Abstract
Background
The use of prosthetic grafts such as polytetrafluorethylene (PTFE) or Dacron to bypass occluded arteries in the lower leg is an accepted practice in the absence of suitable autologous vein. The aim is limb salvage or functional improvement in critical limb ischaemia, but patency rates for below knee prosthetic bypasses are low. Creating a vein cuff at the distal anastomosis is thought to improve outcomes. Other techniques including the use of pre‐cuffed synthetic grafts, spliced segments of vein and the creation of an arterio‐venous fistula (AVF) are also used to improve patency.
Objectives
To compare the beneficial effects of using vein cuffed prosthetic grafts for below knee bypass in critical limb ischaemia with other types of reconstruction.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched May 2012) and CENTRAL (2012, Issue 5) for publications comparing prosthetic infragenicular bypass using vein cuffs with other bypass techniques.
Selection criteria
Randomised controlled trials comparing interposition vein cuff prosthetic graft with autologous vein graft and non‐cuffed prosthetic graft for infragenicular bypass in patients with critical limb ischaemia were included. Trials comparing vein cuff prosthetic grafts with or without AVF and vein cuff prosthetic grafts with pre‐cuffed prosthetic grafts were also included.
Data collection and analysis
The trials were selected and assessed independently by two review authors.
Main results
Six trials with a combined total of 885 patients were included in this review. Only studies using prosthetic PTFE grafts were identified.
Two trials compared PTFE graft with or without a vein cuff. In one underpowered trial for below knee bypass the cumulative primary patency rate was statistically significantly higher in the vein cuff group (80.3% versus 65.3% at 12 months and 51.8% versus 29.1% at 24 months, P = 0.03). There was no statistically significant difference in secondary patency (82.9% versus 72.5% and 58.6% versus 34.9%, P = 0.14) and limb salvage rates (86.3% versus 71.8% and 82.6% versus 62.2%, P = 0.08) at 12 and 24 months respectively. The other trial showed no statistically significant difference between the groups at three years in the below knee femoro‐popliteal bypasses (primary patency rate 26% (95% confidence interval (CI) 18 to 38) and 43% (95% CI 33 to 58), secondary patency rate 32% (95% CI 23 to 44) and 42% (95% CI 31 to 56) and limb salvage rate 64% (95% CI 54 to 75) and 61% (95% CI 50 to 74) in the collar and no collar groups respectively). In the femoro‐distal bypass group, the differences in primary patency, secondary patency and limb salvage rates were also not statistically significant at three years (primary patency rate 20% (95% CI 11 to 38) and 17% (95% CI 9 to 33), secondary patency rate 22% (95% CI 12 to 39) and 20% (95% CI 11 to 35) and limb salvage rate 59% (95% CI 46 to 76) and 44% (95% CI 32 to 61) in the collar and no collar groups respectively).
One trial compared pre‐cuffed PTFE grafts with vein cuffed grafts. There was no statistically significant difference in primary patency rate (62% pre‐cuffed PTFE versus 52% vein cuff PTFE and 49% versus 44%, P = 0.53), secondary patency rate (66% pre‐cuffed PTFE versus 53% vein cuff PTFE and 55% versus 50%, P = 0.30) or limb salvage rate (75% pre‐cuffed PTFE versus 72% vein cuff PTFE and 62% versus 65%, P = 0.88) at 12 and 24 months respectively.
One trial compared spliced vein grafts with vein cuffed PTFE grafts. At 24 months, the secondary patency rate was statistically significantly higher in the spliced vein group (86% in the spliced vein and 52% in the vein cuff group, P < 0.05). There was no statistical significant difference in primary patency rate (44% versus 50%, P > 0.05) and limb salvage rate (94% versus 85%, P > 0.05).
Two trials compared vein cuff PTFE grafts with and without AVF. There was no statistical significant difference at 24 months in primary patency rate (29% versus 36%, P = 0.77; 32% versus 28%, P = 0.2), secondary patency rate (40% versus 40%, P = 0.89; 28% versus 24%, P = 0.2) and limb salvage rate (65% versus 70%, P = 0.97; 62% versus 71%, P = 0.3).
Authors' conclusions
There is some evidence that a vein cuff at the distal anastomosis site improves primary graft patency rates for below knee PTFE graft, but this does not reduce the risk of limb loss. Evidence for this beneficial effect of vein cuffed PTFE grafts is weak and based on an underpowered trial. Pre‐cuffed PTFE grafts have comparable patency and limb salvage rates to vein cuff PTFE grafts. The use of spliced veins improved secondary patency but this did not translate into improved limb salvage. The use of an AVF alone showed no added benefits. A large study with a specific focus on below knee vein cuff prosthetic grafts, including PTFE, is required.
Keywords: Humans, Blood Vessel Prosthesis, Blood Vessel Prosthesis Implantation, Blood Vessel Prosthesis Implantation/methods, Ischemia, Ischemia/surgery, Leg, Leg/blood supply, Limb Salvage, Limb Salvage/methods, Prosthesis Design, Randomized Controlled Trials as Topic, Vascular Patency
Plain language summary
Do additional surgical techniques at the lower end of bypasses in the leg improve the chance of the bypass working and saving the leg from amputation?
If the artery to the lower leg is blocked, replacement of the blocked segment with a bypass graft can save the leg from amputation and reduce the pain resulting from inadequate blood supply to the leg. The best material to use for a bypass graft is the patient's own vein (autologous vein). If a suitable vein is not available, then an artificial tube (synthetic graft) is used. The outcome from these synthetic grafts is less favourable than with autologous veins if the graft extends to below the knee. This review looked at six trials, with a combined total of 885 patients, which compared different methods of making these grafts. Results from two trials which looked at the effect of inserting a cuff of vein at the lower end of the synthetic graft before attaching it to the artery below the knee are conflicting. With one study showing that the bypass graft remains functional for a longer period of time and in the other study no benefit was seen. If a synthetic graft is made in a fashion imitating the shape of a vein cuff, then the same benefit can be achieved. The results also show that when short lengths of vein are joined together to form a sufficiently long graft, the bypass works for longer, although this does not result in fewer amputations. Finally, there is no added benefit for graft patency or amputation rate if a connection is made between the artery and the vein (fistula) when constructing a vein cuff with the synthetic graft but the operation takes longer.
Background
Description of the condition
The consequences of amputation on the quality of life in patients with critically ischaemic legs have encouraged surgeons to attempt revascularisation and limb salvage whenever possible. Autologous veins are universally accepted as the conduit of choice because of their better long term patency rates (four years patency rate: 68% autologous veins versus 47% polytetrafluorethylene (PTFE), P < 0.025) (Veith 1986). The long saphenous vein is the graft of choice, followed by the short saphenous vein and upper limb veins either as a single length or spliced together if additional length is required (Chang 1994; Darling 1993). However, in some patients a suitable vein is not available due to the patient having varicose veins; previous removal because of varicosities, for other bypasses or for phlebitis, fibrosis, or trauma; or if the vein is too small.
The introduction of PTFE and Dacron prostheses in the 1970s offered an alternative in managing these patients. However, it became clear by the mid 1980s that the patency rates for prosthetic below knee bypass was lower than for autologous vein bypass (Ascer 1985; Veith 1986). Intimal hyperplasia (an abnormal thickening in the inner layer of a blood vessel) at the distal anastomosis resulting in narrowing of the lumen is thought to be the main cause for the poor results (O'Donnell 1984).
Description of the intervention
Vein cuff grafts were first introduced by Siegman in 1979 (Siegman 1979) to facilitate joining grafts to small arteries. Later on, Miller modified this technique and introduced the technique of vein cuffed PTFE grafts (Miller 1984). Since then, many case series and non‐randomised controlled trials have shown an improvement in both primary and secondary patency (following further intervention) rates of prosthetic bypasses when vein cuffs are used.
The technique uses any available vein that is slightly longer than double the length of the distal arteriotomy. The vein is opened longitudinally and then one long edge is sewn around the arteriotomy to form a cuff. The PTFE graft is then sutured to this cuff.
How the intervention might work
The improved patency rates have been attributed to a shift in the site of intimal hyperplasia from the critical areas of the anastomotic heel, toe and floor in a PTFE to artery anastomosis to the graft vein interface when a cuff is used. The wider cross‐sectional area of the latter is thought to more readily accommodate the redistributed site of intimal hyperplasia (Kissin 2000; Leuprecht 2002; Tyrrell 1997). Other possible suggestions include an alteration in blood flow dynamics at the artery‐cuff interface compared with an artery‐prosthesis interface. This then alters endothelial shear stresses, which in turn reduce the stimulus for intimal hyperplasia. Other surgeons suggest that improving blood flow above a certain velocity (the thrombotic threshold velocity (TTV)) at the distal anastomotic site might improve the outcome. Therefore, some investigators have added an arterio‐venous fistula to improve the flow rate as an adjunct to the Miller’s cuff (Ascer 1996; Dardik 1996; Sauvage 1979).
Why it is important to do this review
In the absence of a suitable autologous vein, prosthetic grafts may be the only option for patients suffering from critical lower limb ischaemia. Patency is low in PTFE below knee revascularisation (four years patency rates: 76% for autologous veins versus 54% for PTFE grafts, significant P < 0.05) (Veith 1986). Faries et al reported patency rates at one and four years of 81.6% and 68.3% respectively for autologous vein versus 58% and 41% for PTFE (Faries 2000). Another study by Plecha et al showed four year patency rates of 95% for autologous veins versus 50% for PTFE (Plecha 1996). Miller first showed improved patency for PTFE grafts when a vein cuff was interposed at the distal anastomosis (Miller 1984). Over the following 25 years, several randomised clinical trials have looked further into this technique. This review evaluates the evidence for using vein cuffs in below knee prosthetic bypasses.
Twine and McLain previously reviewed the most effective type of graft for femoropopliteal bypass surgery (Twine 2010). The current review complements and extends this review by focusing on the vein cuff technique used in conjunction with the graft as well as involving bypasses distal to the popliteal artery.
Objectives
The objective of this systematic review is to determine the effect of adding a vein cuff to below knee prosthetic grafts in patients with critical limb ischaemia who have insufficient autologous vein for infragenicular bypass. Outcomes include primary and secondary patency rates and limb salvage rates for the following:
below knee bypass prosthetic graft with or without vein cuff;
below knee bypass vein cuffed prosthetic graft with or without arterio‐venous fistula;
below knee bypass vein cuffed prosthetic graft versus pre‐cuffed prosthetic graft;
below knee bypass vein cuffed prosthetic graft versus other (non‐prosthetic) graft.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of interposition vein cuffs for prosthetic infragenicular bypasses are included. Adequacy of randomisation was independently assessed by two authors (AK and GG). Blinding was not considered as it is impossible to achieve during surgery and at follow up.
