Abstract
Although it is well established that CD4+ T cells are required for the protective immune response against tuberculosis (TB), there is some evidence that CD8+ T cells are also involved in the host response to Mycobacterium tuberculosis. There is, however, a paucity of information on the pulmonary CD8+ T-cell response during infection. We therefore have compared the changes in both CD8+ and CD4+ T cells following aerosol infection with M. tuberculosis. There was an observed delay between the peak of infection and the activated T-cell response in the lung. The kinetics of CD8+ and CD4+ T-cell responses in the lung were identical, both peaking at week 8, 4 weeks later than the peak of cellular response in draining lymph nodes. Similar changes in activation/memory phenotypes occurred on the pulmonary CD8+ and CD4+ T cells. Following in vitro restimulation, both subsets synthesized gamma interferon, a cytokine essential for controlling M. tuberculosis infection. Since lung CD8+ T cells are actively expanded during aerosol M. tuberculosis infection, it is important that both CD8+ and CD4+ T cells be targeted in the design of future TB vaccines.
The major protective immune response against intracellular bacteria, such as Mycobacterium tuberculosis, is cell-mediated immunity (26). It has been well established that CD4+ T cells are the dominant protective T cells (1, 10), but there is evidence that CD8+ T cells also have a role in the response against mycobacteria. Mice deficient in CD8+ T cells are unable to control M. tuberculosis infection (13, 18), and cell transfer experiments have shown that immune CD8+ as well as CD4+ T cells can transfer protective immunity (23, 31, 34). Other studies suggest that CD8+ T cells may contribute to the resolution of infection by the production of gamma interferon (IFN-γ) and cytolysis of infected cells (11, 30, 40).
Changes in cell surface activation markers define specific phases of T-cell activation and may distinguish between naive, effector, and memory T-cell populations (39). Resting naive T cells express low levels of CD44 and the integrins LFA-1 and VLA-4 and high levels of CD45RB and CD62L (L-selectin). Upon antigen stimulation, naive T cells transform to large blastoid cells, and the phenotype of these effector cells becomes CD44hi LFA-1hi VLA-4hi CD45RBlo CD62L− (12, 39). Other activation markers such as CD25 and CD69 are also upregulated (29, 38). The importance of CD4+ T cells bearing activation/memory markers in expressing effective immune response against mycobacterial infection has been reported for both humans and mice (2, 3, 19). Previous studies on the nature of the cellular infiltrate in murine tuberculosis (TB) have focused on changes in the spleen after intravenous infection rather than the lung, the site of natural infection.
We have examined changes in CD8+ as well as CD4+ T cells in lungs following aerosol infection and compared them over a 12-week period. Our results show that CD8+ T cells undergo phenotypic and functional changes comparable to those in CD4+ T cells during the pulmonary infection with M. tuberculosis.
MATERIALS AND METHODS
Mice.
C57BL/6 female mice were supplied by ARC (Perth, Western Australia, Australia) and were maintained in specific-pathogen-free conditions at the Centenary Institute animal facility until infection with M. tuberculosis, when they were transferred and maintained in a level 3 physical containment facility. Mice were used between 6 and 8 weeks of age.
Bacteria and aerosol infection.
M. tuberculosis H37Rv (ATCC 27294) was grown in Proskauer-Beck liquid medium (Difco, Detroit, Mich.) for 14 days at 37°C. The bacteria were washed and then enumerated on supplemented Middlebrook 7H11 agar (Difco). Mice were exposed to M. tuberculosis H37Rv in a Middlebrook airborne infection apparatus (Glas-Col, Terre Haute, Ind.) at a predetermined infective dose. Approximately 100 viable bacilli were delivered to the lungs of each mouse, as determined by culture of lung homogenates 24 h later.
Antibodies.
