Abstract
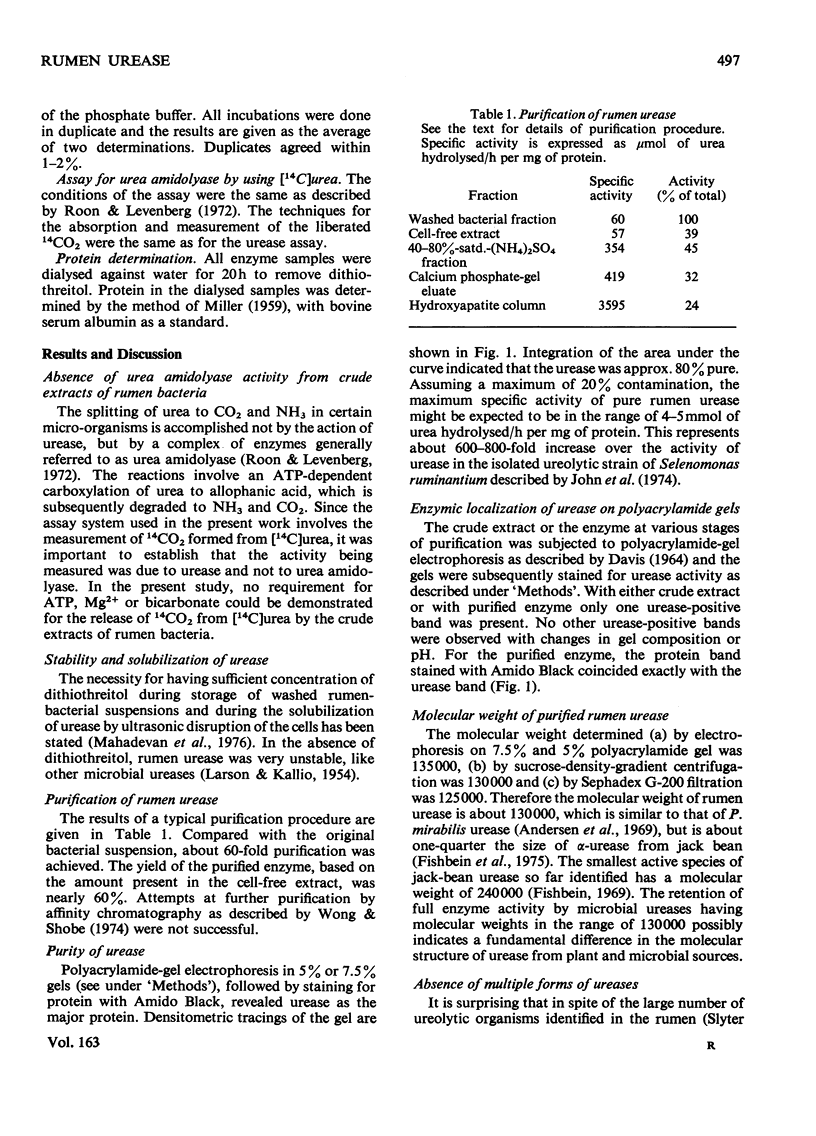
Urease (urea amidohydrolase, EC 3.5.1.5) was extracted from the mixed rumen bacterial fraction of bovine rumen contents and purified 60-fold by (NH4)2SO4 precipitation, calcium phosphate-gel adsorption and chromatography on hydroxyapatite. The purified enzyme had maximum activity at pH 8.0. The molecular weight was estimated to be 120000-130000. The Km for urea was 8.3 X 10(-4) M+/-1.7 X 10(-4) M. The maximum velocity was 3.2+/-0.25 mmol of urea hydrolysed/h per mg of protein. The enzyme was stabilized by 50 mM-dithiothreitol. The enzyme was not inhibited by high concentrations of EDTA or phosphate but was inhibited by Mn2+, Mg2+, Ba2+, Hg2+, Cu2+, Zn2+, Cd2+, Ni2+ and Co2+. p-Chloromercuribenzenesulfphonate and N-ethylmaleimide inhibited the enzyme almost completely at 0.1 mM. Hydroxyurea and acetohydroxamate reversibly inhibited the enzyme. Polyacrylamide-gel electrophoresis showed that the mixed rumen bacteria produce ureases which have identical molecular weights and electrophoretic mobility. No multiple forms of urease were detected.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley R. L., Webb E. C., Zerner B. Jack bean urease (EC 3.5.1.5). A new purification and reliable rate assay. Biochemistry. 1969 May;8(5):1984–1990. doi: 10.1021/bi00833a031. [DOI] [PubMed] [Google Scholar]
- Blattler D. P., Contaxis C. C., Reithel F. J. Dissociation of urease by glycol and glycerol. Nature. 1967 Oct 21;216(5112):274–275. doi: 10.1038/216274b0. [DOI] [PubMed] [Google Scholar]
- Brent B. E., Adepoju A., Portela F. In vitro inhibition of rumen urease with acetohydroxamic acid. J Anim Sci. 1971 Apr;32(4):794–798. doi: 10.2527/jas1971.324794x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Cook A. R. The elimination of urease activity in Streptococcus faecium as evidence for plasmid-coded urease. J Gen Microbiol. 1976 Jan;92(1):49–58. doi: 10.1099/00221287-92-1-49. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Subunit constitution of proteins: a table. Arch Biochem Biophys. 1975 Feb;166(2):651–682. doi: 10.1016/0003-9861(75)90432-4. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola C., Blakeley R. L., Zerner B. Metal ions in enzymes using ammonia or amides. Science. 1976 Mar 19;191(4232):1144–1150. doi: 10.1126/science.769157. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola C., Watters J. J., Blakely R. L., Zerner B. Inhibition of Jack Bean urease (EC 3.5.1.5) by acetohydroxamic acid and by phosphoramidate. An equivalent weight for urease. J Am Chem Soc. 1975 Jul 9;97(14):4130–4131. doi: 10.1021/ja00847a044. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola T. C., blakeley R. L., Zermer B. Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc. 1975 Jul 9;97(14):4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- FISHBEIN W. N., CARBONE P. P. UREASE CATALYSIS. II. INHIBITION OF THE ENZYME BY HYDROXYUREA, HYDROXYLAMINE, AND ACETOHYDROXAMIC ACID. J Biol Chem. 1965 Jun;240:2407–2414. [PubMed] [Google Scholar]
- FISHBEIN W. N., WINTER T. S., DAVIDSON J. D. UREASE CATALYSIS. I. STOICHIOMETRY, SPECIFICITY, AND KINETICS OF A SECOND SUBSTRATE: HYDROXYUREA. J Biol Chem. 1965 Jun;240:2402–2406. [PubMed] [Google Scholar]
