Abstract
The rapid modulation of ligand-binding affinity (“activation”) is a central property of the integrin family of cell adhesion receptors. The Ras family of small GTP-binding proteins and their downstream effectors are key players in regulating integrin activation. H-Ras can suppress integrin activation in fibroblasts via its downstream effector kinase, Raf-1. In contrast, to H-Ras, a closely related small GTP-binding protein R-Ras has the opposite activity, and promotes integrin activation. To gain insight into the regulation of integrin activation by Ras GTPases, we created a series of H-Ras/R-Ras chimeras. We found that a 35-amino acid stretch of H-Ras was required for full suppressive activity. Furthermore, the suppressive chimeras were weak activators of the ERK1/2 MAP kinase pathway, suggesting that the suppression of integrin activation may be independent of the activation of the bulk of ERK MAP kinase. Additional data demonstrating that the ability of H-Ras or Raf-1 to suppress integrin activation was unaffected by inhibition of bulk ERK1/2 MAP kinase activation supported this hypothesis. Thus, the suppression of integrin activation is a Raf kinase induced regulatory event that can be mediated independently of bulk activation of the ERK MAP-kinase pathway.
INTRODUCTION
Interactions of integrin cell adhesion receptors with their extracellular ligands are important for cell migration, growth, and survival (Schwartz et al., 1995; Schwartz, 1997; Clark et al., 1998). A characteristic feature of many integrins is their ability to alter their affinity for ligands in response to intracellular signals, a process termed “activation”(Hughes and Pfaff, 1998).
Presently, the signal transduction cascades controlling integrin activation are incompletely understood (Hughes and Pfaff, 1998). However, several observations suggest that members of the Ras family of small GTP-binding proteins and their downstream effectors are critically involved in the regulation of integrin activation (Zhang et al., 1996; Hughes et al., 1997; Reedquist et al., 2000). Activated H-Ras can suppress the activation of certain β1 and β3 integrins in fibroblasts via its effector serine/threonine kinase Raf-1 (Hughes et al., 1997). This activity of H-Ras is implicated in the control of cell morphology, cell movement, and assembly of the extracellular matrix (Hughes et al., 1997; Brenner et al., 2000). The suppressive activity of H-Ras does not require protein synthesis or mRNA transcription, and furthermore, suppression can be reversed by MAP kinase phosphatase 1 (Hughes et al., 1997). Thus, suppression appears to be mediated by a MAP kinase and correlates with activation of the ERK1/2 MAP kinase pathway.
In contrast, to H-Ras other closely related small GTP-binding proteins, such as R-Ras and Rap1, have the opposite activity, promoting rather than suppressing integrin activation (Zhang et al., 1996; Osada et al., 1999; Caron et al., 2000; Reedquist et al., 2000; Shimizu, 2000). In CHO cells, activated R-Ras can antagonize the Ras/Raf suppressor pathway, and in fibroblasts, myeloid cells and bone marrow–derived mast cells activated R-Ras stimulates integrin activation and integrin-dependent adhesion (Zhang et al., 1996; Osada et al., 1999; Sethi et al., 1999; Kinashi et al., 2000). Activation of PI 3-kinase is involved in R-Ras stimulation of integrins in hematopoietic cells, but in fibroblasts, the critical effectors are as yet unidentified (Osada et al., 1999; Kinashi et al., 2000; Oertli et al., 2000). Thus, H-Ras and R-Ras have opposing effects on integrin activation in fibroblasts.
The exact mechanisms by which H-Ras and R-Ras exert their opposing effects on integrin function are uncertain. Indeed, both of these small GTPases have remarkably similar effector domains, and interact with many of the same downstream targets (Bos, 1998; Campbell et al., 1998; Reuther and Der, 2000). To gain insight into this question, we mapped the regions of H-Ras responsible for suppression of integrin activation by creating a series of H-Ras/R-Ras chimeras. We found that a 35-amino acid stretch of H-Ras encompassing residues 149–175 is required for full suppressive activity. Significantly, certain suppressive chimeras had little effect on the activation of ERK1/2 MAP kinases. Furthermore, blockade of ERK1/2 activation by either a pharmacological inhibitor of MEK kinase or by coexpression of MAP kinase phosphatase 3 (MKP3) did not alter the ability of H-Ras to suppress integrin activation. Thus, activation of the bulk of ERK1/2 is not required for integrin suppression. In addition, ERK activation per se is insufficient for integrin suppression because an activated variant of MEK1 was unable to suppress integrin activation. These results raise the possibilities that Raf-1 can activate ERK1/2- and MEK1/2-independent pathways to suppress integrins. Alternatively, our data do not eliminate the possibility that a small pool of ERK1/2, acting at a discrete subcellular location, mediates integrin suppression.