The following monoclonal antibodies (MAbs) were used for flow cytometry. Anti-CD44-fluorescein isothiocyanate (FITC), anti-CD49d (α4 integrin)-FITC, anti-CD69-FITC, anti-CD45RB-phycoerythrin (PE), anti-CD11a-PE, and anti-β7 integrin-PE were purchased from Pharmingen (San Diego, Calif.). Anti-CD62-PE, anti-CD4-tricolor, anti-CD8-tricolor, and isotype control antibodies were purchased from Caltag (San Francisco, Calif.).
Preparation of single-cell suspensions from lung and lymphoid organs.
Animals were sacrificed by carbon dioxide narcosis. The lungs were perfused with heparin (Fisons Pharmaceuticals, New South Wales, Australia), 20 U/ml in phosphate-buffered saline. Lung tissues were minced and then incubated for 90 min at 37°C with shaking in complete medium (5 ml/lung) supplemented with collagenase I (50 U/ml; type 4197; Worthington, Freehold, N.J.) and DNase I (13 μg/ml; Boehringer, Mannheim, Germany). After incubation, a single-cell suspension was collected by removing large aggregates and debris by passage though a 100-μm-pore-size mesh. A single-cell suspension from lymphoid organs was obtained by passing spleens or lymph nodes through a stainless steel sieve, and erythrocytes in the spleen suspensions were lysed in a hypotonic buffer.
Cell surface staining and flow cytometry.
Cells were stained for 20 min on ice then washed in FACS (fluorescence-activated cell sorting) buffer (2% bovine serum albumin and 0.1% NaN3 in phosphate-buffered saline). The data were collected by using a FACScan with CELL-Quest program and analyzed with the same program (Becton Dickinson, Mountain View, Calif.). Total lymphocyte number was obtained by multiplying the number of viable cells by the percentage of lymphocytes as determined by forward and side scatter. The number of CD4+ and CD8+ T cells was further calculated by multiplying the number of lymphocytes by percentage of each T-cell subset. The quantitation of positively stained population was based on samples stained with isotype control antibodies.
In vitro stimulation of T cells.
Lung cells were prepared by incubating lung single-cell suspensions in a six-well plate to remove macrophages. After 1 h of incubation at 37°C, nonadhering cells (106/ml) were stimulated with plate-bound anti-CD3 MAb (10 μg/ml; Pharmingen) for 16 h in complete RPMI (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 10 mM HEPES, 0.5 μM 2-mercaptoethanol, 100 U of penicillin per ml, and 100 μg of streptomycin per ml).
Intracellular staining for IFN-γ.
Brefeldin A (10 μg/ml; Sigma, St. Louis, Mo.) was added to the culture for the final 4 h of culture. Cells were washed and surface stained with rat anti-mouse CD4 or CD8 MAb (Caltag). The cells were fixed and permeabilized by using a Cytotofix/Cytoperm kit as instructed by the manufacturer (Pharmingen). Briefly, cells were resuspended in a fix buffer for 20 min at room temperature and then washed with permeabilization buffer. Cells were then stained with anti-IFN-γ-FITC (AN18) in permeabilization buffer at 4°C for 30 min. Cells were washed in permeabilization buffer, resuspended in FACS buffer, and analyzed on a FACScan flow cytometer (Becton Dickinson).
RESULTS
Kinetics of cellular response in lungs, MLN and spleens.
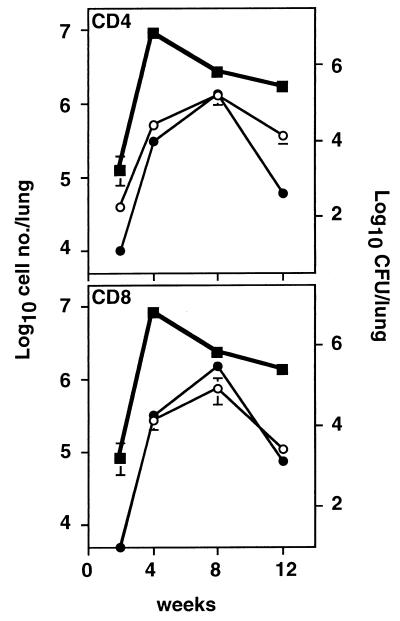
The kinetics of T-cell recruitment and proliferation differed between the three organs. The number of T cells in the lung and mediastinal lymph nodes (MLN) had increased 5- to 10-fold by 4 weeks postinfection (Fig. 1). This cellular influx in the infected lung peaked at week 8 and began to declined by week 12. By contrast, the cell expansion in the MLN peaked earlier at week 4 and was maintained until 12 weeks postinfection, suggesting that the MLN is the site of induction for T-cell activation. There was a small, gradual increase in T-cell number in the spleen over 12 weeks. Both CD4+ and CD8+ T cells were more abundant, and there was no preferential accumulation of either subset in the three organs at the time points examined (Fig. 1).
