Abstract
1. The patterns of discharge of spontaneous GABAA-mediated inhibitory postsynaptic currents (sIPSCs), originating from the nucleus reticularis thalami (NRT), and their modulation by GABAB autoreceptors, were studied in rat thalamocortical (TC) neurones using whole-cell voltage-clamp recordings in brain slices. 2. sIPSCs were recorded in all ventro-basal (VB) and dorsal lateral geniculate (LGN) neurones. In VB neurones, in the presence of tetraethylammonium (TEA, 5 mM), these sIPSCs can occur in bursts at frequencies of either 0.1 or 1-2 Hz. In the presence of tetrodotoxin (TTX), these bursting activities are replaced by the continuous discharge of miniature IPSCs (mIPSCs), recorded in the absence of TEA, at a frequency of 4 Hz. The kinetic properties of mIPSCs were similar in VB and LGN TC neurones. 3. In VB TC neurones the GABAB receptor agonist (+/-)-baclofen, at a concentration of 0.05 microM, decreased the mIPSC frequency by 22% without affecting their amplitude distribution. Increasing the (+/-)-baclofen concentration to 1 and 10 microM caused similar reductions (41 and 47%, respectively) in the mIPSCs frequency: these values were significantly different from the one observed with 0.05 microM (+/-)-baclofen. In LGN TC neurones, where mIPSCs originate from both NRT and local interneurone terminals, 1 microM (+/-)-baclofen produced a 66% reduction in the mIPSC frequency. 4. The GABAB receptor antagonist CGP55845A (50 nM) not only blocked the baclofen-mediated decrease in mIPSC frequency, but also produced a 52% increase in the mIPSC frequency compared with control in three out of seven neurones. Application of CGP55845A (50-500 nM) alone produced a 77% increase in the mIPSC frequency in three out of nine VB neurones, and in the LGN, CGP55845A (100 nM) produced a 53% increase in four out of nine neurones. CGP55845A (100 nM) also reversibly increased the amplitude of evoked GABAA IPSCs by 74 and 57% in three out of three VB and three out of five LGN neurones, respectively. 5. Application of GABA (1.5-5 microM) decreased the mIPSC frequency in VB TC neurones by a similar extent (48%) as 1-10 microM (+/-)-baclofen. 6. In the presence of 100 microM Cd2+, (+/-)-baclofen still decreased the mIPSC frequency by about 40%, indicating that the effect of presynaptic GABAB receptor activation on spontaneous GABA release did not occur through a reduction of voltage-dependent Ca2+ currents. 7. Cd2+ (100 microM) decreased the amplitude of both mIPSCs and isoguvacine-induced current by 30 and 19%, respectively, indicating an effect of this divalent cation on postsynaptic GABAA receptors. 8. We conclude that GABAB autoreceptors are present on the GABAergic terminals within the thalamic sensory nuclei and that these receptors can be tonically activated by the ambient GABA.
Full text
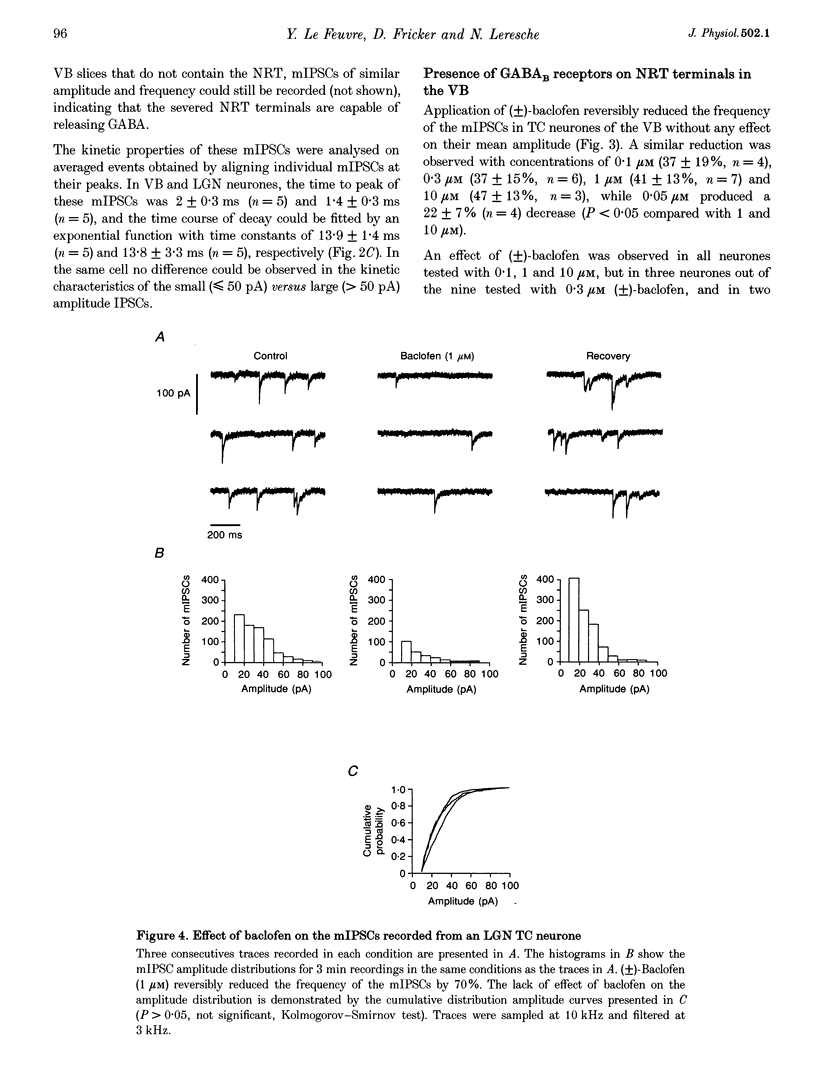
PDF




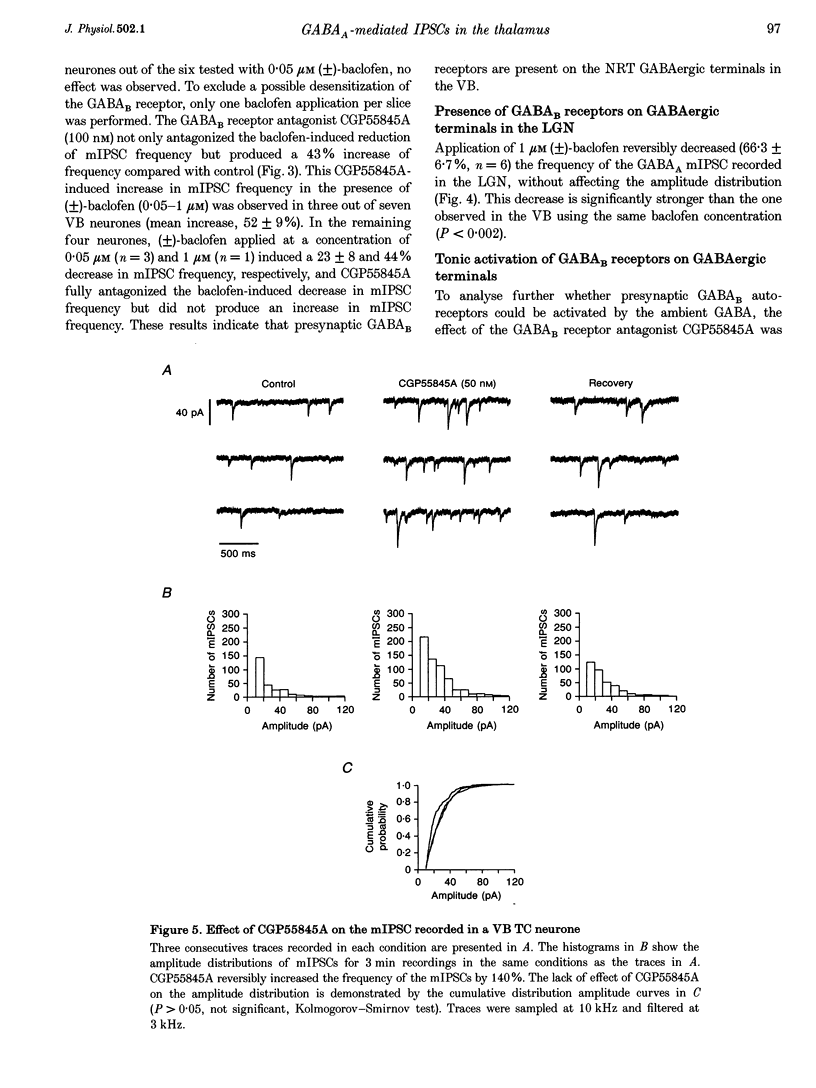







