Abstract
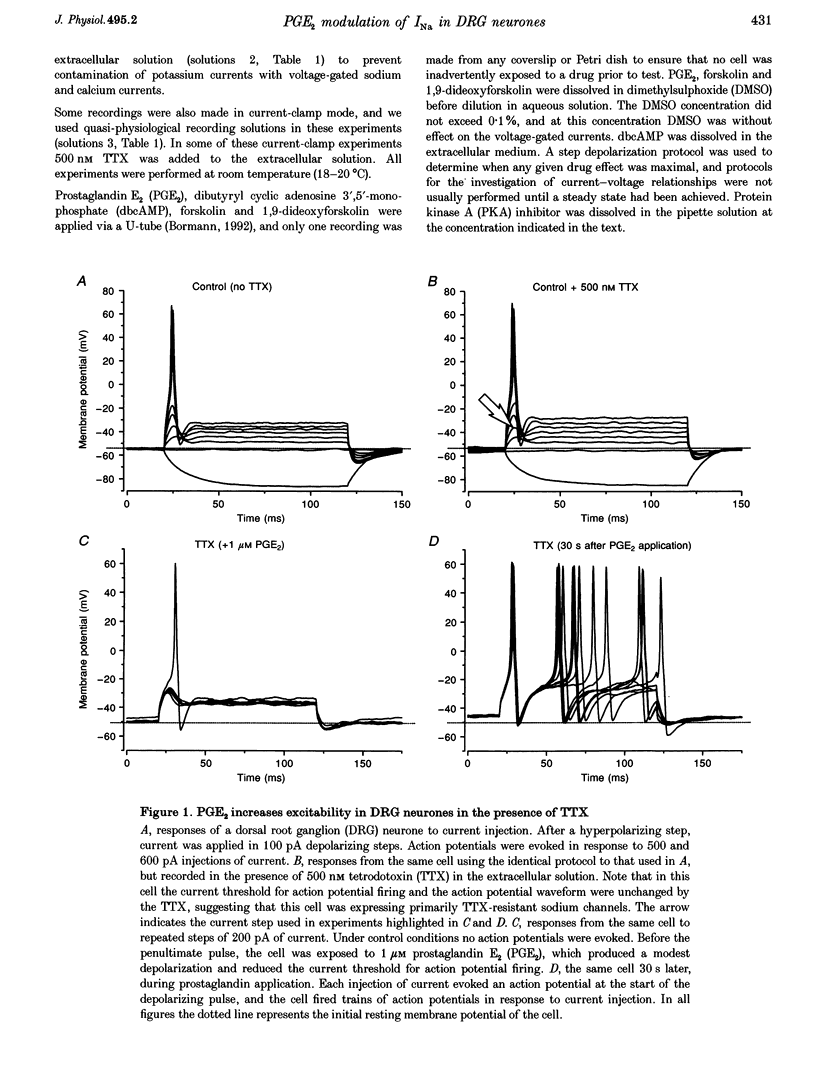
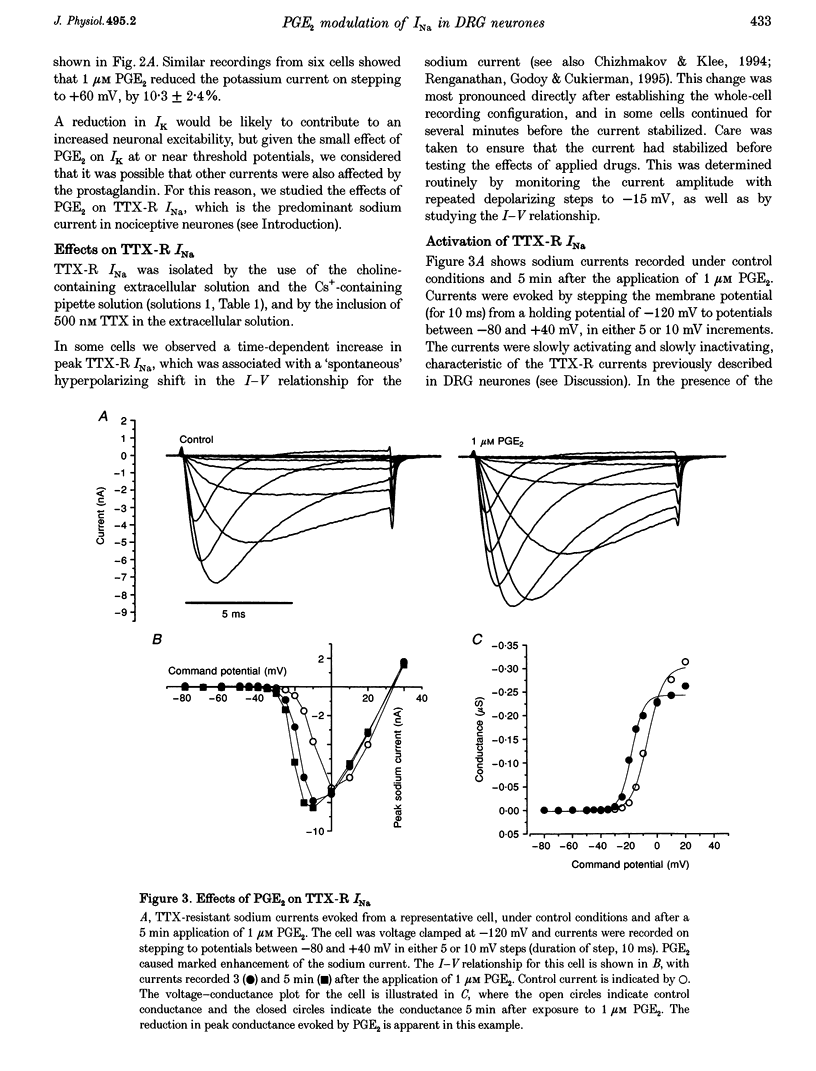
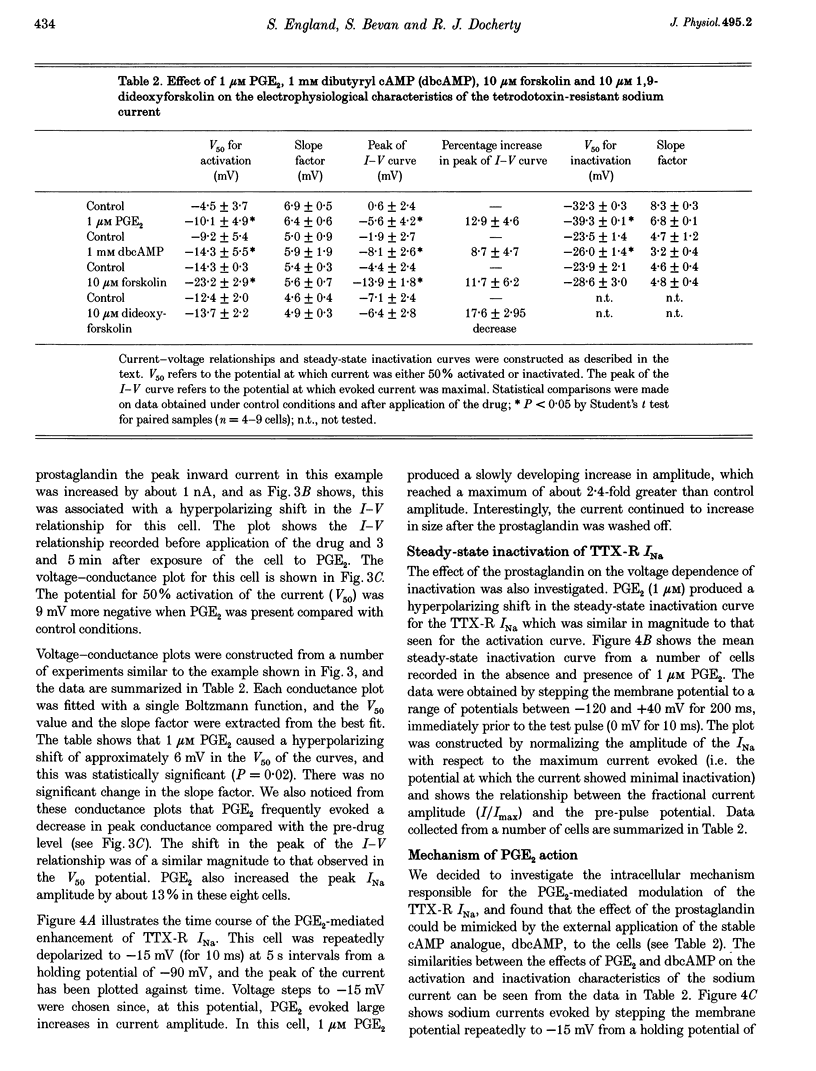
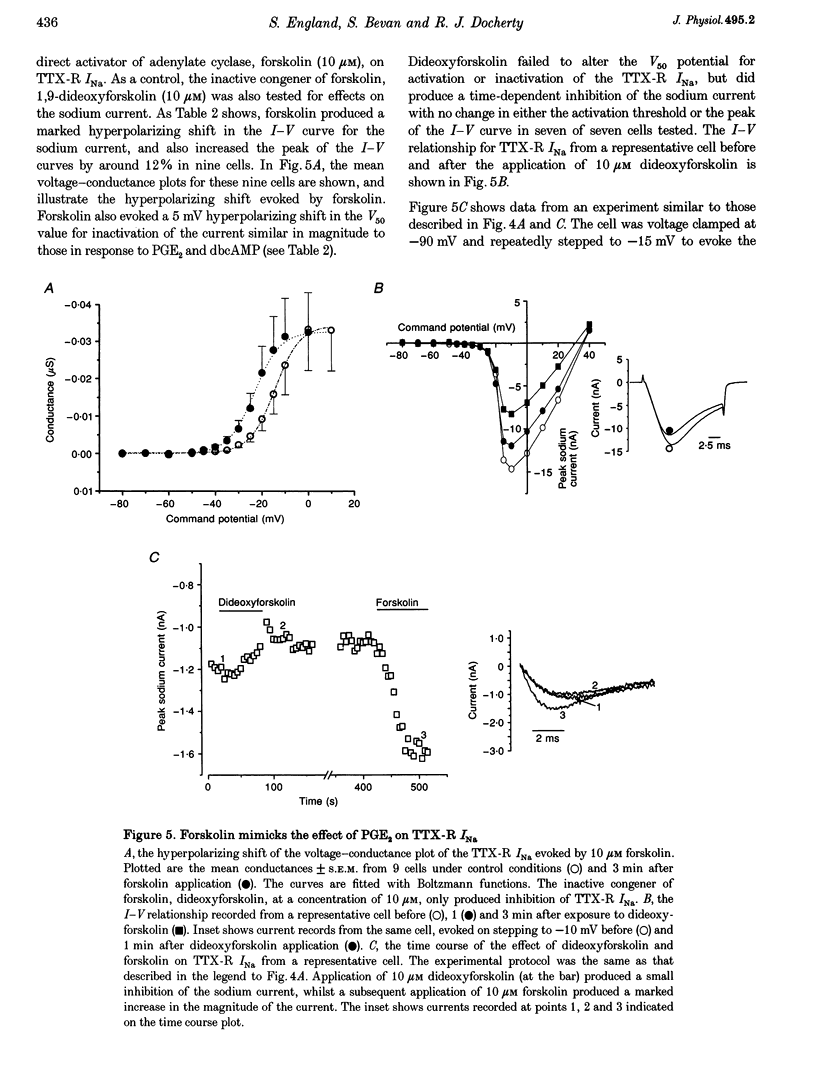
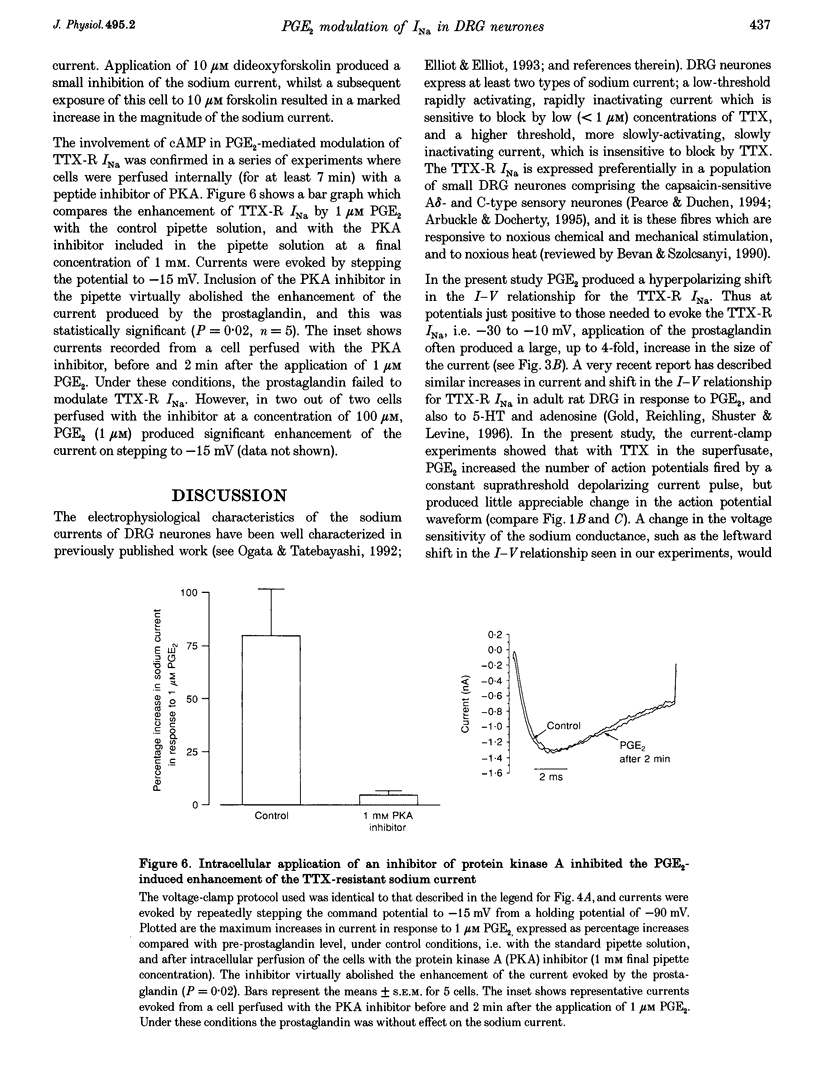
1. In current-clamp recordings, 1 microM prostaglandin E2 (PGE2) increased the excitability of neonatal rat dorsal root ganglion neurones. The current threshold for firing was reduced, and the response to a constant suprathreshold stimulation was modified such that a single evoked action potential was converted to a train of action potentials. The excitatory action of PGE2 was still apparent when action potentials were evoked in the presence of 500 nM tetrodotoxin. 2. In voltage-clamp experiments 1 microM PGE2 frequently increased the magnitude of the peak currents recorded, and caused a hyperpolarizing shift (of approximately 6 mV) in the activation curve for the tetrodotoxin-resistant sodium current (TTX-R INa). In some cells, the hyperpolarizing shift in the activation curve was accompanied by a decrease in peak conductance. PGE2 also caused a hyperpolarizing shift in the steady-state inactivation curve for the sodium current. 3. Extracellular application of the cAMP analogue dibutyryl cAMP (dbcAMP) at a concentration of 1 mM produced effects on both the current-voltage relationship and the steady-state inactivation curve for the TTX-R INa which were indistinguishable from those observed with PGE2. Prior exposure of the neurones to dbcAMP occluded the effect of a subsequent treatment with PGE2. 