FIG. 1.
Kinetics of cellular responses in the lung, MLN, and spleen. Cell numbers in the lung, MLN, and spleen were examined at 0, 4, 8, and 12 weeks after aerosol M. tuberculosis infection. Data are presented as the mean and standard error for three mice in one of two separate experiments. The significance of the differences in cell number between weeks 4 and 8 postinfection was determined by unpaired Student’s t test. The number of CD4+ and CD8+ T cells in the lung at 8 weeks was significantly greater (P < 0.05) than at 4 weeks, while there was no significant difference in the numbers of CD4+ and CD8+ T cells in MLN between 4 and 8 weeks.
Changes in activation markers on T cells during infection.
To investigate whether CD8+ T cells have patterns of activation markers similar to those of CD4+ T cells, we compared the levels of expression of activation/homing markers on the two subsets in the three organs from infected and age-matched control mice. The proportions of CD4+ and CD8+ T cells expressing activated phenotypes in the infected lungs were significantly greater than in control mice, although the changes for CD8+ T cells were not as dramatic as those for CD4+ T cells (Fig. 2).
FIG. 2.
Change in the expression of activation markers on CD4+ and CD8+ T cells in infected lungs. Cells isolated from the lung at week 8 postinfection were analyzed by multiparameter FACS. The histogram profiles show the expression of activation markers after gating on lymphocytes and CD4+ and CD8+ T cells. The profiles are representative of three to six mice. Numbers in the histograms indicate percentages of CD4+ and CD8+ T cells for each molecule. The differences between control and infected mice were significant for all markers analyzed (P < 0.05, unpaired Student’s t test).
The two subsets exhibited comparable patterns of expression, with few exceptions. The levels of CD45RB expressed on CD8+ T cells were more heterogeneous than those on CD4+ T cells, consistent with previous reports (5, 6). Since β7 has been implicated as a component of mucosa-related integrin (37), we examined the expression of this molecule in the lungs of control and infected mice. In contrast to the majority of CD4+ T cells, which expressed low level of β7 integrin, half of the CD8+ T cells expressed high levels of β7 in uninfected lungs. A dramatic change was observed following infection. While the majority of CD4+ and CD8+ T cells expressed no or a low level of β7 integrin, minor populations of CD4+ and CD8+ T cells which expressed higher levels of β7 emerged during infection. A similar change of β7 did not occur on T cells in MLN and spleen throughout the course of infection (data not shown).
To investigate whether the increase in cell numbers was due to the preferential accumulation of activated T cells following infection, activation/memory markers were examined on CD4+ and CD8+ T cells over the course of infection. Cells which had an upregulated level of CD69 or which showed dual expression of CD44hi and CD45RBlo markers were defined as activated cells. There was no significant difference in both percentage and absolute number of activated T cells in lung, MLN, and spleen between control mice and mice at 2 weeks postinfection. The maximum number of organisms (CFU) in lung was observed at week 4, whereas the number of activated lung T cells bearing these markers did not peak until week 8. The total number of activated lung CD4+ and CD8+ T cells decreased by week 12 (Fig. 3). Compared to the lung, the change in the majority of surface markers on T cells in MLN and spleens of infected mice was minimal although a substantial rise in the absolute number of activated (CD44hi CD45RBlo and CD69+) CD4+ and CD8+ T cells occurred in MLN (Fig. 4), owing to the increased cellularity of MLN following infection (Fig. 1). A significant downregulation of CD62L, however, occurred on T cells in both infected lungs and MLN (Fig. 5), and by week 12 of infection, more than 90% CD4+ and 70% of CD8+ lung T cells had downregulated CD62L.
