Abstract
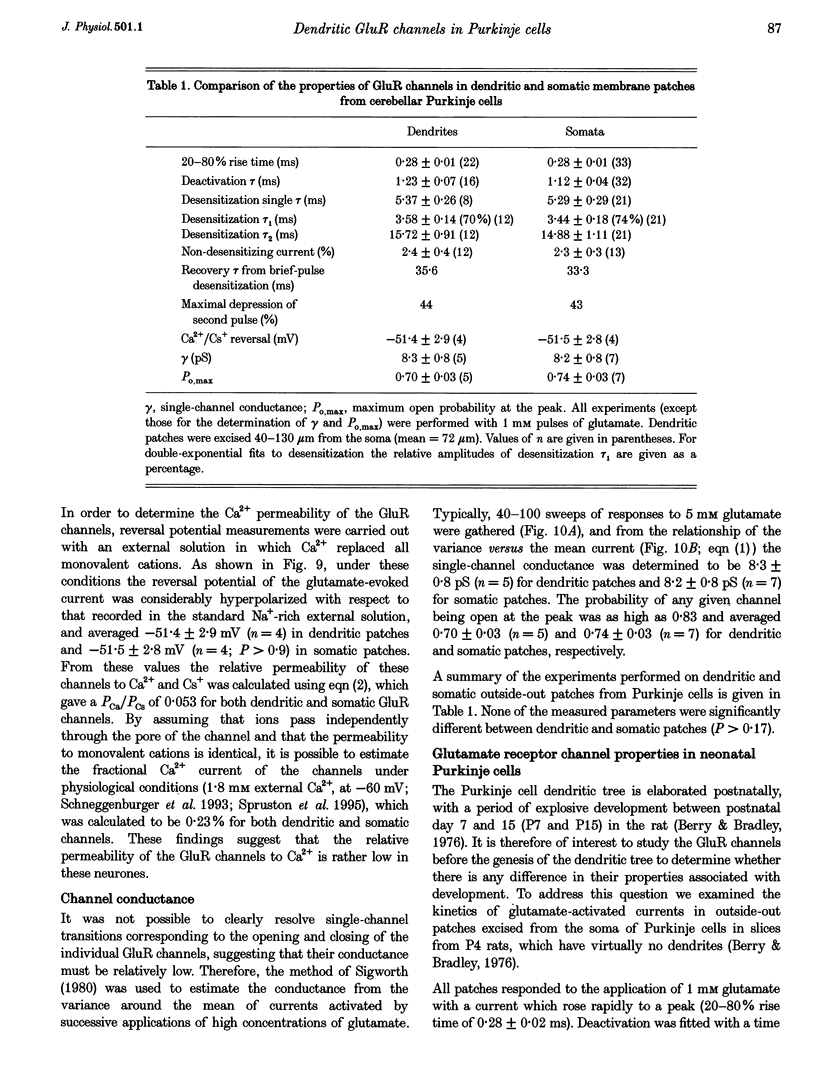
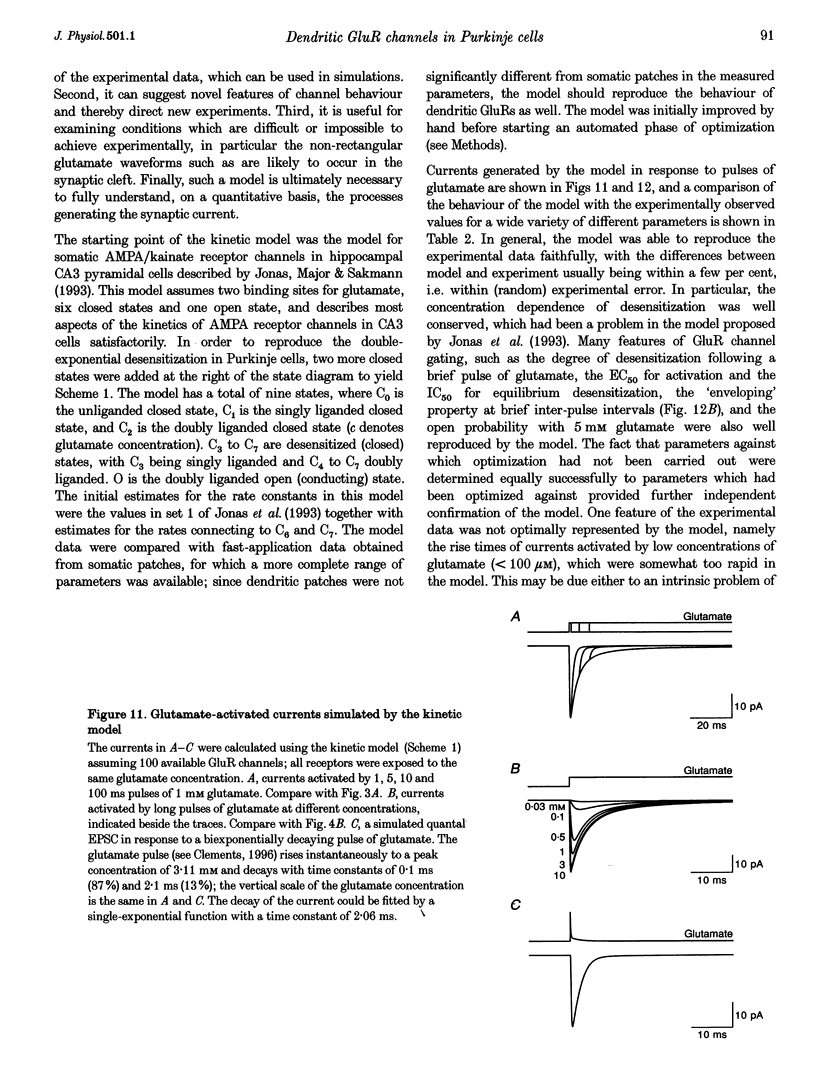
1. The properties of glutamate receptor (GluR) channels in outside-out patches from the dendrites and somata of rat cerebellar Purkinje cells in brain slice were studied using fast agonist application techniques. Dendritic patches were isolated 40-130 micronm from the soma. 2. Outside-out patches from both dendrites and somata of Purkinje cells responded to application of glutamate with a current which desensitized rapidly and nearly completely. Currents evoked by glutamate application were blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), were mimicked by L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), and were modulated by cyclothiazide. Kainate produced small, non-desensitizing currents. No currents were observed in response to aspartate application. Responses characteristic of NMDA receptor activation were not observed. These findings indicate that glutamate-activated currents were mediated by the AMPA subtype of GluR. 3. Deactivation of the GluR channels following 1 ms pulses of glutamate occurred with a time constant of 1.23 +/- 0.07 ms in dendritic and 1.12 +/- 0.04 ms in somatic patches. Desensitization occurred with a time constant of 5.37 +/- 0.26 ms in dendritic and 5.29 +/- 0.29 ms in somatic patches. The time constant of recovery from desensitization caused by a 1 ms application of 1 mM glutamate was 36 ms in dendritic patches and 33 ms in somatic patches. 4. Half-maximal activation of the GluR channels was achieved at a glutamate concentration of 432 microM. Deactivation kinetics were not dependent on the glutamate concentration, while desensitization became slower at lower glutamate concentrations. 5. Pre-equilibration of patches with low concentrations of glutamate reduced the peak current activated by 1 mM glutamate. The IC50 for this effect was 8.7 microM. Equilibrium desensitization did not affect the kinetics of the current activated by 1 mM glutamate. 6. The current-voltage relationship of the peak current was linear in normal Na(+)-rich external solution, with a reversal potential near 0 mV. In Ca(2+) -rich external solution, the reversal potentials were -51.4 +/- 2.9 and -51.5 +/- 2.8 mV for dendritic and somatic patches, respectively, indicating that these glutamate channels have a low permeability to Ca2+ (PCa/PCa = 0.053). 7. The mean single-channel conductance of the GluR channels measured using non-stationary fluctuation analysis was approximately 8 pS in dendritic and somatic patches, and the maximum open probability was at least 0.7 with 5 mM glutamate. 8. GluR channel kinetics in patches excised from the soma of neonatal (postnatal day 4; P4) Purkinje cells, before the development of the dendritic arborization of the Purkinje cell, were similar to those in patches excised from more mature (P12-18) Purkinje cells. 9. Dendritic and somatic GluR channels in Purkinje cells appear to be functionally identical, are AMPA-subtype receptors containing the GluR-B subunit, and have rapid kinetics and low permeability to Ca2+. A kinetic model was constructed which faithfully reproduces the gating characteristics of the GluR channels.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour B., Keller B. U., Llano I., Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994 Jun;12(6):1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Barbour B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron. 1993 Oct;11(4):759–769. doi: 10.1016/0896-6273(93)90085-6. [DOI] [PubMed] [Google Scholar]