- Fishbein W. N., Nagarajan K., Scurzi W. Urease catalysis and structure. VI. Correlation of sedimentation coefficients and electrophoretic mobilities for the polymeric urease isozymes. J Biol Chem. 1970 Nov 25;245(22):5985–5992. [PubMed] [Google Scholar]
- Fishbein W. N. The structural basis for the catalytic complexity of urease: interacting and interconvertible molecular species (with a note on isozyme classes). Ann N Y Acad Sci. 1969 Nov 5;147(20):857–881. doi: 10.1111/j.1749-6632.1969.tb41296.x. [DOI] [PubMed] [Google Scholar]
- Hughes R. B., Katz S. A., Stubbins S. E. Inhibition of urease by metal ions. Enzymologia. 1969 Jun 30;36(6):332–334. [PubMed] [Google Scholar]
- JONES G. A., MACLEOD R. A., BLACKWOOD A. C. UREOLYTIC RUMEN BACTERIA. II. EFFECT OF INORGANIC IONS OF UREASE ACTIVITY. Can J Microbiol. 1964 Jun;10:379–387. doi: 10.1139/m64-051. [DOI] [PubMed] [Google Scholar]
- John A., Isaacson H. R., Bryant M. P. Isolation and characteristics of a ureolytic strain of Selenomonas ruminatium. J Dairy Sci. 1974 Sep;57(9):1003–1014. doi: 10.3168/jds.s0022-0302(74)85001-0. [DOI] [PubMed] [Google Scholar]
- KOBASHI K., HASE J., UEHARA K. Specific inhibition of urease by hydroxamic acids. Biochim Biophys Acta. 1962 Dec 4;65:380–383. doi: 10.1016/0006-3002(62)91067-3. [DOI] [PubMed] [Google Scholar]
- LARSON A. D., KALLIO R. E. Purification and properties of bacterial urease. J Bacteriol. 1954 Jul;68(1):67–73. doi: 10.1128/jb.68.1.67-73.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAL J. A., VILLANUEVA J. R. An improved selective medium for the formation of ascospores by Aspergillus nidulans. Nature. 1962 Mar 17;193:1106–1106. doi: 10.1038/1931106a0. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance plasmids of Staphylococcus aureus and their clinical importance. Bacteriol Rev. 1975 Mar;39(1):1–32. doi: 10.1128/br.39.1.1-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mahadevan S., Sauer F., Erfle J. D. Studies on bovine rumen bacterial urease. J Anim Sci. 1976 Mar;42(3):745–753. doi: 10.2527/jas1976.423745x. [DOI] [PubMed] [Google Scholar]
- Pearson R. M., Smith J. A. The utilization of urea in the bovine rumen. 2. The conversion of urea to ammonia. Biochem J. 1943 Apr;37(1):148–153. doi: 10.1042/bj0370148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Levenberg B. Urea amidolyase. I. Properties of the enzyme from Candida utilis. J Biol Chem. 1972 Jul 10;247(13):4107–4113. [PubMed] [Google Scholar]
- Slyter L. L., Oltjen R. R., Kern D. L., Weaver J. M. Microbial species including ureolytic bacteria from the rumen of cattle fed purified diets. J Nutr. 1968 Feb;94(2):185–192. doi: 10.1093/jn/94.2.185. [DOI] [PubMed] [Google Scholar]
- Wong B. L., Shobe C. R. Single-step purification of urease by affinity chromatography. Can J Microbiol. 1974 Apr;20(4):623–630. doi: 10.1139/m74-095. [DOI] [PubMed] [Google Scholar]
- Zwaan J. Estimation of molecular weights of proteins by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Nov;21(2):155–168. doi: 10.1016/0003-2697(67)90177-7. [DOI] [PubMed] [Google Scholar]