Types of participants
All patients who underwent a femoro‐distal bypass (femoral to below knee popliteal bypass, femoro‐tibial, femoro‐peroneal and femoro‐pedal) for critical limb ischaemia. Patients were included whatever the cause of their ischaemia and regardless of their diabetes control, smoking or use of aspirin or anticoagulants in the follow‐up period.
Types of interventions
Bypass procedures to below knee vessels using a prosthetic graft with a vein cuff at the distal anastomotic site were considered and compared with prosthetic bypasses without cuffs, autologous bypasses and pre‐cuffed hooded prosthetic grafts. Modification to the technique of Miller’s cuff was included when authors adequately described the formation of a vein cuff at the distal anastomosis site. Additional procedures to the primary operation, such as the formation of an arterio‐venous fistula (AVF), were also considered.
Types of outcome measures
The outcome measures used in this review were the primary and secondary (after further intervention) patency rates and limb salvage rates. The patency rate following specific intervals of time from the intervention was reported in all trials included and was directly measured at the follow‐up examination for each patient. The limb salvage rate is the ultimate outcome measure, being the principle aim of the intervention.
Primary outcomes
Primary patency rate (patency rate after surgery without additional intervention)
Secondary patency rate (patency rate when an additional procedure was performed to restore blood flow in an occluded graft)
Limb salvage rate
All methods of graft patency assessment were accepted when they were clearly described by the study, whether clinical, Doppler ankle‐brachial pressure measurement or graft patency on duplex scan.
Secondary outcomes
We did not look at any secondary outcomes, however they are reported when mentioned in individual studies.
Search methods for identification of studies
There was no language restriction on publication searches.
Electronic searches
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched May 2012) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 5), part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases (PVD) Group module in The Cochrane Library (www.thecochranelibrary.com).
The following trial database was searched by the TSC for details of ongoing and unpublished studies:
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Searching other resources
We searched for further reports from the studies identified from the electronic searches.
Data collection and analysis
Authors of the studies identified from the completed search were contacted for data. We were unable to obtain raw data and a reply from authors is awaited.
Selection of studies
Two authors, Ahmed Khalil (AK) and Gareth Griffiths (GG), independently selected trials for inclusion in the review and assessed the quality of the trials using quality assessment checklists provided by the Cochrane PVD Group. These are included in the 'Characteristics of included trials' table. Trials which meet most of the essential criteria were discussed between authors.
The assessment of the quality of included studies was based on adequacy of the randomisation process and concealment of allocation as reported by each study, as well as the 'Risk of bias' tool (Higgins 2011). Blinding was not considered because the nature of the problem, the intervention and the follow up make it impossible to blind the investigator.
Data extraction and management
AK and GG extracted data according to a fixed protocol, recorded on data extraction forms provided by the Cochrane PVD Group. Any disagreement was resolved by discussion and, where necessary, AB was consulted. AK entered the data into RevMan 5.1 for analysis.
Assessment of risk of bias in included studies
We rated the quality of the included trials according to the rating system used by the Cochrane Peripheral Vascular Diseases Group. We assessed the following domains: adequacy of the randomisation process, concealment of allocation, intention‐to‐treat analysis (ITT) and completeness of follow up, by allocating a score of A (clearly yes), B (unclear) or C (clearly no). If possible, a funnel plot (trial effect against trial size) was used to investigate publication bias. We contacted authors if evidence of ITT analysis was not clearly mentioned in the report.
In addition, we used the 'Risk of bias' tool from The Cochrane Collaboration to assess risk of bias of the following domains: selection bias, attrition bias, reporting bias and other bias. The domains were judged to be at low risk of bias, high risk of bias or unclear risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Blinding was not considered as it is impossible to achieve during surgery and follow up.
Measures of treatment effect
Primary and secondary patency rates and limb salvage rates were the measures of treatment effect. They are included in the tables and reported in the text of the review.
Unit of analysis issues
Primary and secondary patency rates and limb salvage rates reported as percentage per year were compared between studies. We used bypasses as the unit of analysis.
Dealing with missing data
We contacted the corresponding authors of included studies asking for explanations of missing data and for any new data which may be available. Additional techniques were used to study the effects of any missing data (see assessment of reporting bias section).
Assessment of heterogeneity
Where appropriate, and depending on data availability, we planned to use a Chi2 test of heterogeneity to investigate the heterogeneity between studies. When significance was encountered, we planned to perform a sensitivity analysis.
Assessment of reporting biases
Reporting bias was addressed when dealing with missing data. ITT was expected in the report and, if not mentioned, attempts were made to clarify this issue with the corresponding author. When missing data became available, the worst‐case scenario or sensitivity analysis techniques were followed and reported. Other forms of reporting bias such as selective reporting of data or stressing the desired outcome were reported when encountered. We contacted the authors for any unpublished data and looked for unpublished studies.
Data synthesis
We planned to pool data using a random‐effects model analysis. However, because sufficient data could not be compiled, we were unable to pool the data. All patency and limb salvage rates from different studies were organised at specific time intervals as reported by individual studies. Analyses were grouped in these reported intervals, usually by the number of months following the intervention.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were not performed in this review, but subgroup analyses from the individual studies are reported in the text.
Sensitivity analysis
In general, this analysis is performed when heterogeneity is encountered between the studies. It is also one of the methods used when missing data that cannot be replaced are encountered (see dealing with missing data section). Sensitivity analysis is also performed if significance is encountered to determine whether exclusion of individual studies would change the results of the analysis. We planned to perform sensitivity analyses if sufficient data were available.
Results
Description of studies
Results of the search
See Figure 1. Eleven reports of six studies which met the inclusion criteria were identified, the remainder were not relevant. Multiple reports from the same study were reviewed to include the most up to date data. Only studies reporting on PTFE grafts were identified. Studies using other prosthetic grafts such as Dacron grafts were not identified.
1.

Study flow diagram.
Included studies
See: Characteristics of included studies.
PTFE with vein cuff versus PTFE without vein cuff
Two trials were identified in this group. One was a Category A trial (Stonebridge 1997) and a second trial was assigned Category B (SCAMICOS 2010) because of the method used in randomisation, heterogeneity of technique and use of differing PTFE grafts (externally supported versus not externally supported grafts).
Stonebridge 1997 randomised 261 patients and included both above and below knee bypass, 133 patients underwent cuffed PTFE bypass (cuff group) and 128 patient had PTFE without a cuff. One hundred and fifty bypasses were to the popliteal artery above the knee, 76 in the cuff group and 74 in the no cuff group. Ninety six bypasses were to the popliteal below the knee, but 48 in the cuff group and 47 in the no cuff group were available for analysis. Another 15 bypasses were to tibial vessels with eight in the cuff group and seven in the no cuff group. There were 179 males and 82 females. The mean age was 67.2 years in the cuff group and 69.3 years in the no cuff group. Two hundred and nineteen patients were diabetic and 227 were smokers. One hundred and forty six had rest pain, 83 had an ulcer or gangrene and 28 had claudication. Forty six patients had previous graft failure. Postoperatively, 193 patients received aspirin and 40 patients were anticoagulated.
SCAMICOS 2010 randomised 353 patients requiring both above and below knee bypass. One patient with unknown group and all data missing was excluded from analysis. Two hundred and two patients received femoro‐popliteal below knee bypasses. Follow‐up data were available for 199 patients, 114 in the vein collar group and 85 in the no collar group. One hundred and fifty patients were randomised to femoro‐distal bypass. Follow‐up data were available for 146 patients, 69 patients in the collar group and 77 patients in the no collar group. One hundred and thirty nine patients were males and 152 were females, mean age was 78 years. One hundred and twenty one patients were diabetics and 97 patients were smokers. Two hundred and five patients had cardiac disease, 187 had previous vascular surgery, 33 had pulmonary disease and 34 had renal disease; 86.3% in the collar group received some form of anticoagulation versus 89.2% in the no collar group. The three Danish centres in the study used externally supported PTFE grafts in all patients, while the 29 Swedish centres were independently randomised to receive externally supported grafts or not in a factorial design, resulting in equal numbers in each group (50% with externally supported graft and 50% with non‐supported graft). This is regarded as another independent study.
Pre‐cuffed PTFE versus vein cuff PTFE
Only one study was identified in this category (Panneton 2004). This study was assigned Category B for randomisation and allocation concealment. Eighty nine patients and 91 bypasses were reported in this study after exclusion of 13 patients for violation of the protocol. The mean age was 73.2 years in the pre‐cuffed group and 72.6 years in the vein cuffed group. Fifty five per cent in the pre‐cuffed and 64% in the vein cuffed groups were males. Forty five per cent in the pre‐cuffed and 36% in the vein cuffed groups were females. Eighty five per cent in the pre‐cuffed and 75% in the vein cuffed groups had hypertension. Fifty seven per cent in the pre‐cuffed and 61% in the vein cuffed groups had coronary artery disease. Eighty five per cent in the pre‐cuffed and 84% in the vein cuffed group were smokers. Sixty per cent in the pre‐cuffed and 68 % in the vein cuffed groups were diabetic. Twenty one per cent in the pre‐cuffed and 20% in the vein cuffed groups had congestive heart failure. Thirteen per cent in the pre‐cuffed and 23% in the vein cuffed groups had chronic obstructive pulmonary disease (COPD). Sixty six per cent in the pre‐cuffed and 82% in the vein cuffed groups had prior infrainguinal bypass. Fifty five per cent of pre‐cuffed and 50% of vein cuffed bypasses were re‐do procedures. The bypasses were for chronic limb ischaemia in 81% of the pre‐cuffed and in 95.5% of the vein cuffed groups. Acute limb ischaemia was the indication in 19% of the pre‐cuffed and in 4.5% of the vein cuffed groups (P = 0.33). Patients were excluded if they suffered only from claudication or had an above knee reconstruction.
Spliced vein bypass graft versus PTFE graft with a distal vein cuff
One study (Kreienberg 2002) was identified and included. Although the design of this trial was good, with a description of patients who dropped out from follow up, we assigned it Category B because of an unclear description of the randomisation process and of concealment. The study included 36 patients with critical limb ischaemia who underwent 39 bypasses, 20 in the vein cuff group and 19 in the spliced vein group. Mean age was 68.2 ± 2.7 years in the vein cuff group and 71.4 ± 2.3 years in the spliced vein group. Sixty per cent of the vein cuff and 52.6% of the spliced vein groups were males, and 40% of the vein cuff and 47.4% of the spliced vein groups were females. Both groups have comparable ischaemic categories but more patients in the spliced vein group had high risk cardiac status compared with the vein cuff group (P < 0.0001). All the PTFE vein cuff group were anticoagulated postoperatively and the Wolfe boot technique was used to construct the vein cuffs.
