Abstract
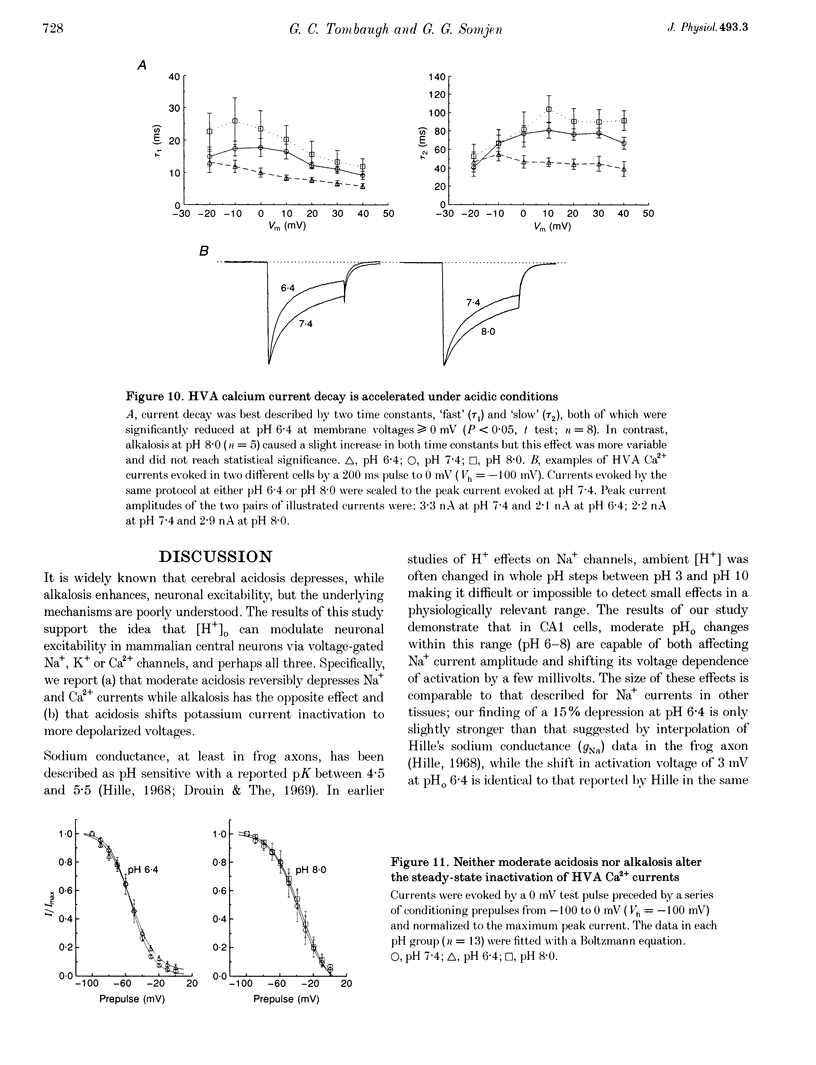
1. The effects of extracellular H+ (pHo) in the pathophysiological range (pH 6-8) on voltage-gated sodium, potassium, and calcium currents were examined in acutely dissociated rat hippocampal CA1 neurons using the whole-cell patch clamp technique. All experiments were conducted in Hepes-buffered solutions and were performed at room temperature (21-23 degrees C). 2. TTX-sensitive sodium currents, evoked by both step and ramp depolarization, were reversibly depressed by moderate acidosis and enhanced slightly by alkaline exposure. Changes in current amplitude were coincident with small reversible shifts (+/- 3 mV) in the voltage dependence of activation. In contrast, sodium current activation and decay kinetics as well as steady-state inactivation were unaffected by acidosis. 3. Outward potassium currents could be separated into a transient, rapidly inactivating current (IA) and a sustained, slowly inactivating component (IK). Steady-state activation of both currents was unaffected by an increase or decrease in pHo. Similarly, IK activation and IA decay kinetics remained stable during pHo exchange. In contrast, the steady-state inactivation (h infinity) of both potassium currents was reversibly shifted by approximately +10 mV during acid exposure, but remained unchanged during alkaline treatment. 4. Calcium currents were found to be predominantly of the high-voltage-activated (HVA) type, which could be carried by Ba2+ and inhibited completely by cadmium. Moderate acidosis (pH 6.9-6.0) reversibly depressed HVA Ca2+ current amplitude and caused a positive shift in its voltage dependence. For both of these parameters, alkaline treatment (pH 8.0) had the opposite effect. The depression of HVA Ca2+ currents by low pHo was unaffected by raising the internal Hepes concentration from 10 to 50 mM in the patch pipette. A Hill plot of the effect of pH on Ca2+ current amplitude revealed a pK value (defined as the mid-point of the titration curve) of 7.1 and a slope of 0.6. 5. The rate of Ca2+ current activation was unaffected by pHo at positive potentials, but below 0 mV the activation rate increased at low pH and decreased at high pH, becoming significant at -20 mV. At this membrane voltage, a second HVA current was revealed during acid exposure as the whole-cell HVA current was depressed. Ca2+ current decay was described by two time constants, both of which were significantly reduced at pH 6.4 and slightly enhanced at pH 8.0. Steady-state Ca2+ current inactivation reached 50% near -50 mV and was not affected at either pH extreme. 6. These results demonstrate that extracellular pH shifts within the pathophysiological range are capable of modulating both the conductance and gating properties of voltage-gated ion channels in hippocampal CA1 neurons. The effects we describe are consistent with the wellknown effects of pHo on neuronal excitability and strengthen the idea that endogenous pHo shifts may help regulate cell activity in situ.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balestrino M., Somjen G. G. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988 Feb;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S., Bui Q. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J Neurosci. 1991 Dec;11(12):4015–4023. doi: 10.1523/JNEUROSCI.11-12-04015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Membrane currents of internally perfused neurones of the snail, Lymnaea stagnalis, at low intracellular pH. J Physiol. 1986 Jul;376:477–491. doi: 10.1113/jphysiol.1986.sp016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers H., Speckmann E. J. Cerebral pO 2 , pCO 2 and pH: changes during convulsive activity and their significance for spontaneous arrest of seizures. Epilepsia. 