Abstract
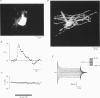
1. The subcellular characteristics of an ATP-induced elevation of the cytoplasmic free calcium concentration ([Ca2+]i) were studied in cultured cells of the oligodendrocyte lineage obtained from mouse cortex and rabbit retina, as well as in oligodendrocytes from mouse corpus callosum slices, using laser scanning confocal microfluorometry. 2. With the stage- and lineage-specific antibodies O4 and O10, three developmental stages within the oligodendrocyte lineage were distinguished prior to Ca2+ recording. 3. Bath application of 1-100 microM ATP induced a transient increase of [Ca2+]i in late precursors and oligodendrocytes but not in early glial precursor cells from retinal and cortical cultures and from corpus callosum slices. This effect of ATP was observed in Ca(2+)-free extracellular solution, suggesting that the ATP-mediated elevation of [Ca2+]i is due to a Ca2+ liberation from intracellular stores. 4. In both late precursors and oligodendrocytes from retina, the amplitude of ATP-induced [Ca2+]i transients was significantly higher in processes as compared with the soma; in cortical cultures such an uneven response was only observed in oligodendrocytes, while in immature cells responses in soma and processes were of similar amplitude. 5. The rank order of potency for the purine and pyrimidine nucleotides was UTP > or = ATP > ADP >> AMP = adenosine = Me-ATP for retinal oligodendrocytes, and ADP > or = ATP >> UTP = AMP = adenosine = Me-ATP for cortical oligodendrocytes. The response to ATP and related nucleotides was blocked by suramin, indicating the involvement of a P2-purinoreceptor in the ATP-mediated [Ca2+]i response. 6. ATP-induced elevation of the cytosolic Ca2+ concentration was inhibited by incubating cells with thapsigargin (10 microM) and by intracellular administration of heparin (1 microM). These findings indicate that ATP triggers a release of Ca2+ ions from InsP3-sensitive internal stores. 7. The ATP receptors may play a role in neuron-glial signal transfer; ATP is released as neurotransmitter, but also under pathological conditions from damaged cells.
Full text
PDF
















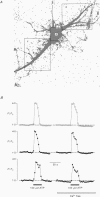
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banik N. L., McAlhaney W. W., Hogan E. L. Calcium-stimulated proteolysis in myelin: evidence for a Ca2+-activated neutral proteinase associated with purified myelin of rat CNS. J Neurochem. 1985 Aug;45(2):581–588. doi: 10.1111/j.1471-4159.1985.tb04026.x. [DOI] [PubMed] [Google Scholar]
- Berger T., Schnitzer J., Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J Neurosci. 1991 Oct;11(10):3008–3024. doi: 10.1523/JNEUROSCI.11-10-03008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blankenfeld Gv Gabriela v., Verkhratsky Alexej N., Kettenmann Helmut. Ca2+ Channel Expression in the Oligodendrocyte Lineage. Eur J Neurosci. 1992 Oct;4(11):1035–1048. doi: 10.1111/j.1460-9568.1992.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Cejka J. C., Bidet M., Tauc M., Poujeol P. Nucleotides mobilize intracellular calcium stores of renal proximal cells in primary culture: existence of a suramin-sensitive mechanism. Biochim Biophys Acta. 1993 Mar 10;1176(1-2):7–12. doi: 10.1016/0167-4889(93)90170-t. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Fine J., Cole P., Davidson J. S. Extracellular nucleotides stimulate receptor-mediated calcium mobilization and inositol phosphate production in human fibroblasts. Biochem J. 1989 Oct 15;263(2):371–376. doi: 10.1042/bj2630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S. M. Glial calcium. Glia. 1993 Oct;9(2):83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A. L., Pfeiffer S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989 May;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Kalthof B., Bechem M., Flocke K., Pott L., Schramm M. Kinetics of ATP-induced Ca2+ transients in cultured pig aortic smooth muscle cells depend on ATP concentration and stored Ca2+. J Physiol. 1993 Jul;466:245–262. [PMC free article] [PubMed] [Google Scholar]
- Kastritsis C. H., McCarthy K. D. Oligodendroglial lineage cells express neuroligand receptors. Glia. 1993 Jun;8(2):106–113. doi: 10.1002/glia.440080206. [DOI] [PubMed] [Google Scholar]
- Kastritsis C. H., Salm A. K., McCarthy K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem. 1992 Apr;58(4):1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff Frank, Kettenmann Helmut. GABA Triggers a [Ca2+]i Increase in Murine Precursor Cells of the Oligodendrocyte Lineage. Eur J Neurosci. 1992 Oct;4(11):1049–1058. doi: 10.1111/j.1460-9568.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Magoski N. S., Walz W. Ionic dependence of a P2-purinoceptor mediated depolarization of cultured astrocytes. J Neurosci Res. 1992 Aug;32(4):530–538. doi: 10.1002/jnr.490320408. [DOI] [PubMed] [Google Scholar]
- Maire J. C., Medilanski J., Straub R. W. Uptake of adenosine and release of adenine derivatives in mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1982 Feb;323:589–602. doi: 10.1113/jphysiol.1982.sp014093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Pearce B., Murphy S., Jeremy J., Morrow C., Dandona P. ATP-evoked Ca2+ mobilisation and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J Neurochem. 1989 Mar;52(3):971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Petersen C. C., Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Reber B. F., Stucki J. W., Reuter H. Unidirectional interaction between two intracellular calcium stores in rat phaeochromocytoma (PC12) cells. J Physiol. 1993 Aug;468:711–727. doi: 10.1113/jphysiol.1993.sp019796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J., Downie J. W., Reid A. R., Cahill C. M., White T. D. ATP release from dorsal spinal cord synaptosomes: characterization and neuronal origin. Brain Res. 1993 Apr 30;610(1):32–38. doi: 10.1016/0006-8993(93)91213-c. [DOI] [PubMed] [Google Scholar]
- Scherer J., Schnitzer J. The rabbit retina: a suitable mammalian tissue for obtaining astroglia-free Müller cell cultures. Neurosci Lett. 1989 Feb 13;97(1-2):51–56. doi: 10.1016/0304-3940(89)90138-9. [DOI] [PubMed] [Google Scholar]
- Shmigol A., Kirischuk S., Kostyuk P., Verkhratsky A. Different properties of caffeine-sensitive Ca2+ stores in peripheral and central mammalian neurones. Pflugers Arch. 1994 Jan;426(1-2):174–176. doi: 10.1007/BF00374686. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Trotter J., Schachner M., Kettenmann H. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989 Feb;2(2):1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Volpe P. Ryanodine receptors: how many, where and why? Trends Pharmacol Sci. 1993 Mar;14(3):98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Trotter J., Bitter-Suermann D., Schachner M. Differentiation-regulated loss of the polysialylated embryonic form and expression of the different polypeptides of the neural cell adhesion molecule by cultured oligodendrocytes and myelin. J Neurosci Res. 1989 Apr;22(4):369–383. doi: 10.1002/jnr.490220402. [DOI] [PubMed] [Google Scholar]
- Walz W., Ilschner S., Ohlemeyer C., Banati R., Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993 Oct;13(10):4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Blankenfeld G., Kettenmann H. Glutamate and GABA receptors in vertebrate glial cells. Mol Neurobiol. 1991 Spring;5(1):31–43. doi: 10.1007/BF02935611. [DOI] [PubMed] [Google Scholar]