- Baude A., Molnár E., Latawiec D., McIlhinney R. A., Somogyi P. Synaptic and nonsynaptic localization of the GluR1 subunit of the AMPA-type excitatory amino acid receptor in the rat cerebellum. J Neurosci. 1994 May;14(5 Pt 1):2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Bradley P. The growth of the dendritic trees of Purkinje cells in the cerebellum of the rat. Brain Res. 1976 Aug 6;112(1):1–35. doi: 10.1016/0006-8993(76)90331-0. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Lodder J. C., Kits K. S. Large amplitude variability of GABAergic IPSCs in melanotropes from Xenopus laevis: evidence that quantal size differs between synapses. J Neurophysiol. 1994 Feb;71(2):639–655. doi: 10.1152/jn.1994.71.2.639. [DOI] [PubMed] [Google Scholar]
- Brorson J. R., Bleakman D., Chard P. S., Miller R. J. Calcium directly permeates kainate/alpha-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid receptors in cultured cerebellar Purkinje neurons. Mol Pharmacol. 1992 Apr;41(4):603–608. [PubMed] [Google Scholar]
- Clark B. A., Farrant M., Cull-Candy S. G. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. J Neurosci. 1997 Jan 1;17(1):107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996 May;19(5):163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Jonas P., Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J Physiol. 1992 Dec;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W., Sugimori M., Llinás R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8279–8282. doi: 10.1073/pnas.92.18.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers J., Augustine G. J., Konnerth A. Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature. 1995 Jan 12;373(6510):155–158. doi: 10.1038/373155a0. [DOI] [PubMed] [Google Scholar]
- Farrant M., Cull-Candy S. G. Excitatory amino acid receptor-channels in Purkinje cells in thin cerebellar slices. Proc Biol Sci. 1991 Jun 22;244(1311):179–184. doi: 10.1098/rspb.1991.0067. [DOI] [PubMed] [Google Scholar]
- Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995 Jul;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Different glutamate receptor channels mediate fast excitatory synaptic currents in inhibitory and excitatory cortical neurons. Neuron. 1993 Dec;11(6):1083–1091. doi: 10.1016/0896-6273(93)90221-c. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Llano I., Armstrong C. M. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B., Audinat E., Bochet P., Crépel F., Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992 Aug;9(2):247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- Lambolez B., Ropert N., Perrais D., Rossier J., Hestrin S. Correlation between kinetics and RNA splicing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in neocortical neurons. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1797–1802. doi: 10.1073/pnas.93.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Herranz A. S., Herreras O., Abraira V., Martín del Río R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986 Oct 1;384(1):145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Linden D. J. Long-term synaptic depression in the mammalian brain. Neuron. 1994 Mar;12(3):457–472. doi: 10.1016/0896-6273(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Wall P. D., Yaksh T. L. Properties of two unmyelinated fibre tracts of the central nervous system: lateral Lissauer tract, and parallel fibres of the cerebellum. J Physiol. 1978 Nov;284:127–145. doi: 10.1113/jphysiol.1978.sp012531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A., Feldmeyer D., Cull-Candy S. G. Identification of a native low-conductance NMDA channel with reduced sensitivity to Mg2+ in rat central neurones. J Physiol. 1996 Jul 15;494(Pt 2):479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo P. G., Palestini M., Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol. 1982 Nov;332:187–202. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z., Mulvihill E., Streit P., Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994 Aug;61(3):421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Winters C. A., Mayer M. L., Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993 Dec;11(6):1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990 Jul;10(7):2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. J., Hestrin S., Sah P., Nicoll R. A. Excitatory synaptic currents in Purkinje cells. Proc Biol Sci. 1990 Aug 22;241(1301):116–121. doi: 10.1098/rspb.1990.0074. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Raman I. M., Trussell L. O. The mechanism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor desensitization after removal of glutamate. Biophys J. 1995 Jan;68(1):137–146. doi: 10.1016/S0006-3495(95)80168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano D., Lambolez B., Rossier J., Paternain A. V., Lerma J. Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron. 1995 May;14(5):1009–1017. doi: 10.1016/0896-6273(95)90339-9. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., Zhou Z., Konnerth A., Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993 Jul;11(1):133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N., Jonas P., Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995 Jan 15;482(Pt 2):325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart G., Häusser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron. 1994 Sep;13(3):703–712. doi: 10.1016/0896-6273(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kovalchuk Y., Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci. 1995 Aug;15(8):5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991 Dec;7(6):965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Tempia F., Kano M., Schneggenburger R., Schirra C., Garaschuk O., Plant T., Konnerth A. Fractional calcium current through neuronal AMPA-receptor channels with a low calcium permeability. J Neurosci. 1996 Jan 15;16(2):456–466. doi: 10.1523/JNEUROSCI.16-02-00456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]