PTFE graft with interposition vein cuff at the distal anastomosis with adjuvant arterio‐venous fistula (AVF) versus without adjuvant AVF
Two trials were identified in this group. One was a Category A trial (Laurila 2004) and the other was allocated Category B (Hamsho 1999) because of an unclear description of randomisation or concealment.
Laurila 2004 reported a pilot trial in which 59 patients with critical limb ischaemia were randomised, 31 patients to the AVF group and 28 patients to the control group. The average age was 73.7 years, 74.3 years in the AVF group and 73 years in the control group. Fifty eight per cent were females and 42% were males. Forty one per cent were diabetic, 61% had coronary heart disease, 19% of the AVF group and 29% of the control group suffered from cerebrovascular disease. Nineteen per cent of the AVF group and 7% of the control group suffered from pulmonary disease. Six per cent of the AVF group and 7% of the control group suffered from renal failure. Twenty nine per cent of the AVF group and 43% of the control group were smokers. Seventy one per cent of patients had previous vascular surgery and 58% of the bypasses were a re‐do procedure. Patients were excluded if they only had claudication or if their bypass was to the tibial or pedal arteries. Vancomycin and cefuroxime or cefuroxime alone were given as prophylaxis to all patients. All patients were heparinised intraoperatively and received oral antiplatelets postoperatively. Vein cuff construction techniques were: Miller cuff in 33 patients, St. Mary's Boot in 19 patients, modified Miller cuff in seven patients. The AVF group had a common ostium AVF (25 patients) or a proximal AVF (six patients) according to the surgeon's discretion.
Hamsho 1999 randomised 89 femoro‐infrapopliteal bypasses using ePTFE with 48 having an AVF and 41 not having an AVF; 87 patients had critical limb ischaemia. The mean age was 70.3 years, 71.5 years in the AVF group and 69.1 years in the control group. The male to female ratio was 3.2:1; 72.5% of the AVF group and 64.4% of the control group were smokers. Seventeen and a half per cent of the AVF group and 15.7% of the control group were diabetics. Patients were excluded in the presence of suitable autologous vein, venous hypertension (deep vein obstruction) and severely compromised cardiac function. All patients received cefotaxime and heparin 5000 IU at induction of anaesthesia, then heparin for five days postoperatively. Aspirin 300 mg was started 48 hours after the operation and all patients were discharged on aspirin and warfarin, indefinitely.
Excluded studies
No randomised trials were excluded.
Risk of bias in included studies
The risk of bias, judged according to the rating from the Cochrane PVD group, was not constant in the included trials. Randomisation was mentioned by all studies, yet only three studies adequately described the method used. The same was true for allocation concealment. However, the overall assessment of the study quality was used as a guide to judge unclear randomisation as probably having been satisfactorily performed but not clearly described, until a response from the authors is received.
The trial comparing PTFE grafts with vein cuffed PTFE grafts (Stonebridge 1997) has a clear randomisation method and allocation concealment, using a phone call to a central randomisation office. It was considered Category A.
The trial comparing PTFE graft with vein collar PTFE graft (SCAMICOS 2010) used an old though acceptable randomisation technique utilising blocks of envelopes, with equal numbers in each group, that were randomly selected at each centre. This method is vulnerable to bias when selecting envelopes within the block. There was heterogeneity in the type of PTFE grafts (externally supported or not) used in different centres; and another randomisation was used in the Swedish centres. It was considered Category B.
The pre‐cuffed prosthetic graft versus vein cuff trial (Panneton 2004) had an unclear method of randomisation and allocation concealment although clear reporting of randomisation was mentioned. We therefore allocated it to category B.
The spliced vein versus vein cuffed PTFE graft study (Kreienberg 2002) again had an unclear description of randomisation and allocation concealment. The report clearly mentioned the random allocation of patients to the trial groups, and an allocation of category B was given.
One trial of vein cuffed PTFE with AVF versus no AVF (Laurila 2004) mentioned randomisation of patients very clearly. The allocation concealment was also clear and this trial was allocated to category A. The other trial (Hamsho 1999) mentioned randomisation of patients without a description of the method of randomisation or the allocation concealment, and was therefore allocated to category B.
See Figure 2 and Figure 3, and the individual domains below, for a graphical representation of risk of bias according to Higgins 2011.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
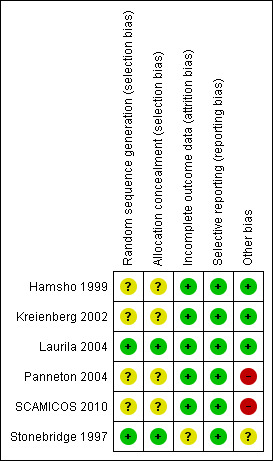
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Only two trials have a clear description of allocation concealment within the text. Stonebridge 1997 randomised patients at operation by a telephone call to the Scottish Cancer Trial Office, University of Edinburgh, and patient were stratified for each level of distal reconstruction. Laurila 2004 utilised a central block stratified randomisation by the study centre.
We were unable to assess allocation bias in three trials (Hamsho 1999; Kreienberg 2002; Panneton 2004) due to lack of text describing the method used. We have written to the contact authors of these trials and a reply is awaited. SCAMICOS 2010 used blocks of sealed envelopes with equal numbers of randomisation allocation. This method is prone to selection bias in some circumstances and many modern techniques avoid this risk.
Blinding
Blinding was not considered as it is impossible to achieve during surgery and at follow up.
Incomplete outcome data
All authors clearly reported the number of patients randomised and included the basic characteristics of each group in the study. Hamsho 1999 included all the randomised patients in the analysis with none missing or withdrawn from the study. Kreienberg 2002 reported loss to follow up of one patient, but the analysis included all randomised patients. Laurila 2004 accounted for all randomised patients but six were lost to follow up and none were excluded from the analysis. Panneton 2004 excluded 13 patients from the analysis with a clear explanation of the reasons (protocol violation, claudication in five patients, pedal vessels for distal anastomosis in four patients, vein patch used concomitantly with the pre‐cuffed graft in three patients and "others" in one patient). All included patients were accounted for. Stonebridge 1997 accounted for all patients with ninety patients out of original ninety five available for extended follow‐up (Griffiths 2004). A clear description was given for data censoring during the extended follow up analysis; however the effect of this is unclear. SCAMICOS 2010 excluded one patient from randomisation due to unknown group and seven patients in total were reported to have missing data.
Selective reporting
All outcomes as stated in the methods have been addressed in all included studies, with no concerns about selective reporting. SCAMICOS 2010 declared another independent study which randomised patients to externally supported PTFE graft versus non‐supported grafts, with no data reported.
Other potential sources of bias
We did not identify any other issues or sources of bias in included studies except in three studies (Panneton 2004; SCAMICOS 2010, Stonebridge 1997). The Panneton 2004 and SCAMICOS 2010 studies were industry sponsored. Panneton 2004 has been contacted for further clarification and a reply is awaited. SCAMICOS 2010 reported that the sponsoring body had no access to data and no influence in preparing the manuscript. SCAMICOS 2010 recruited more patients to FemPopBK group than required according to the power analysis but failed to recruit an adequate number to the FemDist group leaving this group underpowered. Both Stonebridge 1997 and SCAMICOS 2010 studies had high numbers of participating surgeons (18 and 33 respectively). It is unclear what effect this may have on the outcome. In personal communication, SCAMICOS trialists stated that they investigated whether study centre size effected outcome and found none. While both study groups in SCAMICO 2010 were comparable, the whole study population had a high number of females and non‐smokers in the study.
Effects of interventions
Five studies (Hamsho 1999; Kreienberg 2002; Laurila 2004; Panneton 2004; Stonebridge 1997) reported primary patency rates, secondary patency rates and limb salvage rates at 24 months following the intervention. SCAMICOS 2010 reported rates at 36 months, with Kaplan‐Meier figures up to 60 months. Stonebridge 1997 included figures at 12 months and the extended follow‐up report of the same study quoted rates at 36 months. Panneton 2004 also included results at 12 months as well as at 24 months. The rates reported in this review are sometimes estimated figures from Kaplan Meier curves and this is indicated by an asterisk in the relevant table (see additional tables).
PTFE with vein cuff versus PTFE without vein cuff
We identified two trials, Stonebridge 1997 (with extended follow up reported by Griffiths 2004) and SCAMICOS 2010, which compared PTFE (both externally supported and not externally supported) with and without a vein collar at the distal anastomosis.
Stonebridge 1997 included distal anastomoses to the above knee popliteal artery (150 grafts), below knee popliteal artery (96 grafts) and tibial vessels (15 grafts). For the below knee bypass group, the primary patency rate was higher in the vein cuff group (80.3% versus 65.3% and 51.8% versus 29.1% at 12 and 24 months respectively, P = 0.03). The difference in secondary patency rate was not statistically significant (82.9% versus 72.5% and 58.6% versus 34.9% at 12 and 24 months respectively, P = 0.14). The limb salvage rate was also not statistically different (86.3% versus 71.8% and 82.6% versus 62.2% at 12 and 24 months respectively, P = 0.08). Another 15 bypasses were to the tibial vessels, and if they are added to the 95 bypasses to the below knee popliteal artery, then the 24 months patency rate becomes 58% in the vein cuff group versus 27.6% in the non‐cuff group (P = 0.022). Data for 90 below knee bypasses were available in the extended follow‐up report. The data were censored when the grafts at risk number had fallen to 30% and 10% of the original total number of procedures, to give cumulative secondary patency rates of 63% versus 35% (P = 0.026) and 45% versus 19% (P = 0.018) at 24 and 36 months respectively. The difference in limb salvage rate at 36 months was not statistically significant (78% versus 61%, P = 0.08). See Table 1 and Table 2.
1. Below knee bypass vein cuff PTFE versus PTFE without vein cuff: primary patency, secondary patency and limb salvage rates.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | ||||
| Time | 12 months | 24 months | 12 months | 24 months | 12 months | 24 months |
| Vein cuff | 80.3 | 51.8 | 82.9 | 58.6 | 86.3 | 83.6 |
| No vein cuff | 65.3 | 29.1 | 72.5 | 34.9 | 71.8 | 62.2 |
| P | 0.03 | 0.14 | 0.08 | |||
2. Below knee bypass vein cuff PTFE versus PTFE without vein cuff: cumulative patency and limb salvage rates.
| Time |
24 months patency rate (%) (30% of original number of bypasses still at risk) |
36 months patency rate (%) (10% of original number of bypasses still at risk) |
36 months limb salvage rate (%) |
| Vein cuff | 63 | 45 | 78 |
| No vein cuff | 35 | 19 | 61 |
| P | 0.026 | 0.018 | 0.08 |
Stonebridge 1997/Griffiths 2004: Extended follow up. Data censored to 30 per cent (24 months) and 10 per cent (36 months) of original number of grafts at risk.