4. Forskolin (10 microM), a direct activator of adenylate cyclase, mimicked the effects of PGE2 and dbcAMP on TTX-R INa. The inactive congener of forskolin, 1, 9-dideoxyforskolin (10 microM), reduced the amplitude of TTX-R INa, but did not evoke a hyperpolarizing shift in the activation curve. 5. Intracellular perfusion of the neurones with an inhibitor of protein kinase A inhibited the effect of PGE2 on TTX-R INa. 6. PGE2 also reduced the amplitude of voltage-gated potassium currents (IK), which will contribute to the excitatory action. The mechanisms underlying the changes in IK have yet to be elucidated. 7. We propose that the PGE2-mediated increase in excitability in sensory neurones may be due, at least in part, to the cAMP-protein kinase A-dependent modulation of the tetrodotoxin-resistant sodium channel.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins P. T., McCleskey E. W. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993 Oct;56(3):759–769. doi: 10.1016/0306-4522(93)90372-m. [DOI] [PubMed] [Google Scholar]
- Akopian A. N., Sivilotti L., Wood J. N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996 Jan 18;379(6562):257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Arbuckle J. B., Docherty R. J. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995 Feb 6;185(1):70–73. doi: 10.1016/0304-3940(94)11227-a. [DOI] [PubMed] [Google Scholar]
- Bevan S., Szolcsányi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990 Aug;11(8):330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- Birrell G. J., McQueen D. S., Iggo A., Grubb B. D. Prostanoid-induced potentiation of the excitatory and sensitizing effects of bradykinin on articular mechanonociceptors in the rat ankle joint. Neuroscience. 1993 May;54(2):537–544. doi: 10.1016/0306-4522(93)90273-i. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Chizhmakov I. V., Klee M. R. The action of a phorbol ester on voltage-dependent parameters of the sodium current in isolated hippocampal neurons. Neuroscience. 1994 Mar;59(2):285–290. doi: 10.1016/0306-4522(94)90596-7. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Smith W. L., Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994 Jun;46(2):205–229. [PubMed] [Google Scholar]
- Cui M., Nicol G. D. Cyclic AMP mediates the prostaglandin E2-induced potentiation of bradykinin excitation in rat sensory neurons. Neuroscience. 1995 May;66(2):459–466. doi: 10.1016/0306-4522(94)00567-o. [DOI] [PubMed] [Google Scholar]
- Del Bianco E., Maggi C. A., Santicioli P., Geppetti P. Modulation of calcitonin gene-related peptide release evoked by bradykinin and electrical field stimulation in guinea-pig atria. Neurosci Lett. 1994 Mar 28;170(1):163–166. doi: 10.1016/0304-3940(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995 Aug;75(2):125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- Elliott A. A., Elliott J. R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993 Apr;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972 Dec 13;240(102):200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Gold M. S., Dastmalchi S., Levine J. D. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996 Mar;71(1):265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Gold M. S., Reichling D. B., Shuster M. J., Levine J. D. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega D. S., Macdonald R. L. Activators of adenylate cyclase and cyclic AMP prolong calcium-dependent action potentials of mouse sensory neurons in culture by reducing a voltage-dependent potassium conductance. J Neurosci. 