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal T., McCormick D. A. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993 Aug;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T., von Krosigk M., McCormick D. A. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol. 1995 Mar 15;483(Pt 3):665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G., Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993 Jul;14(7):259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., De Murtas M., Bernardi G. Involvement of GABA systems in feedback regulation of glutamate-and GABA-mediated synaptic potentials in rat neostriatum. J Physiol. 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic inhibition of calcium-dependent and -independent release elicited with ionomycin, gadolinium, and alpha-latrotoxin in the hippocampus. J Neurophysiol. 1996 May;75(5):2017–2028. doi: 10.1152/jn.1996.75.5.2017. [DOI] [PubMed] [Google Scholar]
- Chu D. C., Albin R. L., Young A. B., Penney J. B. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34(2):341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Dittman J. S., Regehr W. G. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996 Mar 1;16(5):1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doze V. A., Cohen G. A., Madison D. V. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J Neurophysiol. 1995 Jul;74(1):43–53. doi: 10.1152/jn.1995.74.1.43. [DOI] [PubMed] [Google Scholar]
- Emri Z., Turner J. P., Crunelli V. Tonic activation of presynaptic GABA(B) receptors on thalamic sensory afferents. Neuroscience. 1996 Jun;72(3):689–698. doi: 10.1016/0306-4522(95)00590-0. [DOI] [PubMed] [Google Scholar]
- Guyon A., Leresche N. Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurones. J Physiol. 1995 May 15;485(Pt 1):29–42. doi: 10.1113/jphysiol.1995.sp020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford D. A., Clark S., Cao Z., Wilson W. A., Jr, Lin F. H., Morrisett R. A., Huin A. The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science. 1992 Jul 17;257(5068):398–401. doi: 10.1126/science.1321503. [DOI] [PubMed] [Google Scholar]
- Häusser M. A., Yung W. H. Inhibitory synaptic potentials in guinea-pig substantia nigra dopamine neurones in vitro. J Physiol. 1994 Sep 15;479(Pt 3):401–422. doi: 10.1113/jphysiol.1994.sp020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U. GABAB receptor-mediated inhibition of tetrodotoxin-resistant GABA release in rodent hippocampal CA1 pyramidal cells. J Neurosci. 1997 Feb 1;17(3):1025–1032. doi: 10.1523/JNEUROSCI.17-03-01025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U. On the inhibitory actions of baclofen and gamma-aminobutyric acid in rat ventral midbrain culture. J Physiol. 1992;451:419–443. doi: 10.1113/jphysiol.1992.sp019171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. G., Allen C. N., North R. A. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience. 1995 Feb;64(3):813–819. doi: 10.1016/0306-4522(94)00429-9. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N. A., Wilson W. A. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron. 1993 Dec;11(6):1057–1067. doi: 10.1016/0896-6273(93)90219-h. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Friedberg M. H., Ebner F. F. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. II. Differential effects of GABAA and GABAB receptor antagonists on responses of VPM neurons. J Neurophysiol. 1994 May;71(5):1716–1726. doi: 10.1152/jn.1994.71.5.1716. [DOI] [PubMed] [Google Scholar]
