Abstract
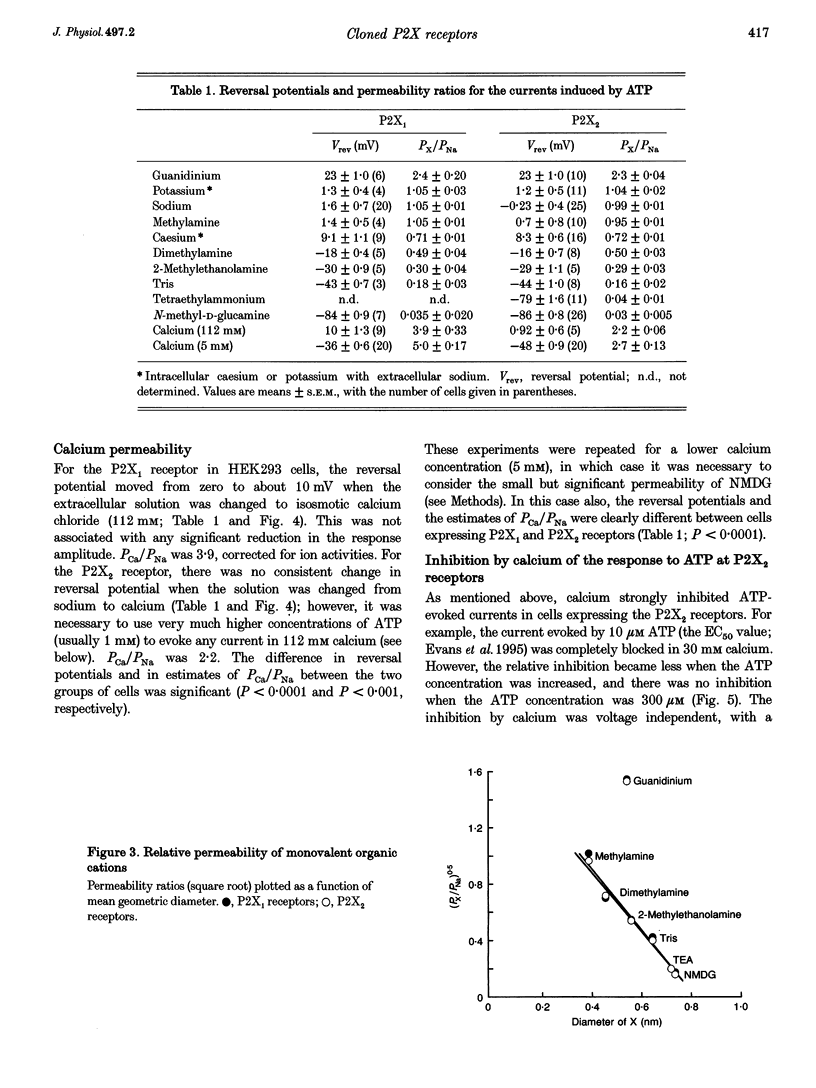
1. Complementary DNAs for the ATP-gated ion channel subunits P2X1 (from human bladder) and P2X2 (from rat phaeochromocytoma (PC12) cells) were used to express the receptors in human embryonic kidney cells by stable transfection, and in Chinese hamster ovary cells by viral infection. 2. Membrane currents evoked by ATP were recorded by the whole-cell patch clamp method. The reversal potential of the current was measured with various intracellular and extracellular solutions and used to compute the relative permeability of the P2X receptor channels. 3. There was no difference between the two receptors with respect to their permeability to monovalent organic cations. The relative permeabilities (PX/PNa) were 2.3, 1.0, 1.0, 0.95, 0.72, 0.5, 0.29, 0.16, 0.04 and 0.03 for guanidinium, potassium, sodium, methylamine, caesium, dimethylamine, 2-methylethanolamine, tris(hydroxymethyl)-aminomethane, tetraethylammonium and N-methyl-D-glucamine, respectively (values for P2X2 receptor). 4. The calcium permeability of P2X1 receptors was greater than that of P2X2 receptors. Under biionic conditions (112 mM calcium outside, 154 mM sodium inside), PCa/PNa values were 3.9 and 2.2, respectively (corrected for ionic activities). 5. ATP-evoked currents in cells expressing the P2X2 receptor were strongly inhibited when the extracellular calcium concentration was increased (0.3-30 mM); the action of ATP could be restored by increasing the ATP concentration. ATP-evoked currents in cells expressing the P2X1 receptor were not inhibited by such increases in the extracellular calcium concentration.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989 Dec;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berglund P., Sjöberg M., Garoff H., Atkins G. J., Sheahan B. J., Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology (N Y) 1993 Aug;11(8):916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Castro N. G., Albuquerque E. X. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995 Feb;68(2):516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G., North R. A., Kawashima E., Merlo-Pich E., Neidhart S., Surprenant A., Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996 Apr 15;16(8):2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Lewis C., Buell G., Valera S., North R. A., Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol Pharmacol. 1995 Aug;48(2):178–183. [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Ishibashi H., Akaike N. ATP-induced inward current in neurons freshly dissociated from the tuberomammillary nucleus. J Neurophysiol. 1994 Mar;71(3):868–873. doi: 10.1152/jn.1994.71.3.868. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Khakh B. S., Humphrey P. P., Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. J Physiol. 1995 Apr 15;484(Pt 2):385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Wakelam M. J. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983 Jun 16;303(5918):621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Lewis C., Neidhart S., Holy C., North R. A., Buell G., Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995 Oct 5;377(6548):432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol. 1991 Jan;65(1):147–154. doi: 10.1128/jvi.65.1.147-154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström K., Mills A., Buell G., Allet E., Adami N., Liljeström P. High-level expression of the human neurokinin-1 receptor in mammalian cell lines using the Semliki Forest virus expression system. Eur J Biochem. 1994 Sep 15;224(3):917–921. doi: 10.1111/j.1432-1033.1994.00917.x. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Akaike N., Kimitsuki T., Komune S., Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990 May;63(5):1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol. 1990 Sep;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Comparison of adenosine triphosphate- and nicotine-activated inward currents in rat phaeochromocytoma cells. J Physiol. 1991 Mar;434:647–660. doi: 10.1113/jphysiol.1991.sp018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Hess P. Block by calcium of ATP-activated channels in pheochromocytoma cells. J Gen Physiol. 1993 Mar;101(3):377–392. doi: 10.1085/jgp.101.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A. Families of ion channels with two hydrophobic segments. Curr Opin Cell Biol. 1996 Aug;8(4):474–483. doi: 10.1016/s0955-0674(96)80023-8. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Raman I. M., Trussell L. O. AMPA receptors with high Ca2+ permeability mediate synaptic transmission in the avian auditory pathway. J Physiol. 1995 Jan 15;482(Pt 2):309–315. doi: 10.1113/jphysiol.1995.sp020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M., Dani J. A. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J. 1995 Feb;68(2):501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Buell G., North R. A. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995 May;18(5):224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Séguéla P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993 Feb;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Harata N., Inoue K., Akaike N. ATP-gated current in dissociated rat nucleus solitarii neurons. J Neurophysiol. 1992 Sep;68(3):778–785. doi: 10.1152/jn.1992.68.3.778. [DOI] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Valera S., Talabot F., Evans R. J., Gos A., Antonarakis S. E., Morris M. A., Buell G. N. Characterization and chromosomal localization of a human P2X receptor from the urinary bladder. Receptors Channels. 1995;3(4):283–289. [PubMed] [Google Scholar]
- Yang J., Mathie A., Hille B. 5-HT3 receptor channels in dissociated rat superior cervical ganglion neurons. J Physiol. 1992 Mar;448:237–256. doi: 10.1113/jphysiol.1992.sp019039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994 Oct;17(10):420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