FIG. 3.
Delay between the peak of infection (CFU) and accumulation of activated CD4+ and CD8+ T cells in infected lungs. The course of infection, expressed as CFU (■), was compared with the kinetics of influx of activated T cells. The expression of CD44hi CD45RBlo (○) or CD69+ (●) was used to define the activated CD4+ and CD8+ T cells. Data are presented as the mean and standard error for three mice from one of two separate experiments.
FIG. 4.
Change in the expression of activation markers on CD4+ and CD8+ T cells in MLN. Cells isolated from the MLN at weeks 2, 4, 8, and 12 postinfection were analyzed by multiparameter FACS. The expression of CD44hi CD45RBlo or CD69+ was used to define the activated CD4+ and CD8+ T cells. Data are presented as the mean and standard errors for three mice from one of two separate experiments. The significance of the differences in each subset between control mice and mice at 2, 4, 8, and 12 weeks postinfection was determined by unpaired Student’s t test. There were no significant differences in both percentage and absolute number of activated T cells between control mice and mice at 4, 8, and 12 weeks postinfection. The change in the proportion of activated T cells in either subset throughout the course of infection was not statistically different. There was a significant difference in absolute number of activated T cells between control mice and mice at 4, 8, and 12 weeks postinfection (P < 0.05).
FIG. 5.
Progressive loss of expression of CD62L on both CD4+ and CD8+ T cells. Cells from lungs and MLN were harvested at weeks 0, 4, 8, and 12 after aerosol infection. The CD62L expression on CD4+ and CD8+ T cells was analyzed after gating on viable lymphocytes. Data are presented as mean and standard error for three mice in one of two separate experiments. There were significant differences between control mice and mice at 4, 8, and 12 weeks postinfection in both lungs and MLN (P < 0.05).
The recruitment of T cells to nonlymphoid tissues such as the lung is dependent on the interaction between integrins on the T cells and their ligands on endothelial cells (7, 8). We therefore examined the expression of CD11a and CD49d, the respective α chains, of LFA-1 and VLA-4 on CD4 and CD8 T cells. Multiparameter FACS analysis demonstrated that both markers were upregulated (Fig. 2), suggesting the possible involvement of LFA-1–ICAM-1 or -2 and VLA-4–VCAM-1 interactions in the recruitment of activated T cells into M. tuberculosis-infected lungs. In agreement with a previous report on the changes of activation markers on spleen CD4+ T cell after intravenous infection (2), CD44hi CD45RBlo pulmonary T cells expressed high levels of CD11a (Fig. 6). There was also an accumulation of CD44hi and CD11ahi T cells in infected lungs.
FIG. 6.
Coexpression of CD44 and CD45RB and CD11a on CD4+ (A) and CD8+ (B) T cells. Cells isolated from lungs at week 8 after infection were analyzed as described for Fig. 2. The profiles shown are representative of three mice from one of two separate experiments. Numbers in the quadrants indicate percentages of CD4+ and CD8+ T cells.
Increase in the number of IFN-γ-producing T cells in infected lungs.
To examine the contribution of T cell subsets to IFN-γ production, lung cells from normal and infected mice were restimulated with anti-CD3 MAb for 16 h. Throughout the course of infection, the numbers of both CD4+ and CD8+ IFN-γ-producing cells were greater in infected lungs than in uninfected lungs (Fig. 7).
FIG. 7.
Recall IFN-γ production by CD4+ and CD8+ T cells in infected and uninfected mice. Lung cells isolated from mice 8 weeks after aerosol infection or from uninfected control mice were cultured at 106/ml with solid-phase anti-CD3 MAb for 24 h. Intracellular staining for IFN-γ was performed after surface staining of CD4 and CD8 molecules. The profiles shown are representative of three mice from one of two separate experiments. Numbers in the quadrants indicate percentages of total viable lymphocytes.