The SCAMICOS 2010 study included below knee femoro‐popliteal (FemPopBK) bypasses (202 grafts) and femoro‐distal (FemDist) bypasses (150 grafts). In the FemPopBK group, the differences in primary patency rate, secondary patency rate and limb salvage rate were not statistically significant at 36 months (primary patency rate 26% (95% CI 18 to 38) in the collar group and 43% (95% CI 33 to 58) in the no collar group, secondary patency rate 32% (95% CI 23 to 44) in the collar group and 42% (95% CI 31 to 56) in the no collar group, and limb salvage rate 64% (95% CI 54 to 75) in the collar group and 61% (95% CI 50 to 74) in the no collar group). Survival rates were not significantly different at 36 months (60%, 95% CI 51 to 73 in the collar group and 67%, 95% CI 56 to 81 in the no collar group).
In the FemDist group, the differences in primary patency rate, secondary patency rate and limb salvage rate were not statistically significant at 36 months (primary patency rate 20% (95% CI 11 to 38) in the collar group and 17% (95% CI 9 to 33) in the no collar group, secondary patency rate 22% (95% CI 12 to 39) in the collar group and 20% (95% CI 11 to 35) in the no collar group, and limb salvage rate 59% (95% CI 46 to 76) in the collar group and 44% (95% CI 32 to 61) in the no collar group). Survival rates were not significantly different at 36 months (66% (95% CI 54 to 82) in the collar group and 53% (95% CI 40 to 72) in the no collar group).
See Table 3, Table 4, Table 5 and Table 6.
3. FemPopBK bypass vein collar PTFE versus PTFE without vein collar: primary patency, secondary patency and limb salvage rates.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | ||||
| Time | 12 months | 24 months | 12 months | 24 months | 12 months | 24 months |
| Vein collar | 48* | 38* | 56* | 45* | 76* | 71* |
| No vein collar | 56* | 45* | 56* | 47* | 74* | 65* |
| P | 0.0853 | 0.317 | 0.757 | |||
* Numbers estimated manually from Kaplan‐Meier curves
4. FemDist bypass vein collar PTFE versus PTFE without vein collar: primary patency, secondary patency and limb salvage rates.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | ||||
| Time | 12 months | 24 months | 12 months | 24 months | 12 months | 24 months |
| Vein collar | 48* | 26* | 40* | 38* | 71* | 71* |
| No vein collar | 39* | 35* | 52* | 29* | 65* | 48* |
| P | 0.228 | 0.280 | 0.187 | |||
* Numbers estimated manually from Kaplan‐Meier curves
5. FemPopBK bypass vein collar PTFE versus PTFE without vein collar: three years patency and limb salvage rates.
| Time |
Primary patency rate (%) (95% CI) |
Secondary patency rate (%) (95% CI) |
Limb salvage rate (%) (95% CI) |
Survival rate (%) (95% CI) |
| Vein collar | 26 (18 to 38) | 32 (23 to 44) | 64 (54 to 75) | 60 (51 to 73) |
| No vein collar | 43 (33 to 58) | 42 (31 to 56) | 61 (50 to 74) | 67 (56 to 81) |
6. FemDist bypass vein collar PTFE versus PTFE without vein collar: three years patency and limb salvage rates.
| Time |
Primary patency rate (%) (95% CI) |
Secondary patency rate (%) (95% CI) |
Limb salvage rate (%) (95% CI) |
Survival rate (%) (95% CI) |
| Vein collar | 20 (11 to 38) | 22 (12 to 39) | 59 (46 to 76) | 66 (54 to 82) |
| No vein collar | 17 (9 to 33) | 20 (11 to 35) | 44 (32 to 61) | 53 (40 to 72) |
Pre‐cuffed PTFE versus vein cuff PTFE
One study was included (Panneton 2004). The difference in primary patency rate between the two groups was not statistically significant (62% pre‐cuffed PTFE versus 52% vein cuff PTFE at 12 months, and 49% versus 44% at 24 months, P = 0.53). The secondary patency rate also showed no statistically significant difference between the groups (66% pre‐cuffed PTFE versus 53% vein cuff PTFE at 12 months, and 55% versus 50% at 24 months, P = 0.30). Limb salvage rates were also similar in the two groups (75% pre‐cuffed PTFE versus 72% vein cuff PTFE at 12 months, and 62% versus 65% at 24 months, P = 0.88). Panneton 2004 reported a trend towards a shorter operative procedure in the precuffed group compared with the vein cuffed group (210 mins versus 242 mins respectively) but this was not statistically significant (P = 0.11). See Table 7.
7. Below knee bypass pre‐cuffed versus vein cuffed PTFE: primary patency, secondary patency and limb salvage rates.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | ||||
| Months | 12 | 24 | 12 | 24 | 12 | 24 |
| Pre‐cuffed PTFE | 62* | 49* | 66* | 55* | 75* | 62* |
| Vein cuff PTFE | 52* | 44* | 53* | 50* | 72* | 65* |
| P | 0.53 | 0.30 | 0.88 | |||
* Numbers estimated manually from Kaplan‐Meier curves
Spliced vein bypass graft versus PTFE graft with a distal vein cuff
One trial was included (Kreienberg 2002). The primary patency rate at 24 months was 44% in the spliced vein group and 50% in the vein cuff group, which was not statistically significant. The difference in secondary patency rate, however, was statistically significant between the two groups at 24 months (86% in the spliced vein and 52% in the vein cuff group, P < 0.05). The limb salvage rate was not significantly different at the same time point, being 94% in the spliced vein group and 85% in the vein cuff group. Kreienberg 2002 also reported a statistically significantly shorter operative time for the vein cuff group compared with the spliced vein group (177 ± 9 mins versus 263 ± 21 mins respectively, P < 0.05), less blood loss (P < 0.05), shorter hospital stay in hospital (P < 0.05) and less need for revision or reoperation for wound complications in the vein cuff group compared with the spliced vein group. See Table 8.
8. Below knee bypass spliced vein versus vein cuff PTFE: primary patency, secondary patency and limb salvage rates at 24 months.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | |
| Spliced vein | 44 | 86 | 94 |
| Vein cuff PTFE | 50 | 52 | 85 |
| P | > 0.05 | < 0.05 | > 0.05 |
PTFE graft with interposition vein cuff at the distal anastomosis with adjuvant AVF versus without adjuvant AVF
Two trials were included (Laurila 2004; Hamsho 1999). The trial by Laurila 2004 showed a primary patency rate at 24 months of 29% in the AVF group versus 36% in the non‐AVF group (P = 0.77). The secondary patency rate was 40% in both groups at 24 months (P = 0.89), and the limb salvage rate in the same period was 65% in the AVF group and 70% in the non AVF group (P = 0.97). The trial by Hamsho 1999 also showed similar statistically not significant results at 24 months with a primary patency rate of 32% versus 28% in the AVF group and non‐AVF group respectively (P = 0.20). The secondary patency rate was 28% in the AVF group and 24% in the non‐AVF group (P = 0.20) and the limb salvage rate was 62% and 71% respectively (P = 0.30). Hamsho 1999 reported that the AVF group had an increased average operative time of approximately 30 minutes compared with the non‐AVF group. Laurila 2004 also concluded that the operative time was increased in the AVF group but did not back this up with data. See Table 9 and Table 10.
9. Below knee bypass vein cuff PTFE with or without AVF: primary patency, secondary patency and limb salvage rates at 24 months.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | |
| Vein cuff PTFE with AVF | 29 | 40 | 65* |
| Vein cuff PTFE without AVF | 36 | 40 | 70* |
| P | 0.77 | 0.89 | 0.97 |
10. Below knee bypass vein cuff PTFE with or without AVF: primary patency, secondary patency and limb salvage rates at 24 months.
| Primary patency rate (%) | Secondary patency rate (%) | Limb salvage rate (%) | |
| Vein cuff PTFE with AVF | 32* | 28* | 62* |
| Vein cuff PTFE without AVF | 28* | 24* | 71* |
| P | > 0.2 | > 0.2 | < 0.3 |
* Numbers estimated manually from Kaplan‐Meier curves
Discussion
Since Miller 1984 described the vein cuff technique, many case series have described a benefit in the primary and secondary patency rates and limb salvage rates for below knee anastomosis compared to using a PTFE graft alone. Because variation in surgical technique, selection bias and additional treatment can impact significantly on patency rates following a bypass, we believe that only randomised trials can reliably confirm any significant benefit in terms of patency rates. The most important clinical outcome is limb salvage, and it is essential that this is included as an end point in these trials.
Summary of main results
PTFE with vein cuff versus PTFE without vein cuff
There is contradictory evidence from the two trials. One randomised trial (Stonebridge 1997) suggests that a vein cuff confers a benefit in the primary patency rate at 12 and 24 months. The secondary patency rate and limb salvage rate were not significantly different in the same period. The cumulative secondary patency rate at 24 and 36 months remains statistically significantly improved when a vein cuff is used, as reported by the extended follow‐up report, however the limb salvage rate at 36 months was not statistically different.
The other trial (SCAMICOS 2010) included a high number of female and non‐smoking patients. The primary patency rate, secondary patency rate and limb salvage rate showed no statistically significant difference at 36 months in either below knee femoro‐popliteal or femoro‐distal bypasses.
Pre‐cuffed PTFE versus vein cuff PTFE
The evidence from a randomised trial of moderate quality (Panneton 2004) suggests no significant difference in primary patency rate, secondary patency rate and limb salvage rate between the two techniques. The operative time was shorter in the pre‐cuffed group, but this was not statistically significant. The authors felt that the vein cuff PTFE graft is more technically demanding and so the pre‐cuffed PTFE grafts can probably offer a technically less demanding alternative with similar benefits.
Spliced vein bypass graft versus PTFE graft with a distal vein cuff
One intermediate quality randomised trial (Kreienberg 2002) showed no statistically significant difference in the primary patency rate and limb salvage rate between the two groups. The secondary patency rate was significantly improved in the spliced vein group at 24 months. Spliced veins required significantly longer operative time, resulted in more blood loss, longer stays in hospital and needed revision or reoperation more often due to wound complications.
