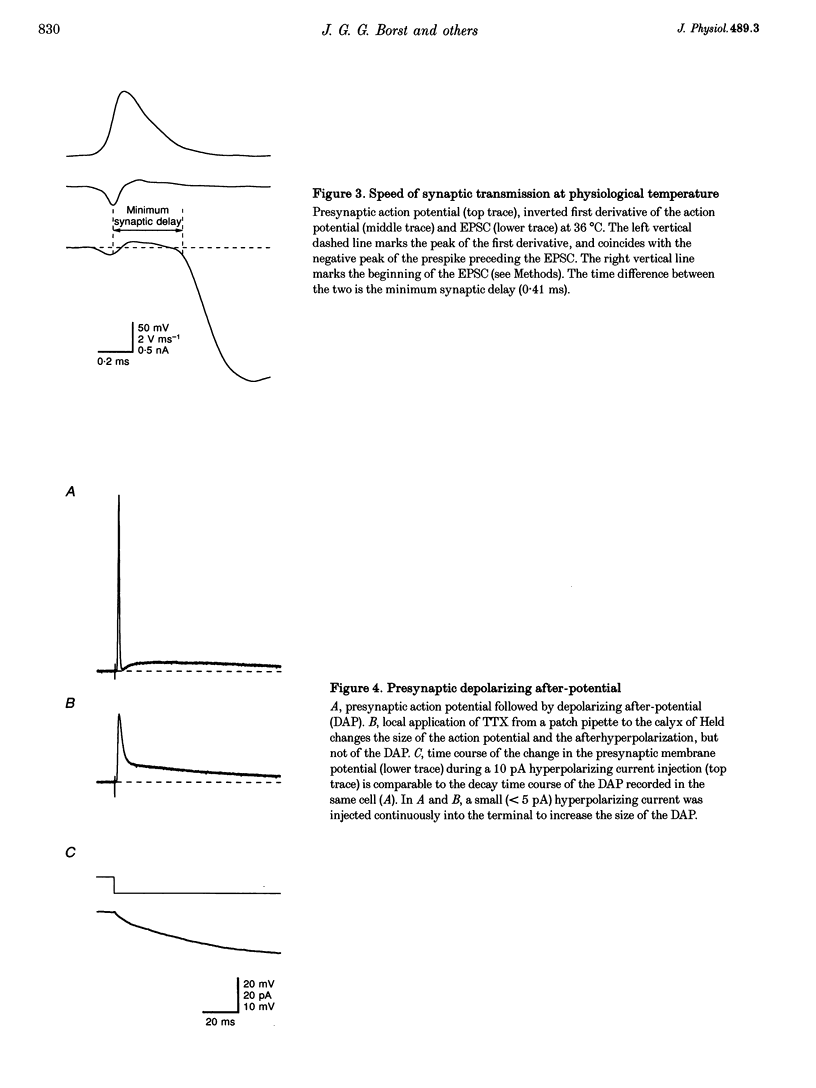
1. Simultaneous whole-cell recordings in a rat brain slice preparation are described from presynaptic terminals (calyces of Held) and postsynaptic somata which form an axosomatic synapse in the medial nucleus of the trapezoid body (MNTB). 2. Presynaptic action potentials evoked suprathreshold excitatory postsynaptic potentials (EPSPs). The minimum synaptic delay was around 0.4 ms at 36 degrees C and 0.9 ms at 23-24 degrees C. The amplitude of the L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor-mediated component of the excitatory postsynaptic currents (EPSCs) was 2-13 nA (at -80 mV). 3. Current-voltage relations showed that presynaptic Ca2+ channels were of the high voltage-activated type. 4. A single action potential evoked a presynaptic fluorescence transient that decayed with a time constant of 0.3-0.7 s, depending on the concentration (60-200 microM) of the Ca2+ indicator Calcium Green-5N (CG-5N). The peak amplitude of the [Ca2+]i transient was severalfold larger in the terminal than in the preterminal axon. 5. EPSC peak amplitudes were stable for more than 30 min after establishing the whole-cell configuration in the presynaptic terminal when the pipette contained 50 microM BAPTA. In contrast, with 1 mM BAPTA, peak amplitudes of EPSCs were reduced to one-third. 6. Trains of presynaptic action potentials evoked EPSCs with progressively smaller amplitudes. Little change was observed in the depression when the terminals were dialysed with 50 microM BAPTA, whereas depression was reduced with 1 mM BAPTA. 7. In low (1 mM) [Ca2+]o, facilitation instead of depression of EPSCs was observed. 8. The effects of presynaptic BAPTA suggest that the endogenous mobile Ca2+ buffer capacity of giant presynaptic terminals in the MNTB is lower than in other terminals of fast transmitting synapses.