DISCUSSION
While the mouse model has been used extensively to study the immune response to M. tuberculosis infection, the phenotype of responding T cells is not fully defined. The majority of such studies have been conducted in intravenous models of infection (2, 19). After a sublethal intravenous injection of M. tuberculosis, the number of CD4+ T cells with CD44hi CD45RBlo phenotype peaked at 2 to 3 weeks in the spleens of infected mice and then gradually declined (19). In this model, the kinetics of T-cell responses mirrored the course of infection in the spleen, which peaked at week 3 after intravenous infection. This CD44hi CD45RBlo population has therefore been suggested to be involved in protective immune responses. Andersen et al. further extended this finding by demonstrating the involvement of this population in the memory CD4+ T-cell response in a rechallenge model (2). After primary infection, the mice were treated with chemotherapy and activation markers were downregulated to the resting state. When these immune mice were rechallenged with M. tuberculosis, an accelerated accumulation of CD44hi CD45RBlo CD4+ T cells occurred, with rapid production of high levels of IFN-γ by this population. However, analysis of changes in the CD8+ T-cell subset during an aerosol infection with M. tuberculosis has not been reported. Our study provides a comprehensive phenotypic and functional study of T-cell responses in the M. tuberculosis-infected lung, which is the primary site of natural infection.
The evidence presented here reveals similarities and differences between aerosol and intravenous models. In agreement with the pattern in spleen after intravenous injection, an expansion of activated CD4+ T cells developed in the lungs, and we further demonstrated that similar changes also occurred among the CD8+ T-cell subset (Fig. 2). A striking difference was the delay between the peak of infection and the activated T-cell response in the lung (Fig. 3). Several factors may be responsible for the differences between the aerosol and intravenous models. First, the size of the inoculum varies, as a higher dose of the bacteria is usually used in the intravenous model. The lower number of organisms delivered by the aerosol route may be less immunogenic than the higher number given by intravenous infection (33). Second, the levels of efficiency of the control of intracellular infection and subsequent presentation of mycobacterial antigens by macrophages in the lung and spleen may be different (33). Finally, the quantity and nature of professional antigen-presenting cells may vary between the lung and spleen (21). A similar pattern of T-cell responses in the lung and MLN was also observed in an aerosol M. bovis BCG infection model (34a).
In contrast to a previous report that the CD8+ T-cell response in the intravenous model peaked later than the CD4+ T cell response (34), the kinetics of CD4+ and CD8+ T-cell responses in the lung appeared similar, both peaking around week 8 of infection. Therefore, as with CD4+ T cells, CD8+ T cells can be primed and activated during M. tuberculosis infection. This activation led to the phenotypic changes observed on the CD8+ cells. The resulting upregulation of activation/homing markers may allow these activated CD8+ T cells to accumulate at the site of inflammation. We cannot rule out that some degree of bystander activation for CD8+ T cells may have occurred, but it is unlikely that nonspecific activation accounts for the majority of activated CD8+ T cells present in the lung. In a model of lymphocytic choriomeningitis virus infection, 50 to 70% of the activated CD8+ T cells were antigen specific (32). This finding questions the traditional view of the magnitude of the bystander effects (41).
In mice, the inhibition of mycobacterial growth by macrophages activated by IFN-γ has been well established (16, 17, 35). CD8+ T cells from IFN-γ-deficient mice failed to transfer protective immunity against M. tuberculosis infection, demonstrating IFN-γ was essential for CD8+ T-cell-mediated protective immunity (40). Following anti-CD3 stimulation in vitro, which has previously been shown to activate cytokine production from in vivo-primed cells (9, 14, 15, 28), the number of IFN-γ-producing CD4+ and CD8+ T cells from M. tuberculosis-infected lungs was greater than that in uninfected lungs (Fig. 7); this result indicates that both subsets appeared primed and predisposed to produce IFN-γ, a crucial cytokine for the protective immunity against mycobacterial infection.