1987 Mar;7(3):700–707. doi: 10.1523/JNEUROSCI.07-03-00700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horrobin D. F., Durand L. G., Manku M. S. Prostaglandin E1 modifies nerve conduction and interferes with local anaesthetic action. Prostaglandins. 1977 Jul;14(1):103–108. doi: 10.1016/0090-6980(77)90158-7. [DOI] [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibers. Brain Res. 1994 Mar 7;639(1):125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Khasar S. G., Ouseph A. K., Chou B., Ho T., Green P. G., Levine J. D. Is there more than one prostaglandin E receptor subtype mediating hyperalgesia in the rat hindpaw? Neuroscience. 1995 Feb;64(4):1161–1165. doi: 10.1016/0306-4522(94)00423-3. [DOI] [PubMed] [Google Scholar]
- Leidenheimer N. J., Browning M. D., Harris R. A. GABAA receptor phosphorylation: multiple sites, actions and artifacts. Trends Pharmacol Sci. 1991 Mar;12(3):84–87. doi: 10.1016/0165-6147(91)90509-q. [DOI] [PubMed] [Google Scholar]
- Manku M. S., Horrobin D. F. Chloroquine, quinine, procaine, quinidine and clomipramine are prostaglandin agonists and antagonists. Prostaglandins. 1976 Nov;12(5):789–801. doi: 10.1016/0090-6980(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Mizumura K., Sato J., Kumazawa T. Effects of prostaglandins and other putative chemical intermediaries on the activity of canine testicular polymodal receptors studied in vitro. Pflugers Arch. 1987 May;408(6):565–572. doi: 10.1007/BF00581157. [DOI] [PubMed] [Google Scholar]