PTFE graft with interposition vein cuff at the distal anastomosis with adjuvant AVF versus without adjuvant AVF
One good quality study (Laurila 2004) and one intermediate quality study (Hamsho 1999) reported no effect of adding an AVF to the distal anastomosis on any of the primary outcomes. Laurila 2004 reported no significant difference between the two groups throughout the study period in the primary and secondary patency rates and limb salvage rates. There were no perioperative deaths. Immediate reoperative and graft occlusion rates were similar between the groups. Hamsho 1999 observed a trend toward improved patency in the first six months in the AVF group, which diminished with time resulting in no significant differences in the primary patency rate, secondary patency rate and limb salvage rate over the observation period of 24 months. The construction of an AVF increased the operative time by about 30 minutes. No conclusion can be drawn from the relationship between AVF occlusion and graft failure.
Overall completeness and applicability of evidence
The search for studies for this review identified only studies using PTFE grafts. All included trials used relevant outcomes in their investigation.
The evidence from two trials is contradictory regarding the benefit of a distal vein cuff with below knee PTFE grafts. However, one of the trials (SCAMICOS 2010), which showed no significant benefits, included a population with a high percentage of females and non‐smokers, unusual for a population with arterial disease. By personal communication, SCAMICOS trialists have stated that this may be explained by the older population recruited in this trial. They have further commented on the interaction of sex and vein collar; and age and vein collar in a recent publication (SCAMICOS 2012). The other trial (Stonebridge 1997), where some benefits were noted, is underpowered for the below knee bypass group. They have addressed the question under review but the evidence is not sufficient.
We found that Hamsho 1999 and Laurila 2004 have answered the AVF question with the vein cuff PTFE graft, showing no significant difference. This is reflected in current practice.
The pre‐cuffed graft (Panneton 2004) and spliced vein graft (Kreienberg 2002) studies are underpowered, but remain the only evidence available for such grafts.
Quality of the evidence
PTFE with vein cuff versus PTFE without vein cuff
Stonebridge 1997 reported only 95 bypasses out of 261 that were to the below knee popliteal artery. As such, this is an underpowered study to look at the differences in this subgroup, and the authors acknowledged this point.The assumption used in the power calculations before the study was that the benefit from below knee vein cuffed PTFE bypass would be higher than in above knee bypasses. The randomisation process and allocation concealment is clear. The study reports no demographic differences between the two groups. The complication rate is not reported separately for below knee bypasses in this trial. In the discussion section, the authors looked further to the effect of a history of previous graft failure and concluded that previous graft failure did not affect the outcome. The risk of bias was low in this study.
SCAMICOS 2010 reported 202 below knee femoro‐popliteal bypasses and 150 femoro‐distal bypasses. The randomisation process is described clearly. Power calculations are discussed but the study failed to recruit the proposed number for the FemDist group. Sufficient numbers were recruited for the FemPopBk group. The two study groups are comparable but the whole study population has a high number of females and non‐smokers. All patients are accounted for, including missing data. The number of participating centres and surgeons is high, which may create a lot of heterogeneity in the procedure performed, and no adjustment has been made for this. The study is industry sponsored. The overall risk of bias is low in this study but some limitations are noted.
Pre‐cuffed PTFE versus vein cuff PTFE
Panneton 2004 organised a randomised study comparing a pre‐cuffed PTFE graft (Impra DistafloTM PTFE graft, Bart Peripheral Vascular Inc., Tempe, AZ) to a vein cuff PTFE graft for below knee bypasses. The patients were randomised before surgery but details of the method used are not described and there is no allocation concealment. Because of the overall quality of the study, we felt that the randomisation probably followed an acceptable method. Power calculations were not mentioned and the number of patients included in the study was low (n = 91). However, the study was well organised and followed a strict protocol. The two groups were comparable in their basic characteristics but the pre‐cuffed group (n = 47) had more patients with acute limb ischaemia than the vein cuff group (n = 44), mainly following graft failure or acute thrombosis (19% versus 4.5%, P = 0.03). The author performed a subgroup analysis by excluding the acute ischaemic patients and found no significant difference in the results. The risk of bias in this study is moderate.
Spliced vein bypass graft versus PTFE graft with a distal vein cuff
Kreienberg 2002 randomised only 39 procedures. Power calculations were not mentioned. The randomisation method and allocation concealment is not clear. The study was well designed and approved by the Committee on Research Involving Human Subjects. For this reason we felt that randomisation was probably adequate and assigned a low risk of bias. The two groups were comparable except in cardiac status and the author suggested that this may have contributed to a higher perioperative mortality and morbidity in the spliced vein group.
PTFE graft with interposition vein cuff at the distal anastomosis with adjuvant AVF versus without adjuvant AVF
Laurila 2004 randomised 59 procedures. The power of the study was probably inadequate and no power calculation was reported. The randomisation process was clearly described and allocation concealment was adequate. Therefore, we allocated this study to a very low risk of bias category. The baseline characteristics were comparable for the two groups. Hamsho 1999 randomised 89 bypasses over six years. There were no power calculations but the author acknowledges that the power of the study was questionable. Overall, the quality of the study is good and we felt that despite no clear description of the randomisation process or allocation concealment this was probably carried out correctly with a low risk of bias.
Potential biases in the review process
We identified all relevant studies in this review using the CENTRAL search strategy (Appendix 1). The studies were reviewed individually by two review authors to identify all included studies that matched the review questions. The two trials comparing PTFE with or without vein cuffs offered potential for meta‐analysis, however we faced two problems. First, the raw data for one study (Stonebridge 1997) is not accessible; second, the other trial (SCAMICOS 2010) contained an unusual group of patients such that, even if the data were available, heterogeneity would be very significant and would prevent combining the data. We contacted the authors for raw data for the AVF trials (Hamsho 1999; Laurila 2004) and replies are awaited.
Agreements and disagreements with other studies or reviews
We could not identify any other review addressing the same question. However, a body of evidence does exist comprising non‐randomised trials and case series which report the same findings as this review but do not add any weight to the evidence, which is sometimes controversial.
Authors' conclusions
Implications for practice.
There is evidence from one underpowered randomised controlled trial that the addition of a vein cuff at the distal anastomosis for a below knee PTFE graft improves graft patency rates (Stonebridge 1997) but does not reduce the risk of limb loss. An effect on limb salvage rates may have been overlooked because of the small numbers (type II error), as this approaches significance in long term follow up. The evidence from one randomised controlled trial of small size, which was also underpowered, suggests that pre‐cuffed PTFE grafts have a comparable effect to vein cuff PTFE grafts (Panneton 2004). The use of spliced veins instead of vein cuff PTFE grafts improved the secondary patency rate but increased operating time and the number of complications. The authors of this study felt that this justified the use of a prosthesis (Kreienberg 2002) but in our opinion this can't be concluded without further trial data for confirmation. The addition of an AVF to vein cuff PTFE grafts had no benefit in graft patency or limb salvage rates according to the evidence from one good quality trial (Laurila 2004) and one intermediate quality trial (Hamsho 1999). The relationship between AVF occlusion and graft patency is unknown.
Limb salvage bypass surgery is universally considered to be better performed with a single length of autologous vein whenever possible. However, in the absence of a suitable autologous vein, prosthetic grafts may be the only option for patients suffering from critical lower limb ischaemia. None of the studies in this review showed any improvement in limb salvage rates. Therefore we conclude that if a prosthetic bypass has to be used to the below knee popliteal artery it is not likely that any addition or modification to the technique as discussed in this review will have any effect on the most important clinical outcome. Individual surgeons may prefer to use additions such as a vein cuff or to use modifications such as a spliced vein to maximise the chances of graft patency but this may not impact on the eventual outcome for the patient.
Implications for research.
The evidence for a beneficial effect of vein cuff PTFE graft is insufficient and based on an underpowered trial. A large study with a specific focus on below knee vein cuff PTFE is required. Extended follow up is important to confirm long term benefits. The focus of future research should be on limb salvage as the primary outcome measure.
Feedback
Feedback SCAMICOS collaborators, 7 January 2013
Summary
We have some critical remarks concerning this Cochrane review. We argue that the evaluation of the three articles concerning the use of vein cuff for infragenicular prosthetic bypass graft does not represent "trustworthy, independent and relevant information".
The three articles:
Stonebridge PA, Prescott RJ, Ruckley CV. Randomized trial comparing infrainguinal polytetrafluoroethylene bypass grafting with and without vein interposition cuff at the distal anastomosis. The Joint Vascular Research Group. Journal of Vascular Surgery 1997;26(4):543–50.
Griffiths GD, Nagy J, Black D, Stonebridge PA. Randomized clinical trial of distal anastomotic interposition vein cuff in infrainguinal polytetrafluoroethylene bypass grafting. British Journal of Surgery 2004;91(5):560–2.
SCAMICOS. PTFE bypass to below‐knee arteries: distal vein collar or not? A prospective randomised multicentre study. European Journal of Vascular and Endovascular Surgery 2010;39(6):747–54.
Two studies (a: JVRG study – Stonebridge et al. & Griffiths et al. and b: SCAMICOS) report conflicting results with respect to patency after femoro‐popliteal below‐knee by‐pass with and without a vein cuff at the distal anastomosis when a PTFE‐graft is used. JVRG found a better patency with a vein cuff while the larger SCAMICOS could not show any such effect.
In the second report (Griffiths et al.) with longer follow‐up from the JVRG's study only 90 patients are presented at risk for occlusion among patients with femoro‐popliteal below‐knee bypass while in the first report (Stonebridge et al.) 95 patients are presented at risk for occlusion. No explanation of why these five patients have been excluded is given in the second report. We think this must be presented and discussed in the review as a potential source of bias of the JVRG's study.
In the second report (Griffiths et al.) patients are censored when 30 or 10% of the original number of patients are at risk – meaning that emphasize is laid on the early follow‐up period and late occlusions are withhold from the statistical analysis, thus the statistical analysis is not made on all available data. The reasons for this procedure is not explained or motivated in the report. We think this must be presented and discussed in the review as a potential source of bias of the JVRG's study.
In the SCAMICOS "Patients were recruited to the FemPopBK group in excess of that which was required according to the power analysis, but the study failed to recruit the stipulated number of patients to the FemDist group." In the review this is described as "Study stopped recruiting before proposed number of recruits was achieved leaving the study with unconfirmed power to detect a difference". Where conflicting results exist – femoro‐popliteal bypass below knee – our study recruited enough patients according to the power analysis. We think this should be presented in fair way in the review, which in its present form question the general power of our study.