CD62L has been widely used in the study of T-cell trafficking, and activated CD62L− T cells have been shown to migrate to sites of inflammation. It has been suggested that the majority of virus-specific cytotoxic T-lymphocyte precursors generated in MLN were CD62L−, and they differentiated into cytotoxic T-lymphocyte effectors after migrating to the site of infection (22). At week 12 after aerosol M. tuberculosis infection, the expression of this marker on both CD4+ and CD8+ T cells was downregulated in the lung (Fig. 4), suggesting that there was an accumulation of activated/memory T cells in the local tissue. Interestingly, the percentage of CD62L− T cells in MLN also began to rise at the later stage of the infection. This may result from local T-cell activation due to the spread of infection to MLN or homing of a population of memory cells to the local lymph nodes. The binding of the CD62L− population to high endothelial venules in the lymph nodes is probably mediated by LFA-1 (20).
TB is a chronic infectious disease characterized by the infiltration of lymphocytes to inflamed tissues. Integrins have a major role in leukocyte-endothelial cell interactions which are important for migration of activated T cells to nonlymphoid tissues (7, 8). The T-cell homing to local tissues mediated by the integrins LFA-1 and VLA-4 has been demonstrated in many chronic inflammatory situations, such as inflamed skin (25), arthritic joints (24, 36), and glomerulonephritis (27). The expansion of CD11ahi and CD49dhi T cells in lung observed in this study (Fig. 2) suggests that these integrins may also contribute to the recruitment of activated T cells to inflamed lungs. Similar to the data presented here, CD44hi LFA-1hi T cells also expanded dramatically in lung during an influenza virus infection (4). The expansion of the T-cell population correlated with increased cytokine production in that model (4), suggesting that these cells may well be the major cytokine-producing cells in the M. tuberculosis-infected lung.
In conclusion, both CD4+ and CD8+ T cells respond to M. tuberculosis infection in the lung, with maximal activation and expansion of both subsets observed 4 weeks after the peak of infection. The CD8+ T-cell response is comparable to that of CD4+ T cells, both phenotypically and functionally, suggesting that CD8+ T cells contribute to the protective immune response against M. tuberculosis. The potential effects of CD8+ T cells warrant strategies for stimulating them in the design of future TB vaccines.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council of Australia. C.G.F. and H.H. are recipients of Australian postgraduate awards.
REFERENCES
- 1.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 3.Barnes P F, Mistry S D, Cooper C L, Pirmez C, Rea T H, Modlin R L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 4.Baumgarth N, Egerton M, Kelso A. Activated T cells from draining lymph nodes and an effector site differ in their responses to TCR stimulation. J Immunol. 1997;159:1182–1191. [PubMed] [Google Scholar]
- 5.Baumgarth N, Kelso A. Functionally distinct T cells in three compartments of the respiratory tract after influenza virus infection. Eur J Immunol. 1996;26:2189–2197. doi: 10.1002/eji.1830260934. [DOI] [PubMed] [Google Scholar]
- 6.Birkeland M L, Johnson P, Trowbridge I S, Pure E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci USA. 1989;86:6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley L M, Watson S R. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr Opin Immunol. 1996;8:312–20. doi: 10.1016/s0952-7915(96)80118-x. [DOI] [PubMed] [Google Scholar]
- 8.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 9.Christensen J P, Stenvang J P, Marker O, Thomsen A R. Characterization of virus-primed CD8+ T cells with a type 1 cytokine profile. Int Immunol. 1996;8:1453–1461. doi: 10.1093/intimm/8.9.1453. [DOI] [PubMed] [Google Scholar]
- 10.Cooper A M, Flynn J L. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 11.De Libero G, Flesch I, Kaufmann S H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 12.Doherty P C. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. doi: 10.1007/978-3-642-85208-4_1. [DOI] [PubMed] [Google Scholar]