MATERIALS AND METHODS
Antibodies and Reagents
The activation-dependent anti-αIIbβ3 mAb, PAC1, and activating antibody, anti-LIBS6, have previously been described (Shattil et al., 1985; Frelinger et al., 1990). The anti-Tac antibody 7G7B6 was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and was biotinylated with biotin-N-hydroxysuccinimide (Sigma, St. Louis, MO). The αIIbβ3 specific peptidomimetic inhibitor Ro43–5054 was a generous gift of Dr. Beat Steiner (Hoffmann-LaRoche, Basel, Switzerland). The MEK kinase inhibitor U0126 was obtained from Promega (Madison, WI) and used according to the manufacturer's instructions. 4-Hydroxy tamoxifen (4′OHT) was obtained from Sigma (St. Louis, MO) and used at a final concentration of 300 nM
cDNA Constructs, Cell Lines, and Transfection
The mammalian expression vectors encoding H-Ras(G12V), R-Ras(G38V), and HA-ERK2 have been described previously (Hughes et al., 1997; Sethi et al., 1999). The H-Ras/R-Ras chimeras were constructed using splice overlap PCR mutagenesis with pSG5-R-Ras(G38V) and pcDR-H-Ras(G12V) as templates. The amplified DNA was ligated into the EcoRI site of pcDNA3.1 (Invitrogen, San Diego, CA). All chimeras and mutant constructs were verified by DNA sequencing before further analysis. The mammalian expression vectors pMCL- MEK1(ΔN3, S222D) and pSG5-MKP3 were generous gifts of Dr. N. Ahn (Howard Hughes Medical Institute, University of Colorado, Boulder, CO) and Dr. Steven Keyse (ICRF Molecular Pharmacology Unit, Ninewells Hospital, Dundee, United Kingdom), respectively. The plasmid pDCR-H-Ras(G12V) was a gift from Dr. M.H. Wigler (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) and pSG5-R-Ras(G38V) was generously provided by Dr. Julian Downward, (Signal Transduction Laboratory, ICRF, London, United Kingdom). PMX-Raf-1:ER vector was obtained from Martin McMahon (University of California, San Francisco, CA). PMX-Raf-1:ER contains a mutated form of the mouse estradiol receptor-binding domain that is sensitive to 4′OHT, but insensitive to 17-β-estradiol and Phenol Red in the cell culture medium (Danielian et al., 1993). The PMX-RAF-1:ER vector express Raf-1:ER and eGFP (enhanced green fluorescent protein) from a single bicistronic mRNA with the translation of the 5′ coding region for eGFP promoted by the presence of an internal ribosomal entry site (IRES) from encephalomyocarditis virus. The pGEX expression vector encoding the central cell-binding domain of fibronectin as a GST-fusion protein has been described previously (Ramos and DeSimone, 1996). GST-fusion proteins were produced as described (Ramos and DeSimone, 1996). Chinese Hamster Ovary (CHO)-K1 cells were obtained from the ATCC (American Type Culture Collection). The generation of CHO αβ-py cells has been described previously (Baker et al., 1997). These cells stably express the polyoma large T antigen and bear a recombinant chimeric integrin that has the extracellular and transmembrane domains of integrin αIIbβ3 joined to the cytoplasmic domains of integrin α6Aβ1A (αIIbα6Aβ3β1). All cells were cultured in DMEM (BioWhittaker, Walkersville, MD) containing 10% FCS, 1% nonessential amino acids, 2 mM glutamine (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin. Raf-1:ER cells were generated by cotransfecting CHO cells with PMX-RAF-1:ER and a G418 resistance vector. After selection with G418, GFP-expressing cells were isolated by FACS.
Flow Cytometry
PAC1 binding was measured by two-color flow-cytometry as described previously (O'Toole et al., 1994; Hughes et al., 1996). Briefly, 48 h after transfection cells were harvested by a brief trypsinization and washed in DMEM/1% BSA. Cells, 5 × 105, were incubated with 0.1% PAC1 ascites in the presence of the competitive inhibitor Ro43–5054 at 1 μM or anti-LIBS6 ascites. After 30-min incubation at room temperature cells were washed with cold DMEM/1% BSA and incubated with the biotinylated anti-Tac antibody 7G7B6 for 30 min on ice. After washing, cells were incubated with 10% FITC-conjugated goat anti-mouse IgM (TAGO) and 4% phycoerythrin-streptavidin (Molecular Probes Inc., Eugene, OR) for another 30 min on ice. Cells were washed in ice-cold PBS and resuspended in PBS. Then cells were analyzed on a FACScan (Becton Dickinson, Mountain View, CA) flow cytometer as described (Hughes et al., 1997), and the collected data were analyzed using CellQuest software (Becton Dickinson). To obtain numerical estimates of integrin activation, we calculated an activation index (AI), defined as 100 × (Fo − Fr)/(FoLIBS6 − Fr), where Fo is the median fluorescence intensity (MFI) of PAC1 binding; Fr is the MFI of PAC1 binding in the presence of competitive inhibitor (Ro43–5054, 1 μM); and FoLIBS6 is the MFI of PAC1 binding in the presence of 2 μM anti-LIBS6 (Hughes et al., 1996). The percentage inhibition was calculated as 100(AIo − AI)/AIo, where AIo is the activation index in the absence of the cotransfected test cDNA and AI is the activation index in its presence.