Full text
PDF




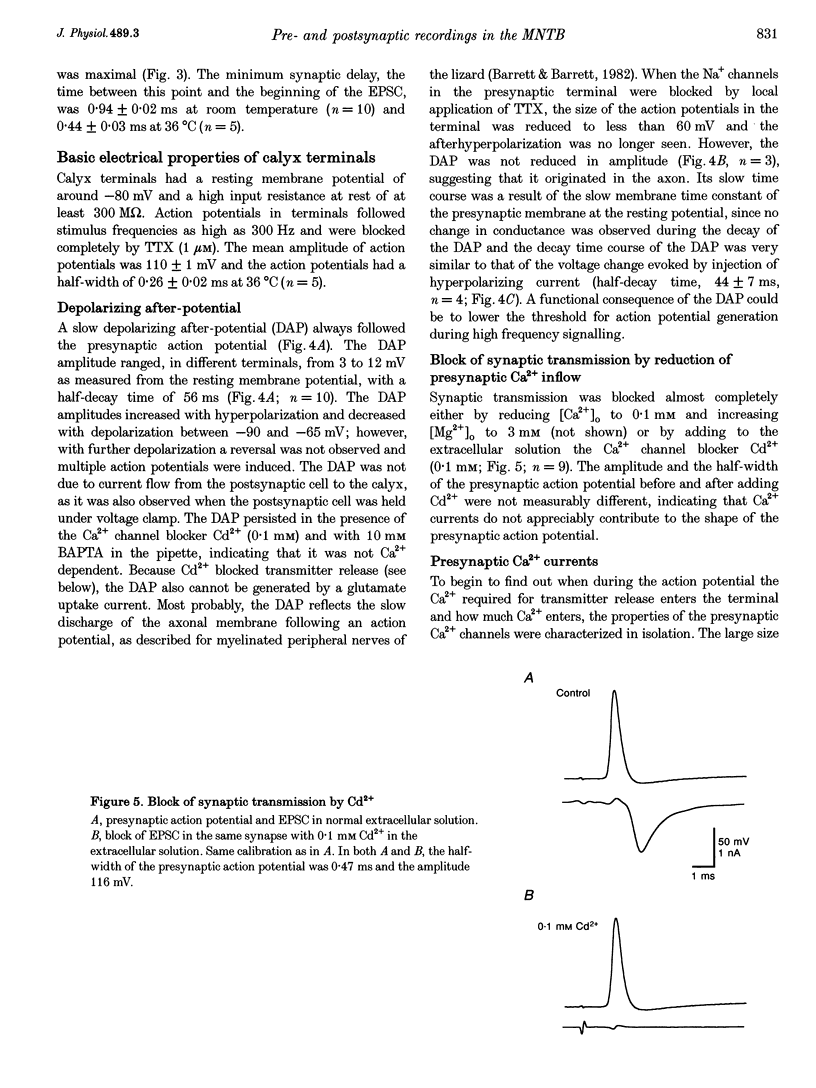

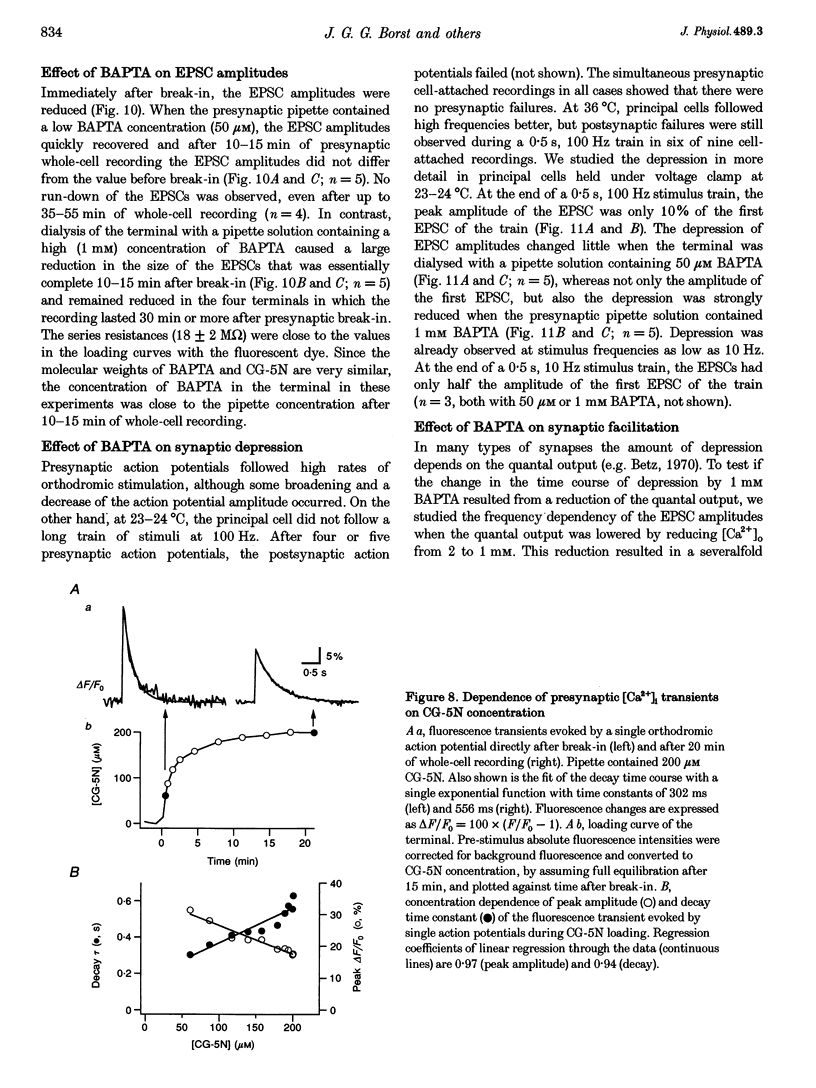
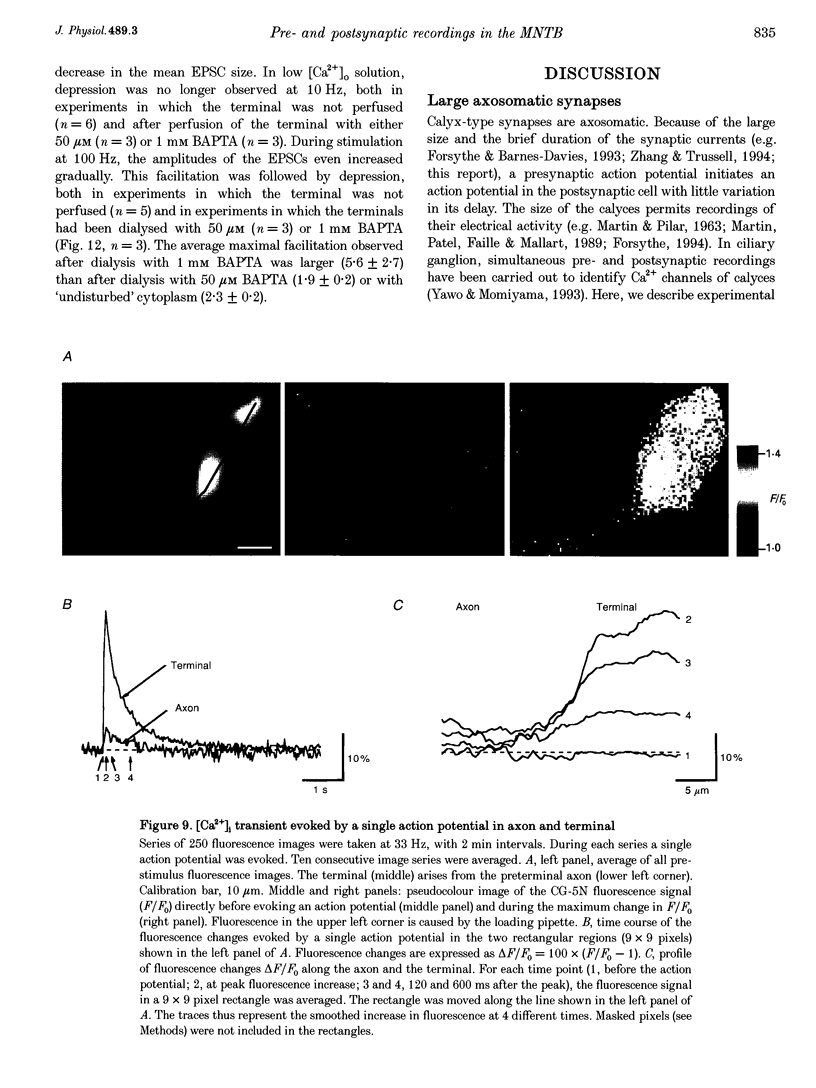
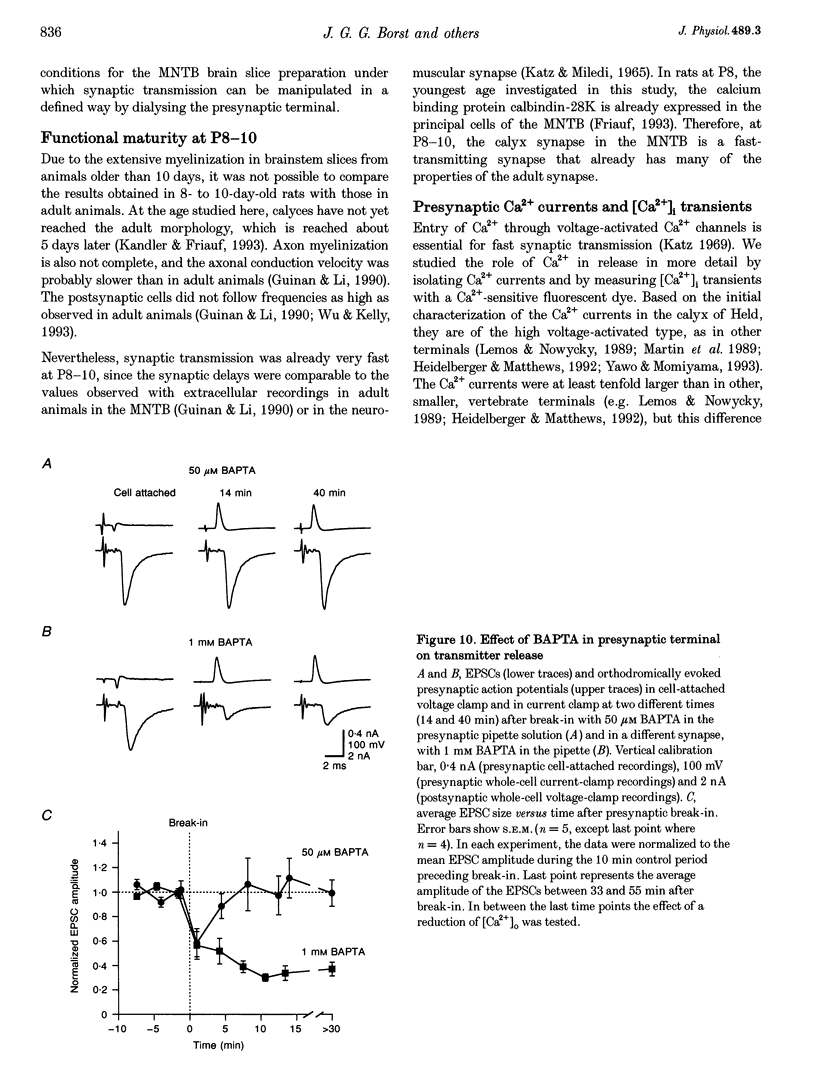




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
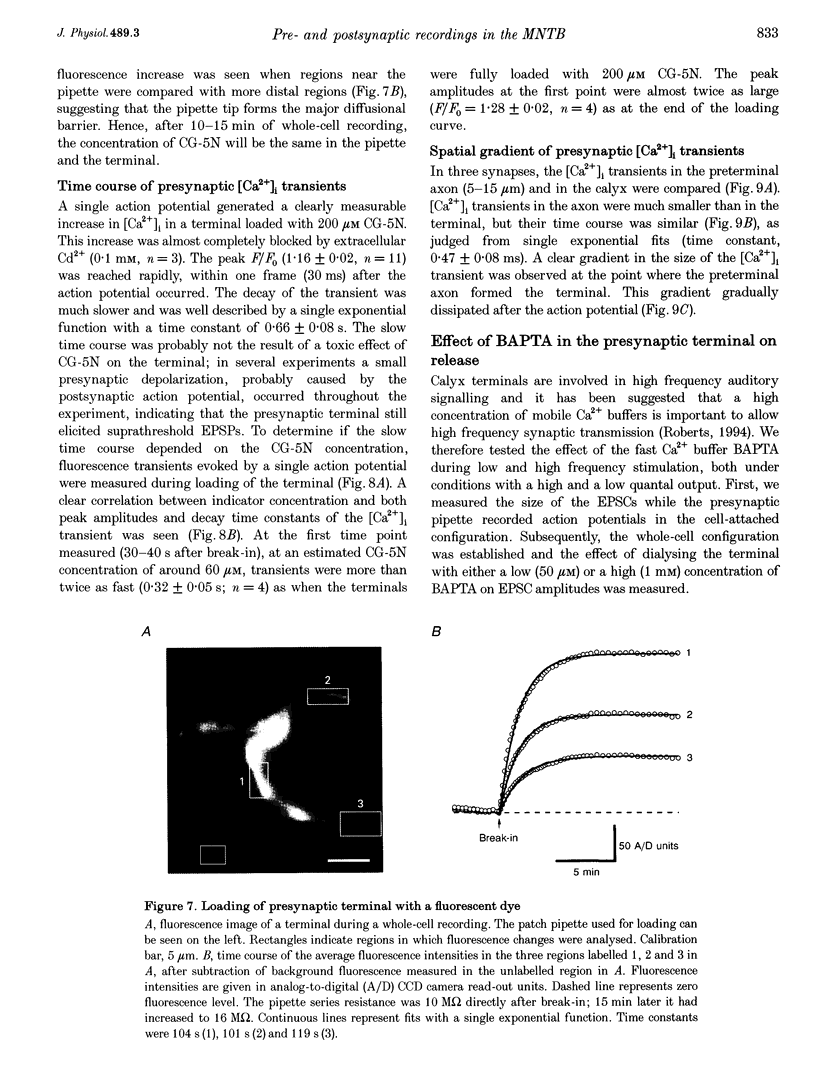
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982 Feb;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon J. A., Wright S. N., Brodwick M. S., Bittner G. D. Presynaptic calcium-activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995 Jan;73(1):178–189. doi: 10.1152/jn.1995.73.1.178. [DOI] [PubMed] [Google Scholar]
- Carlson M. D., Kish P. E., Ueda T. Characterization of the solubilized and reconstituted ATP-dependent vesicular glutamate uptake system. J Biol Chem. 1989 May 5;264(13):7369–7376. [PubMed] [Google Scholar]
- Escobar A. L., Monck J. R., Fernandez J. M., Vergara J. L. Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal muscle fibres. Nature. 1994 Feb 24;367(6465):739–741. doi: 10.1038/367739a0. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Barnes-Davies M. The binaural auditory pathway: excitatory amino acid receptors mediate dual timecourse excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proc Biol Sci. 1993 Feb 22;251(1331):151–157. doi: 10.1098/rspb.1993.0022. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994 Sep 15;479(Pt 3):381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E. Transient appearance of calbindin-D28k-positive neurons in the superior olivary complex of developing rats. J Comp Neurol. 1993 Aug 1;334(1):59–74. doi: 10.1002/cne.903340105. [DOI] [PubMed] [Google Scholar]
- Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995 Jul;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Guinan J. J., Jr, Li R. Y. Signal processing in brainstem auditory neurons which receive giant endings (calyces of Held) in the medial nucleus of the trapezoid body of the cat. Hear Res. 1990 Nov;49(1-3):321–334. doi: 10.1016/0378-5955(90)90111-2. [DOI] [PubMed] [Google Scholar]
- HARRISON J. M., WARR W. B. A study of the cochlear nuclei and ascending auditory pathways of the medulla. J Comp Neurol. 1962 Dec;119:341–379. doi: 10.1002/cne.901190306. [DOI] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992 Feb;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kandler K., Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993 Feb 8;328(2):161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Kuwabara N., DiCaprio R. A., Zook J. M. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol. 1991 Dec 22;314(4):684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Patel V., Faille L., Mallart A. Presynaptic calcium currents recorded from calyciform nerve terminals in the lizard ciliary ganglion. Neurosci Lett. 1989 Oct 23;105(1-2):14–18. doi: 10.1016/0304-3940(89)90004-9. [DOI] [PubMed] [Google Scholar]
- Masterton B., Jane J. A., Diamond I. T. Role of brainstem auditory structures in sound localization. I. Trapezoid body, superior olive, and lateral lemniscus. J Neurophysiol. 1967 Mar;30(2):341–359. doi: 10.1152/jn.1967.30.2.341. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Morest D. K. The growth of synaptic endings in the mammalian brain: a study of the calyces of the trapezoid body. Z Anat Entwicklungsgesch. 