In the review the SCAMICOS is criticised for heterogeneity due to the large number of participating centres. It is difficult to see why the 18 contributing surgeons of the JVRG's study should represent a substantially different problem with heterogeneity than in the SCAMICOS study. In addition SCAMICOS tested for any interaction between study centre size and use of vein collar with respect to patency and no interaction was found. We think that this issue, if actually relevant, should be presented in the review as a potential problem with both studies with the addition that SCAMICOS but not JVRG evaluated if study centre size influenced the outcome.
The review questions the large proportion of female patients and non‐smokers in the SCAMICOS. It is true that the SCAMICOS patients were a decade older than the patients of the JVRG's study and it is probably the reason for the female dominance in the study. Recently we have shown that there is no interaction between age & vein collar on one hand or sex & vein collar on the other hand with respect to patency (SCAMICOS, F, Lundgren. Does patency after a vein collar and PTFE‐bypass depend on sex and age? Re‐analysis of a randomised trial. International Angioliology 2012;31:156‐62). Current smoking was 29% in the JVRG's study and regular smoking during the last five years was 32% in SCAMICOS – this does not appear to be much of a difference. These aspects should be presented in the review.
Several of the above issues have been published in the discussion in connection to the publication of SCAMICOS in EJVES. Finally in the plain language summary of the review: "The results show that if a cuff of vein is inserted at the lower end of the synthetic graft before attaching it to the artery below the knee, then the bypass graft remains functional for a longer period of time.", without any reservations and in spite of the fact that two randomised studies have presented conflicting results with respect to the effect of a distal vein collar in a femoro‐politeal bypass below‐knee. For bypasses to the crural arteries no evidence at all exists to prove any superiority with an added vein collar.
Reply
The authors have made the following responses to the points raised by the SCAMICOS collaborators:
The authors had clearly stated within the ‘Effects of intervention’ section that ‘data for 90 patients were available for extended follow up’. We acknowledge that no explanation of the change from 95 patients in the original study is given. A Khalil and G Griffiths discussed this issue and we could not track why these five patients were lost to follow up.To clarify this we have now added ‘Ninety patients were available for extended follow‐up’ to the Incomplete outcome data (attrition bias) section. We have amended the review text (incomplete outcome data) to account for the fact that these five patients have been lost to follow up in the extended follow up report and we have changed the Risk of bias table judgement from 'low' to 'unclear' to reflect this issue.
Although clearly described, we are aware that the censoring of data may be perceived as a potential bias. We have now amended the sentence ‐ 'the description was clear for data censoring during the extended follow up analysis’ to 'A clear description was given for data censoring during the extended follow up analysis; however the effect of this is unclear' ‐ within the Incomplete outcome data (attrition bias) section and also within the Risk of bias table where the judgement of ‘low’ attrition bias has been changed to ‘unclear’.
The authors agree their description of SCAMICOS patient recruitment is not precise. We have amended the review text from ‐ ‘SCAMICOS 2010 stopped recruiting before the proposed number of recruits was achieved leaving the study with unconfirmed power to detect a difference’ to ‘SCAMICOS 2010 recruited more patients to FemPopBK group than required according to the power analysis but failed to recruit an adequate number to the FemDist group leaving this group underpowered’. This change is also reflected in the Risk of bias table text.
The authors agree that as both Stonebridge 1997 and SCAMICOS 2010 have used multiple surgeons any potential problem caused by this may apply to both studies. Our review did include the text ‘surgical technique not standardised’ within Stonebridge 1997 included study characteristics to acknowledge this. However, to further clarify we have added the text ‐ ‘Both Stonebridge 1997 and SCAMICOS 2010 studies had high numbers of participating surgeons (18 and 33 respectively). It is unclear what effect this may have on the outcome. In personal communication, SCAMICOS 2010 stated that they investigated whether study centre size effected outcome and found none’ ‐ to the Other potential sources of bias section. The Risk of bias tables text of both studies have also been amended. Stonebridge: other bias – ‘18 surgeons participated. This is not controlled for and the effect of this on the outcome is not known (judgement changed from ‘low’ to ‘unclear’). SCAMICOS: other bias – ‘33 surgeons participated. This is not controlled for and the effect of this on the outcome is not known (domain judgement remains ‘high’).
Our review has discussed the difference in patient populations between the Stonebridge 1997 study and SCAMICOS 2010 study. We have now added text following the feedback comments from the SCAMICOS trialists which may explain the high number of female and non‐smokers in their patient population and included their recent publication where they have further investigated this ‐ ‘However, one of the trials (SCAMICOS 2010), which showed no significant benefits, included a population with a high percentage of females and non‐smokers, unusual for a population with arterial disease. By personal communication, SCAMICOS trialists have stated that this may be explained by the older population recruited in this trial. They have further commented on the interaction of sex and vein collar; and age and vein collar; in a recent publication (SCAMICOS 2012)'. We do not believe that the SCAMICOS 2010 data for regular smoking in the last five years (32%) and the Stonebridge 1997 current smoking data (29%) is comparable in this case. We believe a more comparable figure would be former+current smokers which was 83% of patients, as expected in a vascular disease patient population.
The authors agree that the plain language summary is not an accurate reflection of what we have reported in both the Summary of main results and Authors Conclusions. We have amended the text within the PLS in respect to this ‐ ‘Results from two trials which looked at the effect of inserting a cuff of vein at the lower end of the synthetic graft before attaching it to the artery below the knee are conflicting. With one study showing that the bypass graft remains functional for a longer period of time and in the other study no benefit was seen’.
Contributors
Feedback: Dr Fredrik Lundgren, principal investigator on behalf of SCAMICOS collaborators.
Reply: A Khalil and G Griffiths.
Feedback SCAMICOS collaborators, 22 July 2013
Summary
It's a good thing that some of the shortcomings of the JVRG’s study have been mentioned in the new version of the review and some misleading descriptions of SCAMICOS have been corrected. (CD007921. DOI: 10.1002/14651858.CD007921.pub2) However, our study is still criticised for industrial sponsoring, use of a factorial design and for investigating a different population of patients compared to the JVRG study. All are presented as sources of bias in the review. The companies sponsoring SCAMICOS had no access to the data and had no influence in preparation of the manuscript and this is also clearly stated in the article. Factorial designs have been used since the late 19th century and are usually regarded as very effective methods rather than as a source of bias. The design is also described in full detail in our SCAMICOS paper. Concerning the patient population, we studied the patients that were available to us. Different patient populations can of course generate different results but at least the influences of age and sex on the effect of a vein collar has been studied by us and appear not to change the outcome of a graft with/without a vein collar. There were, of course, previous smokers also in the SCAMICOS and usually you may assume that most patients with critical ischemia are current or previous smokers. Thus, in all likelihood the distributions of smoking habits are very similar in the two discussed studies – current smoking and regular smoking during the last 5 years being almost the same. The suggestion that different ways to describe the smoking habits of the population should be a source of bias with respect to the outcome of a graft with/without a vein collar appears somewhat farfetched. To sum up, we seriously question the reviewers’ classification of the above discussed circumstances as likely sources of bias concerning the outcome of a graft with/without a vein collar. Finally, after our criticism of the first version of the review and the JVRG's study our methods of randomisation and allocation concealment (which are accepted methods due to the Cochrane Handbook) has been "degraded" from 'Low risk of bias' to 'Unclear risk of bias' without any further argumentation or comment compared to the first version of the review. Cochrane Collaboration has been extremely important for the development of clinical practise and usually has an unsurpassed reputation. However, based on the above arguments we are still concerned that this review does not represent "trustworthy, independent and relevant information". Possibly, authors should not review their own studies to avoid any conflict of interests.
Reply
A reply from the review authors is awaited.
Contributors
Feedback: Dr Fredrik Lundgren, principal investigator on behalf of SCAMICOS collaborators.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2013 | Feedback has been incorporated | Feedback has been submitted for this review |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 10 April 2013 | Feedback has been incorporated | Feedback addressed by review authors. |
| 7 January 2013 | Feedback has been incorporated | Feedback has been submitted for this review. |
Acknowledgements
The amendments and changes to review text and tables made in response to the feedback were discussed with Dr Cathryn Broderick, Assistant Managing Editor, Cochrane Peripheral Vascular Diseases Group and we acknowledge the great help and advice provided to formulate this reply.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor Anastomosis, Surgical, this term only | 614 |
| #2 | MeSH descriptor Arteriovenous Shunt, Surgical, this term only | 208 |
| #3 | MeSH descriptor Blood Vessel Prosthesis, this term only | 427 |
| #4 | MeSH descriptor Blood Vessel Prosthesis Implantation, this term only | 449 |
| #5 | MeSH descriptor Leg explode all trees with qualifiers: BS,SU | 1233 |
| #6 | MeSH descriptor Popliteal Artery explode all trees with qualifiers: SU,BS | 128 |
| #7 | MeSH descriptor Femoral Artery explode all trees with qualifiers: BS,SU | 231 |
| #8 | MeSH descriptor Ischemia explode all trees with qualifier: SU | 185 |
| #9 | MeSH descriptor Limb Salvage explode all trees | 46 |
| #10 | MeSH descriptor Vascular Patency explode all trees | 733 |
| #11 | MeSH descriptor Graft Occlusion, Vascular explode all trees | 502 |
| #12 | (ischaem* or ischem*):ti,ab,kw | 14503 |
| #13 | (bypass or graft* or anastomos* or surg*):ti,ab,kw | 96112 |
| #14 | leg* or knee* or femoro* or popliteal | 21695 |
| #15 | (vein near3 cuf*):ti,ab,kw | 18 |
| #16 | (veincuf*):ti,ab,kw | 0 |
| #17 | (vein‐cuf*):ti,ab,kw | 9 |
| #18 | (vein near3 collar*):ti,ab,kw | 3 |
| #19 | (interposition):ti,ab,kw | 133 |
| #20 | (vein‐col*):ti,ab,kw | 5 |
| #21 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) | 123184 |
| #22 | (#15 OR #16 OR #17 OR #18 OR #19 OR #20) | 152 |
| #23 | (#21 AND #22) | 123 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hamsho 1999.