FN 9–11 binding was assayed by two-color flow cytometry. Cells were harvested by a brief trypsinization, followed by neutralization with the addition of complete media. Cells were then pelleted by a brief centrifugation, washed, and resuspended in Tyrode's buffer. The harvested cells were then aliquoted into three pools containing either Tyrode's buffer alone, Tyrode's buffer plus 5 mM EDTA, and Tyrode's buffer plus the activating anti-β1 mAb 9EG7 (10 μg/ml; PharMingen, San Diego, CA). The cells were then incubated for 15 min at room temperature. After the addition of biotinylated GST FN 9–11 the cells were incubated at room temperature for an additional 15 min. After washing in ice cold Tyrode's, the cells were incubated on ice for 30 min with 4% phycoerythrin-streptavidin (Molecular Probes Inc.). The cells were then washed in ice cold Tyrode's and analyzed on a FACScan (Becton Dickinson) flow cytometer. The collected data were analyzed using CellQuest software (Becton Dickinson).
Measurement of ERK Phosphorylation
CHO cells were transfected using Lipofectamine (Life Technologies, Rockville, MD) as described (Hughes et al., 1996). Transfections were performed in duplicate to allow for parallel analysis of both ERK phosphorylation and PAC1 binding by flow cytometry. Twenty-four hours after transfection the cells were washed and placed in medium containing 0.5% fetal calf serum. Forty-eight hours after transfection cells were washed and lysed in a buffer containing a mixture of protease and phosphatase inhibitors (Hughes et al., 1997). Phosphorylated ERK was detected by fractionating 20 μg of whole cell lysate on a 4–20% SDS-polyacrylamide gels, transferring to nitrocellulose membranes, and immunoblotting with an mAb that recognizes only the phosphorylated forms of ERK1 and ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA). To determine the total amount of ERK present in each of the lysates, the blots were then stripped and immunoblotted with either polyclonal antibodies recognizing ERK1 and ERK2 (Santa Cruz Biotechnology) or the mAb 12CA5 to detect HA-ERK2.
RESULTS
Residues 148–171 of H-Ras Are Required for Suppression of Integrin Activation
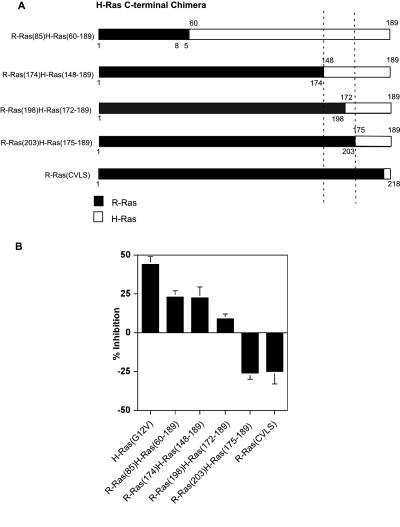
Despite their sequence similarity, the small GTP binding protein R-Ras and H-Ras have opposing effects on integrin activation in CHO cells (Figure 1, A and B; Sethi et al., 1999). H-Ras suppresses integrin activation as assessed by reduction in the binding of the activation-specific mAb, PAC1 to CHO cells expressing the active chimeric integrin, αIIbα6Aβ3β1. In contrast, activated R-Ras does not suppress, but instead reverses Ras-initiated suppression (Figure 1B) through the activation of an as yet unidentified effector. To map the region(s) of H-Ras responsible for suppressing integrin activation, we generated a series of chimeric proteins composed of portions of the C terminus of H-Ras fused to the N-terminus of R-Ras, and reciprocal chimeras composed of portions of the C terminus of R-Ras fused to the N-terminus of H-Ras (Figures 2A and 3A). All chimeras contained either an activating H-Ras(G12V) or R-Ras(G38V) mutations to ensure they were in a GTP-bound state and thus able to engage downstream effectors.
Figure 1.
H-Ras and R-Ras are homologous small GTP-binding proteins with opposing effects on integrin activation. (A) Sequence alignment of H-Ras and R-Ras. asterisk, shared amino acids; arrows, sites where exchanges were made to generate each chimera. Shaded box, the amino acid residues responsible for effector binding; open box, the residues comprising the C-terminal prenylation motif. Prenylation differs between these two proteins; H-Ras is farnesylated, whereas R-Ras is predicted to be geranylgeranylated. (B) The effects of activated H-Ras and R-Ras on integrin activation in CHO cells. αβ-py cells, which express recombinant chimeric integrin αIIbα6Aβ3β1A, were transiently transfected with an expression vector encoding the transfection reporter Tac-α5 alone and Tac-α5 plus H-Ras(G12V). In a separate transfection, Tac-α5 plus H-Ras(G12V) was cotransfected with a plasmid encoding R-Ras(G38V). The cells were harvested and stained for Tac expression (ordinate) to identify transfected cells and PAC1 binding (abscissa) to assess the activation state of the recombinant αIIbα6Aβ3β1A. In the H-Ras(G12V)-transfected cells there is a leftward shift of the dot plot in the upper quadrants as a result of an inhibition of PAC1 binding. This shift is completely reversed by the cotransfection of activated R-Ras(G38V). In the empty vector control transfection there was no suppression of PAC1 binding in the Tac-α5-expressing (transfected) cells.