1968 Nov 4;127(3):201–220. doi: 10.1007/BF00526129. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. Z., Tong G., Jahr C. E. A false transmitter at excitatory synapses. Neuron. 1993 Jul;11(1):85–91. doi: 10.1016/0896-6273(93)90273-t. [DOI] [PubMed] [Google Scholar]
- Raman I. M., Zhang S., Trussell L. O. Pathway-specific variants of AMPA receptors and their contribution to neuronal signaling. J Neurosci. 1994 Aug;14(8):4998–5010. doi: 10.1523/JNEUROSCI.14-08-04998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Atluri P. P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995 May;68(5):2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. E., Pérez-Pinzón M. A., Lee E. J. Ascorbic acid, but not glutathione, is taken up by brain slices and preserves cell morphology. J Neurophysiol. 1994 Apr;71(4):1591–1596. doi: 10.1152/jn.1994.71.4.1591. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994 May;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Shupliakov O., Brodin L., Cullheim S., Ottersen O. P., Storm-Mathisen J. Immunogold quantification of glutamate in two types of excitatory synapse with different firing patterns. J Neurosci. 1992 Oct;12(10):3789–3803. doi: 10.1523/JNEUROSCI.12-10-03789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Wu S. H., Kelly J. B. Response of neurons in the lateral superior olive and medial nucleus of the trapezoid body to repetitive stimulation: intracellular and extracellular recordings from mouse brain slice. Hear Res. 1993 Aug;68(2):189–201. doi: 10.1016/0378-5955(93)90123-i. [DOI] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Preferential inhibition of omega-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993 Sep 16;365(6443):256–258. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Yawo H., Momiyama A. Re-evaluation of calcium currents in pre- and postsynaptic neurones of the chick ciliary ganglion. J Physiol. 1993 Jan;460:153–172. doi: 10.1113/jphysiol.1993.sp019464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Trussell L. O. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol. 1994 Oct 1;480(Pt 1):123–136. doi: 10.1113/jphysiol.1994.sp020346. [DOI] [PMC free article] [PubMed] [Google Scholar]