| Methods | Randomisation technique not mentioned. | |
| Participants |
Country: UK, Multicentre (2 centres) 87 patients (48 with AVF and 41 without) with critical limb ischemias, 89 femoro‐infrapopliteal bypass using ePTFE Age: mean age 70.3 years (AVF 71.5, Control 69.1) Sex: M:F 3.2:1 (AVF 1.4:1, control 4.8:1) smoking (AVF 72.5%, control 64.4%), diabetic (AVF 17.5%, Control 15.7%), outflow vessel (AVF ant tibial 35% peroneal 35% post tibial 30%, control ant tibial 29% peroneal 34% post tibial 37%). Excluded in the presence of suitable autologous vein, venous hypertension (deep vein obstruction) and severely compromised cardiac function. |
|
| Interventions | Femoro‐infrapopliteal PTFE graft with interposition vein cuff at the distal anastomosis with adjuvant AVF versus without adjuvant AVF. | |
| Outcomes | Primary cumulative patency rate, secondary cumulative patency rate and cumulative limb salvage rate. | |
| Source of funding | None declared. | |
| Notes | All received Cefotaxime 1.5 gm iv and heparin 5000 iu at induction, heparin for 5 days postoperative, aspirin 300 mg 48 hours postoperative and discharge on aspirin and warfarin indefinitely. Invitation to author to add, audit and review data: No reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description of method used to randomise patients., no further information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported, all patient initially randomised were included. Analysis of all included patients. |
| Selective reporting (reporting bias) | Low risk | All outcomes as stated in methods have been addressed. |
| Other bias | Low risk | No other issues identified. |
Kreienberg 2002.
| Methods | Randomisation technique not mentioned. | |
| Participants |
Country: USA: single centre 36 patients with critical limb ischaemia and 39 bypasses (19 spliced vein group, 20 PTFE with vein cuff group) Age: 68.2 ± 2.7 vein cuff, 71.4 ± 2.3 spliced vein Sex: male (60% vein cuff, 52.6% spliced vein), female (40% vein cuff, 47.4% spliced vein) Ischaemic category (4.8 ± 0.09 vein cuff, 4.84 ± 0.09 sliced vein "Rutherford et al reporting criteria"), preoperative ABI (0.32 ± 0.07 vein cuff, 0.25 ± 0.07 spliced vein). |
|
| Interventions | Spliced vein bypass graft versus PTFE graft with a distal vein cuff. | |
| Outcomes | Primary patency rate, secondary patency rate, limb salvage rate and over all survival rate. | |
| Source of funding | None declared. | |
| Notes | All PTFE vein cuff group were anticoagulated postoperatively, Wolfe boot to construct vein cuffs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description of method used to randomise patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for, one patient lost to follow up. None excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes as stated in methods have been addressed. |
| Other bias | Low risk | No other issues identified. |
Laurila 2004.
| Methods | Randomisation: Central block stratified randomisation. | |
| Participants |
Country: Finland: Multicentre (4 centres) 59 patients with critical limb ischaemia (31 to AVF group and 28 to control group) Age: Average age 73.7 years (74.3 AVF, 73 control) Sex: 58% F, 42% M (M/F: 15/16 AVF, 10/18 control) Diabetic 41% (39% AVF, 43% control), coronary heart disease 61% (71% AVF, 50% control), cerebrovascular disease (19% AVF, 29% control), pulmonary disease (19% AVF, 7% control), renal failure (6% AVF, 7% control), smoking (29% AVF, 43% control), 71% previous vascular surgery, 58% prosthetic bypass as a re‐do procedure. |
|
| Interventions | Femoro‐infrapopliteal PTFE graft with interposition vein cuff at the distal anastomosis with adjuvant AVF versus without adjuvant AVF. | |
| Outcomes | Primary patency rate, secondary patency rate, limb salvage rate. number of patients alive with a leg, the dependency of graft patency on the patency of the fistula. | |
| Source of funding | None declared. | |
| Notes | Pilot trial, patients excluded if only have claudication or bypass to popliteal or pedal artery. Vancomycin and cefuroxime or cefuroxime alone prophylaxis to all, all heparinised intraoperatively and oral antiplatelets postoperatively. Vein cuff: Miller 33 patients, St Mary's Boot 19, Modified Miller 7. AVF group had common ostium AVF (25) or proximal AVF (6) according to surgeon's discretion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central block stratified randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for, six patients were lost to follow up. None excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes as stated in methods have been addressed. |
| Other bias | Low risk | No other issues identified. |
Panneton 2004.
| Methods | Randomisation technique not mentioned. | |
| Participants |
Country: USA, Multicentre (15 centres) 89 patients and 91 bypasses (47 patients and anastomoses pre‐cuffed and 42 patients and 44 anastomoses vein cuffed), (Initially 104 patients, 13 excluded for violation of protocol). Age: 73.2 pre‐cuffed, 72.6 vein cuffed Sex: Male (55% pre‐cuffed, 64% vein cuffed), Female (45% pre‐cuffed, 36% vein cuffed) hypertension (85% pre‐cuffed, 75% vein cuffed), coronary artery disease (57% pre‐cuffed, 61% vein cuffed), smoking (85% pre‐cuffed 84% vein cuffed), diabetic (60% pre‐cuffed 68 % vein cuffed), congestive heart failure (21% pre‐cuffed, 20% vein cuffed), COPD (13% pre‐cuffed, 23% vein cuffed), prior infrainguinal bypass (66% pre‐cuffed, 82% vein cuffed), redo procedure (55% pre‐cuffed, 50% vein cuffed), chronic limb ischaemia (81% pre‐cuffed, 95.5% vein cuffed), acute limb ischaemia (19% pre‐cuffed, 4.5% vein cuffed), mean angiographic score (13.5 pre‐cuffed, 14.2 vein cuffed), mean ABI (0.39 pre‐cuffed, 0.47 vein cuffed). Excluded if only claudication or above knee reconstruction. |
|
| Interventions | Pre‐cuffed ePTFE versus vein cuffed ePTFE | |
| Outcomes | Primary cumulative patency rate, secondary cumulative patency rate and cumulative limb salvage rate. | |
| Source of funding | Industry sponsored (Bard Peripheral Vascular, Inc.) | |
| Notes | Surgical technique not standardised for graft implantation and antiplatelet and anticoagulation therapy left to discretion of participating centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description of randomisation method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for. Thirteen patients were excluded from the study analysis due to violation of protocol. Description with reasons is clear. |
| Selective reporting (reporting bias) | Low risk | All outcomes as stated in methods have been addressed. |
| Other bias | High risk | Industry sponsored trial. |
SCAMICOS 2010.
| Methods | Concealed envelope stratified randomisation. | |
| Participants |
Country: Denmark, multicentre (3 centres) and Sweden (29 centres). 352 patients with critical limb ischaemia randomised in two groups. Data analysed for 345 patients. 202 (199 analysed) Femoropopliteal below knee (FemPopBK) bypass (114 patients vein collar and 85 patients without vein collar). 150 (146 analysed) Femorodistal (FemDist) bypass (69 patients vein collar and 77 patient without vein collar). Age: 78 years, FemPopBK 76 vein collar and 79 no collar. FemDist 79 both vein collar and no vein collar. Sex: Male sex 40%. FemPopBK Male (41% vein collar, 35% no vein collar). FemDist Male (43% vein collar, 42% no vein collar). Rest Pain/ Ulcer/ Gangrene 94% (FemPopBK 93% vein collar, 94% no vein collar), (FemDist 93% vein collar, 96% no vein collar), Acute ischaemia or severe intermittent claudication 6% (FemPopBK 7% vein collar, 6% no vein collar), (FemDist 7% vein collar, 4% no vein collar), Diabetes 36% (FemPopBK 37% vein collar, 35% no vein collar), (FemDist 37% vein collar, 37% no vein collar), Cardiac disease 61% (FemPopBK 55% vein collar, 61% no vein collar), (FemDist 58%vein collar, 72% no vein collar), Previous vascular Surgery 60% (FemPopBK 66% vein collar, 53% no vein collar), (FemDist 59% vein collar, 59% no vein collar), Pulmonary disease 10% (FemPopBK 7% vein collar, 11% no vein collar), (FemDist 10% vein collar, 12% no vein collar), Renal disease (10% (FemPopBK 8% vein collar, 10% no vein collar), (FemDist 14% vein collar, 11% no vein collar). Smokers 32% (FemPopBK 35% vein collar, 35% no vein collar), (FemDist 24% vein collar, 47% no vein collar). |
|
| Interventions | PTFE with vein collar versus PTFE without vein collar. | |
| Outcomes | Primary patency rate, secondary patency rate and limb salvage rate. | |
| Source of funding | Industry sponsored (IMPRA, Sweden and WL Gore and Associate, Sweden). | |
| Notes | Participating vascular units required to have experience of five reconstructions prior to contribution to the study. Only patients with critical limb ischaemia included and ankle blood pressure and pressure of the first toe not used. Patient with distal anastomosis to foot arteries were excluded. Type of collar left to discretion of surgeons (50% used Millar cuff and 50% used St. Mary Boot). Use of anticoagulant/ antiplatelets left to discretion of surgeons, 86.3% vein collar and 89.2% without vein collar received some form of anticoagulant and/ or antiplatelets. PTFE used either from IMPRA or Gore, any diameter of graft allowed. Danish centres (3 centres) used externally supported PTFE grafts. Swedish centres (29 centres) randomised patients with equal probability to have externally supported graft or not in addition to vein collar or not, resulting in 50% in vein collar and 50% in no vein collar groups having externally supported graft. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes prepared by study centre, in a block of 16 stratified for FemPopBK and FemDist separately, containing equal numbers for collar and no collar for each centre. |
| Allocation concealment (selection bias) | Unclear risk | Envelopes picked randomly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are accounted for with reasons of exclusion from analysis. |
| Selective reporting (reporting bias) | Low risk | Addressed all outcomes, including limitations of the study. |
| Other bias | High risk | Industry sponsored trial. More patients than required according to the power analysis were recruited to the FemPopBK group but the study failed to recruit an adequate number to the FemDist group leaving this group underpowered. 33 surgeons participated. This is not controlled for and the effect of this on the outcome is not known. The two study groups are comparable but the whole study population has high number of females and non‐smokers. |
Stonebridge 1997.
| Methods | Randomisation: Telephone to central randomisation centre, stratified to the level of distal reconstruction. | |
| Participants |
Country: UK, Multicentre (2 centres) 261 patients (133 cuff group, 128 no cuff), 95 patients only had below knee bypass (48 cuff, 47 no cuff) Age: mean age 67.2 cuff, 69.3 no cuff Sex: Male/ Female 96/36 cuff, 83/45 no cuff diabetic (110 cuff, 109 no cuff), smoking 'former or current' (116 cuff, 111 no cuff), rest pain (67 cuff, 79 no cuff), ulcer or gangrene (51 cuff, 32 no cuff), claudication (14 cuff, 14 no cuff), previous graft failure (18 cuff, 28 no cuff), above knee (76 cuff, 74 no cuff), below knee (49 cuff, 47 no cuff), tibial (8 cuff, 7 no cuff). |
|
| Interventions | PTFE with vein cuff versus PTFE without vein cuff. | |
| Outcomes | Primary patency rate, secondary patency rate and limb salvage rate. | |
| Source of funding | Not mentioned. | |
| Notes | Operation for trauma were excluded, surgical technique were not standardised, standardised anticoagulation and antiplatelet therapy only within each centre (103 cuff and 90 no cuff had postoperative aspirin, 20 cuff and 20 no cuff had postoperative anticoagulation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation centre. |
| Allocation concealment (selection bias) | Low risk | Telephone to randomisation centre. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients initially randomised were included. Analysis of all included patients (Stonebridge 1997) All available data were addressed at the extended follow up and methods for censoring data were clearly described, however the effect of this is not clear (Griffiths 2004). |
| Selective reporting (reporting bias) | Low risk | All outcomes as stated in methods have been addressed. |
| Other bias | Unclear risk | 18 surgeons participated. This is not controlled for and the effect of this on outcome is not known. |
Differences between protocol and review
There was a potential for meta‐analysis in the AVF trials. The authors of the two trials were contacted for the data and their reply is awaited. In addition to the planned assessment of quality of the methodology, we used the 'Risk of bias' tool from The Cochrane Collaboration to assess the risk of bias of the included studies.