Figure 2.
Analysis of H-Ras C-terminal chimeras indicates that residues 148–171 of H-Ras are critical for the suppression of integrin activation. (A) A schematic representation of the H-Ras C-terminal chimeras. Open box, H-Ras sequences; filled box, the R-Ras portion of each chimera. (β) αβ-py cells were transiently transfected in duplicate with either a control expression vector or vectors encoding either H-Ras(G12V) or the indicated chimera. After 48 h, integrin activation was measure by PAC1 binding. Depicted is the mean percent inhibition of integrin activation relative to that of the empty vector ±SEM of three independent determinations.
Analysis of both sets of H-Ras/R-Ras chimeras pinpointed residues 148–171 of H-Ras as critical sequences for suppression of integrin activation. Of the H-Ras C-terminal chimeras, all those containing the H-Ras C-terminal 42 residues suppressed PAC1 binding (Figure 2). In contrast, a chimera containing the last 15 residues of H-Ras, R-Ras(203)H-Ras(175–189), failed to suppress PAC1 binding. R-Ras in which the C-terminal prenylation sequence was replaced with that of H-Ras (R-RasCVLS) also did not suppress. Indeed, both R-Ras(203)H-Ras(175–189) and R-RasCVLS appeared to increase activation slightly (Figure 2B), suggesting that they retained R-Ras function. This hypothesis was confirmed by the finding that they could reverse the suppressive effects of H-Ras (our unpublished results). Thus, analysis of chimeras composed of varying portions of the C terminus of H-Ras indicates that sequences C-terminal of Lys147 are necessary for suppressive activity. Furthermore, the C terminus of H-Ras beginning with Asp175 is not sufficient to convey suppressive activity to R-Ras.
Chimeras composed of portions of the C terminus of R-Ras fused to the N-terminus of H-Ras also indicated the importance of residues 148–171 of H-Ras. Chimeras containing H-Ras sequences N-terminal of Leu171, H-Ras(171)R-Ras(199–218), H-Ras(174)R-Ras(204–218), and H-Ras(CVLL), all suppressed PAC1 binding (Figure 3B). In contrast, those chimeras containing H-Ras sequences N-terminal of Lys147 (H-Ras(147)R-Ras(175–218), H-Ras(59)R-Ras(86–218), lacked suppressive activity. Furthermore, both of these constructs seemed to increase activation slightly (Figure 3B) signifying that they retained R-Ras function. Indeed, these chimeras could reverse the suppressive activity of H-Ras (our unpublished results). Thus, the analysis of both the H-Ras and R-Ras C-terminal chimeras pinpointed residues 148–171 of H-Ras as those critical for suppression of integrin activation.
Figure 3.
Analysis of R-Ras C-terminal chimeras indicates the importance of H-Ras residues 148–171 for the suppression of integrin activation. (A) A schematic representation of the R-Ras C-terminal chimeras. Open box, H-Ras sequences, filled box, the R-Ras portion of each chimera. (β) αβ-py cells were transiently transfected in duplicate with either a control expression vector or vectors encoding either H-Ras(G12V) or the indicated chimera. After 48 h, integrin activation was determined by PAC1 binding. Depicted is the mean percent inhibition of integrin activation relative to that of the empty vector ±SEM of three independent determinations.
Suppression of Integrin Activation Does Not Correlate with ERK Activation
Suppression of integrin activation involves activation of a MAP kinase pathway and appeared to be due to the activation of the ERK1/2 MAP kinases (Hughes et al., 1997). Therefore, we examined capacity of the chimeras to activate ERK as assessed by reactivity with a phosphorylation-specific antibody. To our surprise, many of the suppressive chimeras activated ERK poorly (Figure 4). R-Ras(85)H-Ras(60–189) and R-Ras(174)H-Ras(148–189) were potent suppressors of PAC1 binding (Figure 2B), yet they had little effect on ERK phosphorylation. All chimeras were expressed to the same levels and in agreement with the data presented in Figure 4; none of them detectably stimulated ERK kinase activity (our unpublished observations). These data suggest that H-Ras–initiated suppression of integrin activation could occur independently of bulk ERK activation.
Figure 4.
The suppressive chimeras R-Ras(85)H-Ras(60–189) and R-Ras(174)H-Ras(148–189) have little effect on ERK phosphorylation. αβ-py cells were transiently transfected with an expression vector encoding HA-ERK2 alone or in combination with vectors encoding H-Ras(G12V), R-Ras(G38V), R-Ras(85)H-Ras(60–189), or R-Ras(174)H-Ras(148–189). Twenty-four hours after transfection the cells were placed in medium containing 0.5% FCS, and 48 h after transfection the cells were lysed and 30 μg of cell lysate was resolved on 4–20% SDS gel and transferred to a nitrocellulose membrane. The blots were probed with an mAb-specific for phosphorylated ERK (top panel), then stripped, and reprobed with an mAb (12CA5) to verify equal expression of HA-ERK2 (bottom panel).