1972 Sep;13(5):699–725. doi: 10.1111/j.1528-1157.1972.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990 Aug;10(8):2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D. B., Takahashi K., Copenhagen D. R. L-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 1993 Aug;11(2):267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- Gottfried J. A., Chesler M. Endogenous H+ modulation of NMDA receptor-mediated EPSCs revealed by carbonic anhydrase inhibition in rat hippocampus. J Physiol. 1994 Aug 1;478(Pt 3):373–378. doi: 10.1113/jphysiol.1994.sp020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P. E., Nam S. C. High-voltage-activated calcium current in developing neurons is insensitive to nifedipine. Pflugers Arch. 1994 Mar;426(5):402–411. doi: 10.1007/BF00388303. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Karst H., Joëls M., Wadman W. J. Low-threshold calcium current in dendrites of the adult rat hippocampus. Neurosci Lett. 1993 Dec 24;164(1-2):154–158. doi: 10.1016/0304-3940(93)90880-t. [DOI] [PubMed] [Google Scholar]
- Kay A. R. Inactivation kinetics of calcium current of acutely dissociated CA1 pyramidal cells of the mature guinea-pig hippocampus. J Physiol. 1991 Jun;437:27–48. doi: 10.1113/jphysiol.1991.sp018581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. R., Miles R., Wong R. K. Intracellular fluoride alters the kinetic properties of calcium currents facilitating the investigation of synaptic events in hippocampal neurons. J Neurosci. 1986 Oct;6(10):2915–2920. doi: 10.1523/JNEUROSCI.06-10-02915.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Calcium current activation kinetics in isolated pyramidal neurones of the Ca1 region of the mature guinea-pig hippocampus. J Physiol. 1987 Nov;392:603–616. doi: 10.1113/jphysiol.1987.sp016799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M., Bountra C., Voipio J., Kaila K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibres. Neuroscience. 1992;47(4):921–929. doi: 10.1016/0306-4522(92)90040-9. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol. 1989 Jul;94(1):23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994 Jan 6;367(6458):69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Taira T., Smirnov S., Voipio J., Kaila K. Intrinsic proton modulation of excitatory transmission in rat hippocampal slices. Neuroreport. 1993 Jan;4(1):93–96. doi: 10.1097/00001756-199301000-00024. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Wakamori M., Akaike N. Hippocampal CA1 pyramidal cells of rats have four voltage-dependent calcium conductances. Neurosci Lett. 1989 Sep 25;104(1-2):229–234. doi: 10.1016/0304-3940(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh G. C., Sapolsky R. M. Evolving concepts about the role of acidosis in ischemic neuropathology. J Neurochem. 1993 Sep;61(3):793–803. doi: 10.1111/j.1471-4159.1993.tb03589.x. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990 May 24;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Nilius B., Carmeliet E. Modulation of the T-type cardiac Ca channel by changes in proton concentration. J Gen Physiol. 1990 Nov;96(5):973–990. doi: 10.1085/jgp.96.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M., Wadman W. J. Enhancement of calcium currents in rat hippocampal CA1 neurons induced by kindling epileptogenesis. Neuroscience. 1992 Jul;49(2):373–381. doi: 10.1016/0306-4522(92)90103-9. [DOI] [PubMed] [Google Scholar]
- White E. J., Juchniewicz H. J., Clark J. B. Effects of lactic acidosis on the function of cerebral cortical synaptosomes. J Neurochem. 1989 Jan;52(1):154–161. doi: 10.1111/j.1471-4159.1989.tb10910.x. [DOI] [PubMed] [Google Scholar]


