Abstract
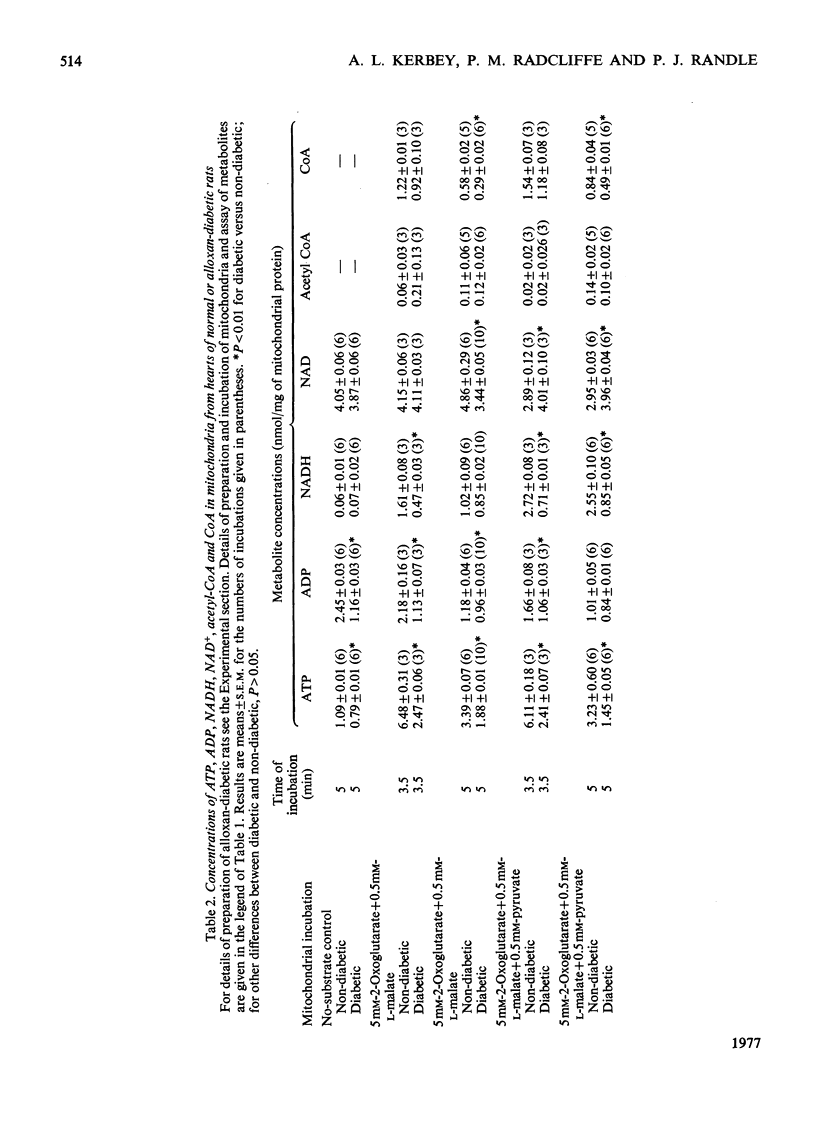
1. The proportion of active (dephosphorylated) pyruvate dehydrogenase in rat heart mitochondria was correlated with total concentration ratios of ATP/ADP, NADH/NAD+ and acetyl-CoA/CoA. These metabolites were measured with ATP-dependent and NADH-dependent luciferases. 2. Increase in the concentration ratio of NADH/NAD+ at constant [ATP]/[ADP] and [acetyl-CoA]/[CoA] was associated with increased phosphorylation and inactivation of pyruvate dehydrogenase. This was based on comparison between mitochondria incubated with 0.4mM- or 1mM-succinate and mitochondria incubated with 0.4mM-succinate+/-rotenone. 3. Increase in the concentration ratio acetyl-CoA/CoA at constant [ATP]/[ADP] and [NADH][NAD+] was associated with increased phosphorylation and inactivation of pyruvate dehydrogenase. This was based on comparison between incubations in 50 micrometer-palmitotoyl-L-carnitine and in 250 micrometer-2-oxoglutarate +50 micrometer-L-malate. 4. These findings are consistent with activation of the pyruvate dehydrogenase kinase reaction by high ratios of [NADH]/[NAD+] and of [acetyl-CoA]/[CoA]. 5. Comparison between mitochondria from hearts of diabetic and non-diabetic rats shows that phosphorylation and inactivation of pyruvate dehydrogenase is enhanced in alloxan-diabetes by some factor other than concentration ratios of ATP/ADP, NADH/NAD+ or acetyl-CoA/CoA.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREMER J. Carnitine in intermediary metabolism. The metabolism of fatty acid esters of carnitine by mitochondria. J Biol Chem. 1962 Dec;237:3628–3632. [PubMed] [Google Scholar]
- Batenburg J. J., Olson M. S. Regulation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. J Biol Chem. 1976 Mar 10;251(5):1364–1370. [PubMed] [Google Scholar]
- Batenburg J. J., Olson M. S. The inactivation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. Biochem Biophys Res Commun. 1975 Sep 16;66(2):533–540. doi: 10.1016/0006-291x(75)90543-4. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Studies on the effects of coenzyme A-SH: acetyl coenzyme A, nicotinamide adenine dinucleotide: reduced nicotinamide adenine dinucleotide, and adenosine diphosphate: adenosine triphosphate ratios on the interconversion of active and inactive pyruvate dehydrogenase in isolated rat heart mitochondria. J Biol Chem. 1976 Sep 25;251(18):5483–5489. [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Walajtys E. I., Williamson J. R. Regulation of glutamate metabolism and interactions with the citric acid cycle in rat heart mitochondria. J Biol Chem. 1973 Oct 25;248(20):7171–7183. [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Tubbs P. K. The role of intermediates in mitochondrial fatty acid oxidation. Biochem J. 1975 Jul;150(1):77–88. doi: 10.1042/bj1500077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E. Determination of subpicomole levels of NADH and FMN using bacterial luciferase and the liquid scintillation spectrometer. Anal Biochem. 1971 Feb;39(2):441–453. doi: 10.1016/0003-2697(71)90434-9. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- TABOR H., MEHLER A. H., STADTMAN E. R. The enzymatic acetylation of amines. J Biol Chem. 1953 Sep;204(1):127–138. [PubMed] [Google Scholar]
- Walsh D. A., Cooper R. H., Denton R. M., Bridges B. J., Randle P. J. The elementary reactions of the pig heart pyruvate dehydrogenase complex. A study of the inhibition by phosphorylation. Biochem J. 1976 Jul 1;157(1):41–67. doi: 10.1042/bj1570041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H. On the mechanism of irreversible pyruvate dehydrogenase inactivation in liver mitochondrial extracts. FEBS Lett. 1975 Mar 15;52(1):44–47. doi: 10.1016/0014-5793(75)80634-x. [DOI] [PubMed] [Google Scholar]