To further test the idea that suppression could be independent of ERK activation, we blocked ERK activation and examined the ability of H-Ras(G12V) to suppress integrin activation. First, we tested the effect of coexpressing MAP kinase phosphatase 3 (MKP3), a phosphatase that specifically binds and dephosphorylates ERK1 and ERK2 (Muda et al., 1996; Keyse, 2000). Second, we used a MEK kinase inhibitor, U0126 (Favata et al., 1998), to block H-Ras–induced MEK activation, and thus, the activation of its downstream target kinases, ERK1 and ERK2. The ability of H-Ras(G12V) to suppress PAC1 binding was largely unaffected by the addition of U0126 or by coexpression of MKP3 (Figure 5A). Nevertheless, both of these treatments inhibited the bulk of ERK1/2 activation (Figure 5B). Thus, bulk ERK1/2 activation can be blocked without reducing the ability of H-Ras to suppress integrin activation.
Figure 5.
H-Ras–initiated suppression is not reversed by an inhibition of ERK MAP kinase activation. (A) αβ-py cells were transiently transfected with a vector encoding H-Ras(G12V). In a separate transfection, a plasmid encoding H-Ras(G12V) was cotransfected with one encoding MKP3. 24 h after transfection, the MEK inhibitor, U0126, was added where indicated at a final concentration of 25 μM. After 48 h, PAC1 binding was assessed by flow cytometry. Depicted is the mean percent inhibition of integrin activation relative to that of the empty vector ±SEM of three independent determinations. (B) ERK phosphorylation was measured in αβ-py cells transfected with the indicated plasmids. Twenty-four hours after transfection the cells were placed in medium containing 0.5% FCS, and 48 h after transfection the cells were lysed and 30 μg of cell lysate was resolved on 4–20% SDS gel and transferred to a nitrocellulose membrane. The blots were probed with an mAb-specific for phosphorylated ERK (top panel), then stripped, and reprobed with the polyclonal antibodies against ERK1/2 to assess the expression of ERK1/2 (bottom panel).
Direct Activation of ERK1/2 Is Insufficient to Suppress Integrin Activation
The previous experiments suggested that ERK activation was not necessary to for Ras to suppress integrin activation. To assess whether ERK activation was sufficient for suppression, we activated ERK by transfecting CHO cells with MEK1(ΔN3, S222D), a constitutively activated variant of MEK1 (Mansour et al., 1994). Unlike H-Ras(G12V), the active variant of MEK1 failed to suppress integrin activation (Figure 6A), whereas it strongly activated ERK1/2 (Figure 6B). Thus, the activation of ERK by this activated MEK1 variant was insufficient to suppress integrin activation.
Figure 6.
Direct activation of ERK1/2 by MEK1 is insufficient to suppress integrin activation. (A) αβ-py cells were transiently transfected with an expression vector encoding HA-ERK2 alone or in combination with vectors encoding H-Ras(G12V) or MEK1(ΔN3, S222D). After 48 h, integrin activation was assessed by PAC1 binding. Depicted is the mean percent inhibition of integrin activation. (B) Twenty-four hours after transfection, the cells were placed in medium containing 0.5% FCS, and 48 h after transfection the cells were lysed and 30 μg of cell lysate was resolved on 4–20% SDS gel and transferred to a nitrocellulose membrane. The blots were probed with an mAb specific for phosphorylated ERK (top panel). To verify equal loading the blots were stripped and reprobed with polyclonal antibodies against ERK1 and ERK2 (bottom panel).
Suppression of Activation of Integrin α5β1 by Activated Raf-1 Is Independent of Bulk ERK Activation
We have previously demonstrated that activated variants of Raf-1 can suppress integrin activation. In addition, analyses of H-Ras effector loop mutants suggest that only those capable of coupling efficiently to Raf-1 are effective suppressors of integrin activation (Hughes et al., 1997; Sethi et al., 1999; our unpublished results). These data suggest that the suppression of integrin activation by H-Ras is via a Raf-1–dependent pathway. The data presented in this article take our understanding of Ras/Raf-mediated suppression further by suggesting that suppression is independent of bulk ERK activation. However, one of the limitations of this data is that we have only analyzed the suppression of chimeric αIIbβ3 integrins. Therefore, to determine if this pathway could suppress the function of native integrins, we examined if Raf-1 could suppress the activation of integrin α5β1.
