Abstract
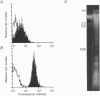
1. During the process of cell death, rises in cytosolic Ca2+ concentration ([Ca2+]i) together with structural changes were investigated in target cells attacked by purified CD3-,CD16+ human natural killer (NK) cells. 2. In the target cell line K562, a rapid [Ca2+]i rise to 1-2 microM occurred a few minutes after NK cell-target cell contact, immediately followed by leakage of the Ca2+ indicator dye fura-2 from the cell. Cells were permeabilized, but their chromatin was not fragmented. The changes were basically consistent with those seen in necrosis induced by activated complement. 3. In the target cell line MOLT-4, which expressed the apoptosis-inducing surface antigen Fas much more strongly than K562, the majority of attacked cells displayed a [Ca2+]i rise to 0.7-1 microM followed by a slow decline, often associated with diminishing [Ca2+]i oscillations. As a whole, [Ca2+]i remained higher than 150 nM for at least 1.5-3 h (approximately 100 nM in control cells). 4. MOLT-4 cells attacked by NK cells became bubble shaped within 20 min of the main [Ca2+]i rise reaching its peak, and then both the cell and chromatin were fragmented into small pieces. These findings were basically consistent with those in apoptosis induced by a monoclonal antibody against the surface antigen Fas. 5. NK cells induced both necrosis and apoptosis in cell lines insensitive to NK cells in the presence of an antibody against the major histocompatibility complex class I (antibody-dependent cell-mediated cytotoxicity, ADCC). The distinct Ca2+ responses patterns described above corresponded to necrosis or apoptosis in different cells stimulated by the common ADCC pathway. 6. Human NK cells were found to be capable of inducing necrosis (membrane damage) or apoptosis (nuclear damage) depending on the target cell types. The characteristic Ca2+ response profile was a good indicator for distinguishing between the modes of cell death induced by the cytotoxicity of the killer cells.
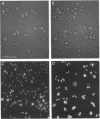
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Verret C. R., Wolley R. C., Eisen H. N. Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med. 1988 Feb 1;167(2):514–527. doi: 10.1084/jem.167.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Arase N., Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995 Mar 1;181(3):1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Harris D. T., Jaso-Friedmann L., Evans D. L. Target-cell sensitivity to natural killer-cell lysis is determined by the expression of a novel antigen in conjunction with major histocompatibility complex class-I molecules. Scand J Immunol. 1994 Jan;39(1):73–78. doi: 10.1111/j.1365-3083.1994.tb03342.x. [DOI] [PubMed] [Google Scholar]
- Jones J., Hallett M. B., Morgan B. P. Reversible cell damage by T-cell perforins. Calcium influx and propidium iodide uptake into K562 cells in the absence of lysis. Biochem J. 1990 Apr 15;267(2):303–307. doi: 10.1042/bj2670303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J., Morgan B. P. Killing of cells by perforin. Resistance to killing is not due to diminished binding of perforin to the cell membrane. Biochem J. 1991 Nov 15;280(Pt 1):199–204. doi: 10.1042/bj2800199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krähenbühl O., Rey C., Jenne D., Lanzavecchia A., Groscurth P., Carrel S., Tschopp J. Characterization of granzymes A and B isolated from granules of cloned human cytotoxic T lymphocytes. J Immunol. 1988 Nov 15;141(10):3471–3477. [PubMed] [Google Scholar]
- Moretta L., Ciccone E., Mingari M. C., Biassoni R., Moretta A. Human natural killer cells: origin, clonality, specificity, and receptors. Adv Immunol. 1994;55:341–380. doi: 10.1016/s0065-2776(08)60513-1. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Dankert J. R., Esser A. F. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987 Jan 1;138(1):246–253. [PubMed] [Google Scholar]
- Nagata S., Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995 Jan;16(1):39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Oshimi K., Oshimi Y., Satake M., Mizoguchi H. Natural killer-mediated lysis of normal and malignant target cells, and its regulation by monocytes. J Exp Med. 1985 Aug 1;162(2):472–486. doi: 10.1084/jem.162.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimi Y., Miyazaki S. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2+ level. J Immunol. 1995 Jan 15;154(2):599–609. [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Cytotoxic T lymphocytes induce different types of DNA damage in target cells of different origins. J Immunol. 1991 Aug 1;147(3):795–803. [PubMed] [Google Scholar]
- Shi L., Kraut R. P., Aebersold R., Greenberg A. H. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992 Feb 1;175(2):553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. M., Luzio J. P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J. 1991 Mar 1;274(Pt 2):381–386. doi: 10.1042/bj2740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita H., Nakata M., Kawasaki A., Shinkai Y., Okumura K. Role of perforin in lymphocyte-mediated cytolysis. Adv Immunol. 1992;51:215–242. doi: 10.1016/s0065-2776(08)60488-5. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D. Killing of target cells by lymphocytes: a mechanistic view. Physiol Rev. 1989 Jan;69(1):250–314. doi: 10.1152/physrev.1989.69.1.250. [DOI] [PubMed] [Google Scholar]