- 13.D’Sousa C D, Cooper A M, Frunk A A, Orme I M. The role of CD8 cells in acquired immunity and pulmonary tuberculosis in the mouse models, p. 47. TB molecular methods and immunological aspects. Silverthorne, Colo: Keystone Symposia; 1998. [Google Scholar]
- 14.Dubey C, Croft M, Swain S L. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 15.Emoto M, Emoto Y, Kaufmann S H. Bacille Calmette Guerin and interleukin-12 down-modulate interleukin-4-producing CD4+ NK1+ T lymphocytes. Eur J Immunol. 1997;27:183–188. doi: 10.1002/eji.1830270127. [DOI] [PubMed] [Google Scholar]
- 16.Fenton M J, Vermeulen M W, Kim S, Burdick M, Strieter R M, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flesch I, Kaufmann S H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987;138:4408–4413. [PubMed] [Google Scholar]
- 18.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin J P, Orme I M. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect Immun. 1994;62:1683–1690. doi: 10.1128/iai.62.5.1683-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher E C, Baisch H, Harder R, Thiele H G. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 21.Holt P G, Oliver J, Bilyk N, McMenamin C, McMenamin P G, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou S, Doherty P C. Partitioning of responder CD8+ T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J Immunol. 1993;150:5494–5500. [PubMed] [Google Scholar]
- 23.Hubbard R D, Flory C M, Collins F M. Memory T-cell-mediated resistance to Mycobacterium tuberculosis infection in innately susceptible and resistant mice. Infect Immun. 1991;59:2012–2016. doi: 10.1128/iai.59.6.2012-2016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iigo Y, Takashi T, Tamatani T, Miyasaka M, Higashida T, Yagita H, Okumura K, Tsukada W. ICAM-1-dependent pathway is critically involved in the pathogenesis of adjuvant arthritis in rats. J Immunol. 1991;147:4167–4171. [PubMed] [Google Scholar]
- 25.Issekutz T B. Inhibition of lymphocyte endothelial adhesion and in vivo lymphocyte migration to cutaneous inflammation by TA-3, a new monoclonal antibody to rat LFA-1. J Immunol. 1992;149:3394–3402. [PubMed] [Google Scholar]
- 26.Kaufmann S H. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–342. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki K, Yaoita E, Yamamoto T, Tamatani T, Miyasaka M, Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993;150:1074–1083. [PubMed] [Google Scholar]
- 28.Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990;145:2167–2176. [PubMed] [Google Scholar]
- 29.Leonard W J, Gnarra J R, Napolitano M, Sharon M. Structure, function, and regulation of the interleukin-2 receptor and identification of a novel immune activation gene. Philos Trans R Soc Lond Ser B. 1990;327:187–192. doi: 10.1098/rstb.1990.0053. [DOI] [PubMed] [Google Scholar]
- 30.Mohagheghpour N, Gammon D, Kawamura L M, van Vollenhoven A, Benike C J, Engleman E G. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400–2406. [PubMed] [Google Scholar]
- 31.Muller I, Cobbold S P, Waldmann H, Kaufmann S H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 33.North R J. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J Infect Dis. 1995;172:1550–1553. doi: 10.1093/infdis/172.6.1550. [DOI] [PubMed] [Google Scholar]
- 34.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 34a.Palendira, U. Personal communication.
- 35.Rook G A, Steele J, Ainsworth M, Champion B R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59:333–338. [PMC free article] [PubMed] [Google Scholar]
- 36.Salmi M, Andrew D P, Butcher E C, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181:137–149. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw S K, Brenner M B. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 38.Swain S L, Bradley L M. Helper T cell memory: more questions than answers. Semin Immunol. 1992;4:59–68. [PubMed] [Google Scholar]
- 39.Swain S L, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley L M. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 40.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tough D F, Sprent J. Viruses and T cell turnover: evidence for bystander proliferation. Immunol Rev. 1996;150:129–142. doi: 10.1111/j.1600-065x.1996.tb00699.x. [DOI] [PubMed] [Google Scholar]