When activated, integrin α5β1 binds soluble fragments of fibronectin containing the cell binding domain with high affinity. The soluble fragment of fibronectin used in these experiments was a fusion protein, composed of glutathione S-transferase (GST) and the 9, 10, and 11 type III repeats of fibronectin, (FN 9–11) that make up the RGD-containing central cell binding domain of fibronectin (Ramos and DeSimone, 1996). We measured FN 9–11 binding to endogenous integrin α5β1 in a CHO cell line stably expressing Raf-1:ER, a conditionally active form of Raf-1. Raf-1:ER is a fusion of a modified form of the hormone-binding domain of the mouse estrogen receptor and the kinase domain of Raf-1 (Samuels and McMahon, 1994; Chen et al., 1999). Raf-1 activity is rapidly induced after the addition of 4′OHT to the culture medium at a final concentration of 300 nM (Chen et al., 1999). In the absence of 4′OHT, the Raf-ER cells bound FN 9–11 (Figure 7A). Binding was through integrin α5β1 as it was inhibited by an anti-α5β1 antibody, PB1 (our unpublished results). FN 9–11 binding was inhibited by Raf-1 activation after the addition of 300 nM 4′OHT (Figure 7B). However, binding could be reconstituted by addition of the exogenous integrin α5β1 activator Mn2+, indicating that it was due to suppression of α5β1 activation. The addition of the MEK kinase inhibitor, U0126, failed to block the capacity of activated Raf-1 to suppress FN 9–11 binding (Figure 7C), even though it blocked detectable Raf-1 initiated ERK activation (Figure 7D). In the absence of 4′OHT the U0126 compound alone occasionally inhibited integrin activation; however, no such inhibition was observed with another MEK inhibitor PD98059 (our unpublished observations). Furthermore, PD98059 also blocked ERK activation but not suppression of FN 9–11 binding (our unpublished results). Thus, the bulk of ERK activation can be blocked without affecting the ability of Raf-1 to suppress the activation of integrin α5β1.
Figure 7.
Raf-1 activation suppresses the binding of soluble FN 9–11 that is not reversed by an inhibition of ERK MAP kinase activation. Depicted are flow cytometry histograms in which fluorescence intensity is plotted on the abscissa and cell number on the ordinate. FN 9–11 binding alone (filled histogram), in the presence of EDTA (dotted line), and MnCl2 (dashed line) are illustrated in panels A–C. (A) Raf-1: ER cells specifically bind FN 9–11 with an activation index (AI) of 35%. (B) FN 9–11 binding is inhibited after treatment with 4-hydroxy tamoxifen (4′OHT) for 18 h before analysis. (C) The inhibition of FN 9–11 binding by 4′OHT is not reversed by the simultaneous addition of the MEK kinase inhibitor U0126 at a final concentration of 25 μM for 18 h before analysis. (D) After the described treatments with 4′OHT and the MEK kinase inhibitor, U0126, the cells were lysed and 20 μg of cell lysate was resolved on 4–20% SDS gel and transferred to a nitrocellulose membrane. The blot was then probed with an mAb specific for phosphorylated ERK (top blot). To verify equal loading the blot was stripped and reprobed with the polyclonal antibodies against ERK1 and ERK2 (bottom blot).
DISCUSSION
Suppression of integrin activation by H-Ras can control cell shape, migration, and assembly of the extracellular matrix. In contrast, the closely related small GTPase, R-Ras, lacks this activity; indeed it promotes rather than suppresses integrin activation (Zhang et al., 1996; Osada et al., 1999; Sethi et al., 1999; Kinashi et al., 2000). With the aim of mapping the regions of H-Ras responsible for the suppression of integrin activation, we created a series of chimeric proteins composed of portions of the C terminus of H-Ras fused to the N-terminus of R-Ras and the reciprocal chimeras composed of portions of the C terminus of R-Ras fused to the N-terminus of H-Ras. Our major findings were as follows: A 24-amino acid stretch of H-Ras (residues 148–171) is required for full suppressive activity. Second, certain suppressive chimeras had little affect on ERK1 and ERK2 MAP kinase activation, suggesting that Ras-induced suppression may be independent of the bulk activation of ERK1/2. Furthermore, blockade of ERK1/2 activation by either a pharmacological inhibitor of MEK kinase or by coexpression of MAP kinase phosphatase 3 (MKP3) did not alter the ability of H-Ras to suppress integrin activation. Thus, ERK1/2 activation per se is not required for integrin suppression. Third, ERK activation is not sufficient for suppression because an activated variant of MEK1 was unable to suppress integrin activation. Thus, the capacity of H-Ras and Raf-1 to suppress integrin activation is not simply due to bulk activation of the ERK MAP kinase pathway.
A 24-amino acid stretch of H-Ras, encompassing residues 148–171, is required for its ability to suppress integrin activation. This conclusion was drawn from two observations; first, a chimera containing H-Ras sequences C-terminal of Lys147, R-Ras(174)H-Ras(148–189), was sufficient to convey suppressive activity to R-Ras. Second, chimeras containing H-Ras residues N-terminal of Leu171 were able to suppress integrin activation. The molecular basis for the dependence on the T148-L171 is not readily apparent. Previous analysis of H-Ras has not implicated this region of the protein as critical for specifying the interaction with or activation of downstream effectors (Reuther and Der, 2000). Indeed, extensive analysis of both H-Ras point mutants and H-Ras/Rap1 chimeras suggested that the critical sequences involved in determining the specificity of effector binding and activation are amino acids 20–48 and amino acids 60–76, which comprise the switch I and switch II regions, respectively (Marshall et al., 1991; Marshall, 1993; Campbell et al., 1998). However, in the present studies exchanges of these regions between H-Ras and R-Ras had only modest effects on integrin activation. Thus, the differences in the ability of H-Ras and R-Ras to exert opposing effects on integrin activation may not be due to simple differences in their well-characterized effector binding domains.