- Leresche N. Synaptic Currents in Thalamo-cortical Neurons of the Rat Lateral Geniculate Nucleus. Eur J Neurosci. 1992;4(7):595–602. doi: 10.1111/j.1460-9568.1992.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Liu Z., Vergnes M., Depaulis A., Marescaux C. Involvement of intrathalamic GABAB neurotransmission in the control of absence seizures in the rat. Neuroscience. 1992;48(1):87–93. doi: 10.1016/0306-4522(92)90340-8. [DOI] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol. 1993 Aug;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean H. A., Caillard O., Khazipov R., Ben-Ari Y., Gaiarsa J. L. Spontaneous release of GABA activates GABAB receptors and controls network activity in the neonatal rat hippocampus. J Neurophysiol. 1996 Aug;76(2):1036–1046. doi: 10.1152/jn.1996.76.2.1036. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Bijak M., Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995 Jul;46(4):423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994 Dec;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992 Jan;67(1):227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992 Jul;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Pinault D., Bourassa J., Deschênes M. The axonal arborization of single thalamic reticular neurons in the somatosensory thalamus of the rat. Eur J Neurosci. 1995 Jan 1;7(1):31–40. doi: 10.1111/j.1460-9568.1995.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Richards D. A., Lemos T., Whitton P. S., Bowery N. G. Extracellular GABA in the ventrolateral thalamus of rats exhibiting spontaneous absence epilepsy: a microdialysis study. J Neurochem. 1995 Oct;65(4):1674–1680. doi: 10.1046/j.1471-4159.1995.65041674.x. [DOI] [PubMed] [Google Scholar]
- Scanziani M., Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992 Nov;9(5):919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Solís J. M., Nicoll R. A. Pharmacological characterization of GABAB-mediated responses in the CA1 region of the rat hippocampal slice. J Neurosci. 1992 Sep;12(9):3466–3472. doi: 10.1523/JNEUROSCI.12-09-03466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Capogna M., Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993 Jun;16(6):222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Turgeon S. M., Albin R. L. Zinc modulates GABAB binding in rat brain. Brain Res. 1992 Nov 20;596(1-2):30–34. doi: 10.1016/0006-8993(92)91528-m. [DOI] [PubMed] [Google Scholar]
- Ulrich D., Huguenard J. R. GABAB receptor-mediated responses in GABAergic projection neurones of rat nucleus reticularis thalami in vitro. J Physiol. 1996 Jun 15;493(Pt 3):845–854. doi: 10.1113/jphysiol.1996.sp021427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Marty A. Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron. 1993 Nov;11(5):885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J Physiol. 1995 Jun 15;485(Pt 3):649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]