- Negus S. S., Butelman E. R., Gatch M. B., Woods J. H. Effects of morphine and ketorolac on thermal allodynia induced by prostaglandin E2 and bradykinin in rhesus monkeys. J Pharmacol Exp Ther. 1995 Aug;274(2):805–814. [PubMed] [Google Scholar]
- Nicol G. D., Cui M. Enhancement by prostaglandin E2 of bradykinin activation of embryonic rat sensory neurones. J Physiol. 1994 Nov 1;480(Pt 3):485–492. doi: 10.1113/jphysiol.1994.sp020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Comparison of two types of Na+ currents with low-voltage-activated T-type Ca2+ current in newborn rat dorsal root ganglia. Pflugers Arch. 1992 Apr;420(5-6):590–594. doi: 10.1007/BF00374638. [DOI] [PubMed] [Google Scholar]
- Ouseph A. K., Khasar S. G., Levine J. D. Multiple second messenger systems act sequentially to mediate rolipram-induced prolongation of prostaglandin E2-induced mechanical hyperalgesia in the rat. Neuroscience. 1995 Feb;64(3):769–776. doi: 10.1016/0306-4522(94)00397-n. [DOI] [PubMed] [Google Scholar]
- Pearce R. J., Duchen M. R. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience. 1994 Dec;63(4):1041–1056. doi: 10.1016/0306-4522(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Pitchford S., Levine J. D. Prostaglandins sensitize nociceptors in cell culture. Neurosci Lett. 1991 Oct 28;132(1):105–108. doi: 10.1016/0304-3940(91)90444-x. [DOI] [PubMed] [Google Scholar]
- Quasthoff S., Grosskreutz J., Schröder J. M., Schneider U., Grafe P. Calcium potentials and tetrodotoxin-resistant sodium potentials in unmyelinated C fibres of biopsied human sural nerve. Neuroscience. 1995 Dec;69(3):955–965. doi: 10.1016/0306-4522(95)00307-5. [DOI] [PubMed] [Google Scholar]