Residues 148–171 of H-Ras overlap with the C-terminal hypervariable region, which spans amino acids 166–189. Significant features of the C-terminal hypervariable region are prenylation and palmitoylation motifs required for the attachment of membrane anchors. H-Ras contains a prenylation motif, which specifies farnesylation, whereas the C-terminal prenylation motif of R-Ras is predicted to specify geranyl-geranylation (Reuther and Der, 2000). However, substitution of the R-Ras prenylation motif with the prenylation motif of H-Ras was not sufficient to convey suppressive activity.
It has recently been proposed that the hypervariable region is responsible for the localization of Ras proteins to distinct microdomains in the plasma membrane (Prior et al., 2001; Prior and Hancock, 2001). Furthermore, Prior et al. suggest that differences in plasma membrane localization may explain the functional differences between different Ras isoforms. Thus, a possible role for residues 148–171 is to specifically target activated H-Ras to the appropriate plasma microdomain for efficient coupling to the downstream effectors driving suppression. To address this hypothesis, it will be necessary to determine if functional distinct H-Ras/R-Ras chimeras localize to different domains of the plasma membrane.
Certain of the suppressive chimeras were weak activators of ERK1 and ERK2, which suggested that the activation of bulk ERK MAP kinase might not be necessary for suppression. This hypothesis was supported by further observations demonstrating that blockade of the H-Ras(G12V)–induced ERK1/2 activation by either a chemical inhibitor of MEK, U0126, or coexpression of MKP3 did not prevent the ability of activated H-Ras to suppress integrin activation. Furthermore, ERK activation is not sufficient for suppression because an activated variant of MEK1 (MEK1(ΔN3, S222D)) was unable to suppress integrin activation despite inducing robust ERK activation. In contrast to the results observed with MEK1(ΔN3, S222D), the activated MEK1 and MEK2 variants, MEK1(ΔN4, S222D) and MEK2(S222/226D), are able to suppress integrin activation (our unpublished observations; Ramos et al., 1998). This apparent contradiction may be explained by differing intracellular localizations of these constitutively activated MEK mutants. The nuclear export sequence has been deleted from MEK1(ΔN3, S222D), suggesting that this protein will remain localized in the nucleus and thus will be unable to phosphorylate cytoplasmic substrates. However, the nuclear export sequence is retained in MEK1(ΔN4, S222D) and in MEK2 (S222/226D), allowing them to phosphorylate cytoplasmic proteins. It has been reported that activated MEK variants, such as MEK(ΔN4 S222D), which retain their nuclear export signal can directly activate Raf-1 (Alessandrini et al., 1996). We have previously found that activated Raf can suppress integrin activation (Hughes et al., 1997). Thus, the ability of certain activated MEK constructs to suppress integrin activation could be due to Raf-1 activation. In summary, sustained activation of bulk ERK MAP kinase is neither necessary nor sufficient for the suppression of integrin activation.
The failure of activated MEK and the capacity of Raf-1 to suppress integrin activation raise the possibility that Raf effectors other than MEK are responsible for this activity. Indeed, several cellular responses to activated Raf-1 appear to be independent of the activation of MEK and subsequently ERK MAP kinase. For example, activated Raf but not MEK can induce the differentiation of hippocampal neuronal cells (Pearson et al., 2000). Furthermore, in Jurkat T cells Raf-mediated activation of NF-κB transcription factors may occur independently of the activation of MEK1/2 (Baumann et al., 2000). Furthermore, all suppressive chimeras stimulated some ERK activation (albeit reduced). The C terminus of H-Ras was critical for suppression and regulates the subcellular localization of Ras and the place of activation of downstream effectors. Thus, the precise localization of activated Raf-1 may be important in its capacity to suppress activation.
In summary, to gain insight into the suppression of integrin activation by H-Ras, we generated a series of chimeric proteins composed of portions of H-Ras fused to its closely related family member R-Ras. We found that a 24-amino acid stretch of H-Ras (residues 148–171) was required for full suppressive activity. Furthermore, we isolated suppressive H-Ras/R-Ras chimeras that were weak activators of the MAP kinase ERK1/2, suggesting that the suppression of integrin activation may not be an effect of the ERK MAP kinase pathway. In addition, we could inhibit bulk ERK activation without affecting the ability of H-Ras or Raf-1 to suppress integrin activation. Finally, we found that ERK1/2 activation alone is not sufficient for suppression, because an activated variant of MEK1 had no effect on integrin activation. Consequently, these studies provide insight into the downstream effectors of H-Ras and Raf-1 responsible for suppressing integrin activation and suggest the existence of novel pathways through which these signaling molecules regulate cell adhesion.