Contributions of authors
Two authors (AK and GG) independently selected the trials, assessed their quality and extracted the data. AB resolved any disagreement between the first two authors. All authors contributed to the writing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
Mr Griffiths is an author in one of the randomised clinical trials addressing the question of interest.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Hamsho 1999 {published data only}
- Hamsho A, Nott D, Harris PL. Prospective randomised trial of distal arteriovenous fistula as an adjunct to femoro‐infrapopliteal PTFE bypass. European Journal of Vascular and Endovascular Surgery 1999;17(3):197‐201. [DOI] [PubMed] [Google Scholar]
- Hamsho A, Nott DM, Harris PL. Prospective randomized trial of distal arteriovenous fistula as an adjunct to femoro‐infrapopliteal bypass using PTFE. 31st Annual Conference, 26‐28th November 1997. Vascular Surgical Society of Great Britain and Ireland. London, 1997.
- Hamsho A, Nott DM, Harris PL. Prospective randomized trial of distal arteriovenous fistula as an adjunct to femoroinfrapopliteal bypass using polytetrafluoroethylene. British Journal of Surgery 1998;85:563‐4. [Google Scholar]
Kreienberg 2002 {published data only}
- Kreienberg PB, Darling RC 3rd, Chang BB, Champagne BJ, Paty PS, Roddy SP, et al. Early results of a prospective randomized trial of spliced vein versus polytetrafluoroethylene graft with a distal vein cuff for limb‐threatening ischemia. Journal of Vascular Surgery 2002;35(2):299‐306. [DOI] [PubMed] [Google Scholar]
Laurila 2004 {published data only}
- Laurila K, Lepantalo M, Teittinen K, Kantonen I, Forssell C, Vilkko P. Does an adjuvant AV‐fistula improve the patency of a femorocrural PTFE bypass with distal vein cuff in critical leg ischaemia? A prospective randomised multicentre trial. European Society for Vascular Surgery, Programme and Abstract Book, XVII Annual Meeting and Course on Vascular Surgical Techniques. 2003:109. [DOI] [PubMed]
- Laurila K, Lepantalo M, Teittinen K, Kantonen I, Forssell C, Vilkko P, et al. Does an adjuvant AV‐fistula improve the patency of a femorocrural PTFE bypass with distal vein cuff in critical leg ischaemia?‐‐a prospective randomised multicentre trial. European Journal of Vascular and Endovascular Surgery 2004;27(2):180‐5. [DOI] [PubMed] [Google Scholar]
Panneton 2004 {published data only}
- Panneton JM, Hollier LH, Hofer JM. Multicenter randomized prospective trial comparing a pre‐cuffed polytetrafluoroethylene graft to a vein cuffed polytetrafluoroethylene graft for infragenicular arterial bypass. Annals of Vascular Surgery 2004;18(2):199‐206. [DOI] [PubMed] [Google Scholar]
SCAMICOS 2010 {published data only}
- SCAMICOS. PTFE bypass to below‐knee arteries: distal vein collar or not? A prospective randomised multicentre study. European Journal of Vascular and Endovascular Surgery 2010;39(6):747‐54. [DOI] [PubMed] [Google Scholar]
- SCAMICOS, Lundgren F. Does patency after a vein collar and PTFE‐bypass depend on sex and age? Re‐analysis of a randomised trial. International Angiology 2012 April;31(2):156‐62. [PubMed] [Google Scholar]
Stonebridge 1997 {published data only}
- Griffiths GD, Nagy J, Black D, Stonebridge PA. Randomized clinical trial of distal anastomotic interposition vein cuff in infrainguinal polytetrafluoroethylene bypass grafting. British Journal of Surgery 2004;91(5):560‐2. [DOI] [PubMed] [Google Scholar]
- Stonebridge PA, Prescott RJ, Ruckley CV. Randomised trial comparing infrainguinal PTFE bypass grafting with and without vein interposition cuff at the distal anastomosis. Proceedings of the 30th Annual Conference of The Vascular Surgical Society of Great Britain and Ireland. 1996:10.
- Stonebridge PA, Prescott RJ, Ruckley CV. Randomized trial comparing infrainguinal polytetrafluoroethylene bypass grafting with and without vein interposition cuff at the distal anastomosis. The Joint Vascular Research Group. Journal of Vascular Surgery 1997;26(4):543‐50. [DOI] [PubMed] [Google Scholar]
Additional references
Ascer 1985
- Ascer E, Veith FJ, Gupta SK, Krasowski G, Samson RH, Scher LA, et al. Six year experience with expanded polytetrafluoroethylene arterial grafts for limb salvage. Journal of Cardiovascular Surgery 1985;26(5):468‐72. [PubMed] [Google Scholar]
Ascer 1996
- Ascer E, Gennaro M, Pollina RM, Ivanov M, Yorkovich WR, Ivanov M, et al. Complementary distal arteriovenous fistula and deep vein interposition: a five‐year experience with a new technique to improve infrapopliteal prosthetic bypass patency. Journal of Vascular Surgery 1996;24(1):134‐43. [DOI] [PubMed] [Google Scholar]
Chang 1994
- Chang BB, Shah DM, Leather RP, Darling RC 3rd. Finding autogenous veins for reoperative lower extremity bypasses: limitations of veins other than the greater saphenous. Seminars in Vascular Surgery 1994;7(3):173‐7. [PubMed] [Google Scholar]
Dardik 1996
- Dardik H, Silvestri F, Alasio T, Berry S, Kahn M, Ibrahim IM, et al. Improved method to create the common ostium variant of the distal arteriovenous fistula for enhancing crural prosthetic graft patency. Journal of Vascular Surgery 1996;24(2):240‐8. [DOI] [PubMed] [Google Scholar]
Darling 1993
- Darling RC 3rd, Kupinski AM. In situ bypass: technical considerations. Preoperative evaluation of veins. Seminars in Vascular Surgery 1993;6(3):155‐8. [PubMed] [Google Scholar]
Faries 2000
- Faries PL, LoGerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, et al. A comparative study of alternative conduits for lower extremity revascularization: all autogenous conduit versus prosthetic grafts. Journal of Vascular Surgery 2000;32(6):1080‐90. [DOI] [PubMed] [Google Scholar]
Griffiths 2004
- Griffiths GD, Nagy J, Black D, Stonebridge PA. Randomized clinical trial of distal anastomotic interposition vein cuff in infrainguinal polytetrafluoroethylene bypass grafting. British Journal of Surgery 2004;91(5):560‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kissin 2000
- Kissin M, Kansal N, Pappas PJ, DeFouw DO, Duran WN, Hobson RW 2nd. Vein interposition cuffs decrease the intimal hyperplastic response of polytetrafluoroethylene bypass grafts. Journal of Vascular Surgery 2000;31(1 pt1):69‐83. [DOI] [PubMed] [Google Scholar]
Leuprecht 2002
- Leuprecht A, Perktold K, Prosi M, Berk T, Trubel W, Schima H. Numerical study of hemodynamics and wall mechanics in distal end‐to‐side anastomoses of bypass grafts. Journal of Biomechanics 2002;35(2):225‐36. [DOI] [PubMed] [Google Scholar]
Miller 1984
- Miller JH, Foreman RK, Ferguson L, Faris I. Interposition vein cuff for anastomosis of prosthesis to small artery. The Australian and New Zealand Journal of Surgery 1984;54(3):283‐5. [DOI] [PubMed] [Google Scholar]
O'Donnell 1984
- O'Donnell TF Jr, Mackey W, McCullough JL Jr, Maxwell SL Jr, Farber SP, Deterling RA, et al. Correlation of operative findings with angiographic and noninvasive hemodynamic factors associated with failure of polytetrafluoroethylene grafts. Journal of Vascular Surgery 1984;1(1):136‐48. [DOI] [PubMed] [Google Scholar]
Plecha 1996
- Plecha EJ, Freischlag JA, Seabrook GR, Towne JB. Femoropopliteal bypass revisited: an analysis of 138 cases. Cardiovascular Surgery 1996;4(2):195‐9. [DOI] [PubMed] [Google Scholar]
Sauvage 1979
- Sauvage LR, Walker MW, Berger K, Robel SB, Lischko MM, Yates SG, et al. Current arterial prostheses. Experimental evaluation by implantation in the carotid and circumflex coronary arteries of the dog. Archives of Surgery 1979;114(6):687‐91. [DOI] [PubMed] [Google Scholar]
SCAMICOS 2012
- SCAMICOS, Lundgren F. Does patency after a vein collar and PTFE‐bypass depend on sex and age? Re‐analysis of a randomised trial. International Angiology 2012 April;31(2):156‐62. [PubMed] [Google Scholar]
Siegman 1979
- Siegman FA. Use of the venous cuff for graft anastomosis. Surgery, Gynecology and Obstetrics 1979;148(6):930. [PubMed] [Google Scholar]
Twine 2010
- Twine CP, McLain AD. Graft type for femoro‐popliteal bypass surgery. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD001487.pub2] [DOI] [PubMed] [Google Scholar]
Tyrrell 1997
- Tyrrell MR, Wolfe JH. Myointimal hyperplasia in vein collars for ePTFE grafts. European Journal of Vascular and Endovascular Surgery 1997;14(1):33‐6. [DOI] [PubMed] [Google Scholar]
Veith 1986
- Veith FJ, Gupta SK, Ascer E, White‐Flores S, Samson RH, Scher LA, et al. Six‐year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. Journal of Vascular Surgery 1986;3(1):104‐14. [DOI] [PubMed] [Google Scholar]