- Renganathan M., Godoy C. M., Cukierman S. Direct modulation of Na+ currents by protein kinase C activators in mouse neuroblastoma cells. J Membr Biol. 1995 Mar;144(1):59–69. doi: 10.1007/BF00238417. [DOI] [PubMed] [Google Scholar]
- Rueff A., Dray A. Sensitization of peripheral afferent fibres in the in vitro neonatal rat spinal cord-tail by bradykinin and prostaglandins. Neuroscience. 1993 May;54(2):527–535. doi: 10.1016/0306-4522(93)90272-h. [DOI] [PubMed] [Google Scholar]
- Schuligoi R., Donnerer J., Amann R. Bradykinin-induced sensitization of afferent neurons in the rat paw. Neuroscience. 1994 Mar;59(1):211–215. doi: 10.1016/0306-4522(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Taiwo Y. O., Bjerknes L. K., Goetzl E. J., Levine J. D. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32(3):577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Taiwo Y. O., Levine J. D. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44(1):131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- Wang J. F., Khasar S. G., Ahlgren S. C., Levine J. D. Sensitization of C-fibres by prostaglandin E2 in the rat is inhibited by guanosine 5'-O-(2-thiodiphosphate), 2',5'-dideoxyadenosine and Walsh inhibitor peptide. Neuroscience. 1996 Mar;71(1):259–263. doi: 10.1016/0306-4522(95)00429-7. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Winter J., James I. F., Rang H. P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988 Sep;8(9):3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Otsuka M., García-Arrarás J. E. E-type prostaglandins depolarize primary afferent neurons of the neonatal rat. Neurosci Lett. 1986 Aug 4;68(3):351–355. doi: 10.1016/0304-3940(86)90515-x. [DOI] [PubMed] [Google Scholar]