ACKNOWLEDGMENTS
We thank our colleagues for their generosity in providing the reagents acknowledged under MATERIALS AND METHODS. P.E.H. was a senior fellow of the Leukemia Society of America. B.O. was supported by grants from the Swiss National Science Foundation and the Novartis Foundation. M.H. and B.M.W. were supported by grants from the Danish Medical Research Council and from the Danish Cancer Society to B.M.W. F-L.C. and M.H.G. were supported by grants from the National Institutes of Health.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–10–0480. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–10–0480.
REFERENCES
- Alessandrini A, Greulich H, Huang W, Erikson RL. Mek1 phosphorylation site mutants activate Raf-1 in NIH 3T3 cells. J Biol Chem. 1996;271:31612–31618. doi: 10.1074/jbc.271.49.31612. [DOI] [PubMed] [Google Scholar]
- Baker EK, Tozer EC, Pfaff M, Shattil SJ, Loftus JC, Ginsberg MH. A genetic analysis of integrin function: glanzmann thrombasthenia in vitro. Proc Natl Acad Sci USA. 1997;94:1973–1978. doi: 10.1073/pnas.94.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B, Weber CK, Troppmair J, Whiteside S, Israel A, Rapp UR, Wirth T. Raf induces NF-kappaB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci USA. 2000;97:4615–4620. doi: 10.1073/pnas.080583397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner KA, Corbett SA, Schwarzbauer JE. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene. 2000;19:3156–3163. doi: 10.1038/sj.onc.1203626. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- Chen D, Heath V, O'Garra A, Johnston J, McMahon M. Sustained activation of the raf-MEK-ERK pathway elicits cytokine unresponsiveness in T cells. J Immunol. 1999;163:5796–5805. [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, White R, Hoare SA, Fawell SE, Parker MG. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993;7:232–240. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Frelinger AL, 3rd, Cohen I, Plow EF, Smith MA, Roberts J, Lam SC, Ginsberg MH. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem. 1990;265:6346–6352. [PubMed] [Google Scholar]
- Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- Hughes PE, Pfaff M. Integrin affinity modulation. Trends Cell Biol. 1998;8:359–364. doi: 10.1016/s0962-8924(98)01339-7. [DOI] [PubMed] [Google Scholar]
- Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr Opin Cell Biol. 2000;12:1861–1892. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Kinashi T, Katagiri K, Watanabe S, Vanhaesebroeck B, Downward J, Takatsu K. Distinct mechanisms of alpha 5beta 1 integrin activation by Ha-Ras and R-Ras. J Biol Chem. 2000;275:22590–22596. doi: 10.1074/jbc.M000633200. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Candia JM, Matsuura JE, Manning MC, Ahn NG. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 1996;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Marshall MS. The effector interactions of p21ras. Trends Biochem Sci. 1993;18:250–254. doi: 10.1016/0968-0004(93)90175-m. [DOI] [PubMed] [Google Scholar]
- Marshall MS, Davis LJ, Keys RD, Mosser SD, Hill WS, Scolnick EM, Gibbs JB. Identification of amino acid residues required for Ras p21 target activation. Mol Cell Biol. 1991;11:3997–4004. doi: 10.1128/mcb.11.8.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- Oertli B, Han J, Marte BM, Sethi T, Downward J, Ginsberg M, Hughes PE. The effector loop and prenylation site of R-Ras are involved in the regulation of integrin function. Oncogene. 2000;19:4961–4969. doi: 10.1038/sj.onc.1203876. [DOI] [PubMed] [Google Scholar]
- Osada M, Tolkacheva T, Li W, Chan TO, Tsichlis PN, Saez R, Kimmelman AC, Chan AM. Differential roles of Akt, Rac, and Ral in R-Ras-mediated cellular transformation, adhesion, and survival. Mol Cell Biol. 1999;19:6333–6344. doi: 10.1128/mcb.19.9.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Bumeister R, Henry DO, Cobb MH, White MA. Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J Biol Chem. 2000;275:37303–37306. doi: 10.1074/jbc.C000570200. [DOI] [PubMed] [Google Scholar]
- Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- Prior IA, Hancock JF. Compartmentalization of Ras proteins. J Cell Sci. 2001;114:1603–1608. doi: 10.1242/jcs.114.9.1603. [DOI] [PubMed] [Google Scholar]
- Ramos JW, Kojima TK, Hughes PE, Fenczik CA, Ginsberg MH. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J Biol Chem. 1998;273:33897–33900. doi: 10.1074/jbc.273.51.33897. [DOI] [PubMed] [Google Scholar]
- Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:227–240. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Samuels ML, McMahon M. Inhibition of platelet-derived growth factor- and epidermal growth factor-mediated mitogenesis and signaling in 3T3 cells expressing delta Raf-1:ER, an estradiol-regulated form of Raf-1. Mol Cell Biol. 1994;14:7855–7866. doi: 10.1128/mcb.14.12.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Sethi T, Ginsberg MH, Downward J, Hughes PE. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol Biol Cell. 1999;10:1799–1809. doi: 10.1091/mbc.10.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- Shimizu Y. Putting the rap on integrin activation. Immunol Today. 2000;21:597. doi: 10.1016/s0167-5699(00)01783-7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]