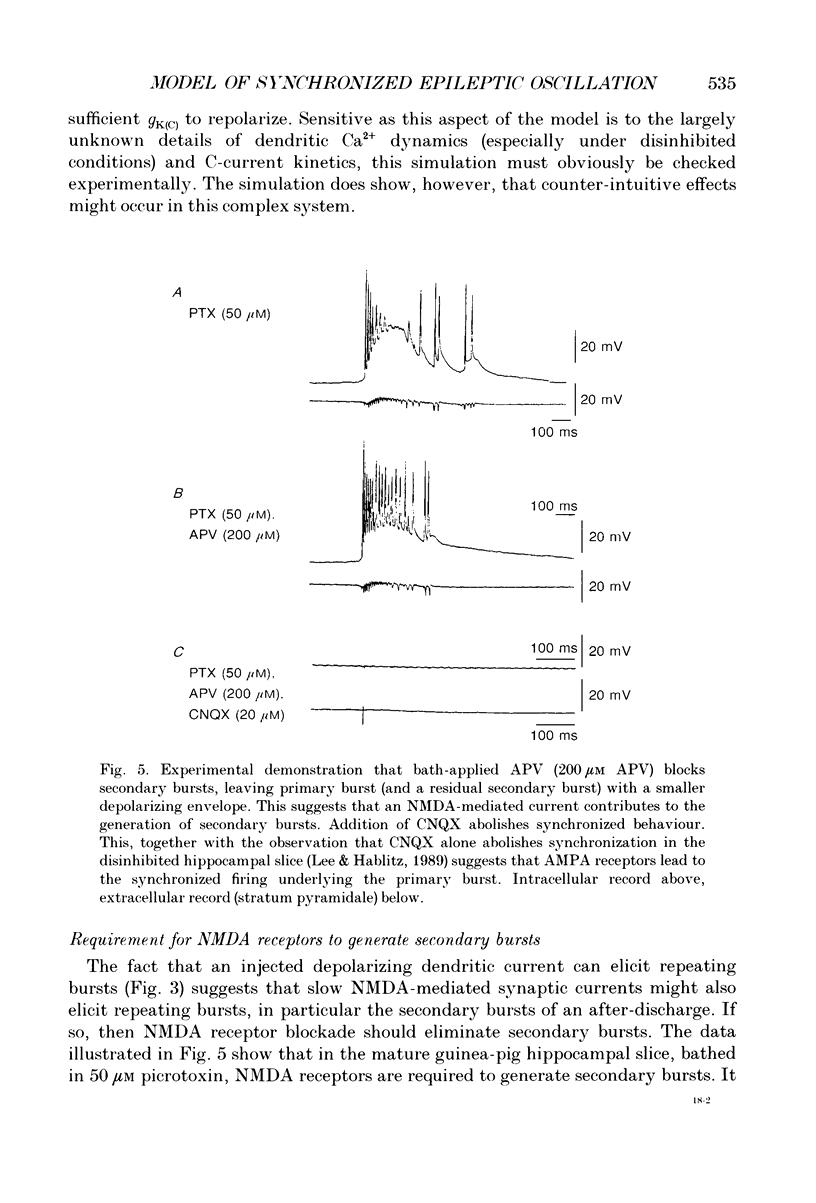
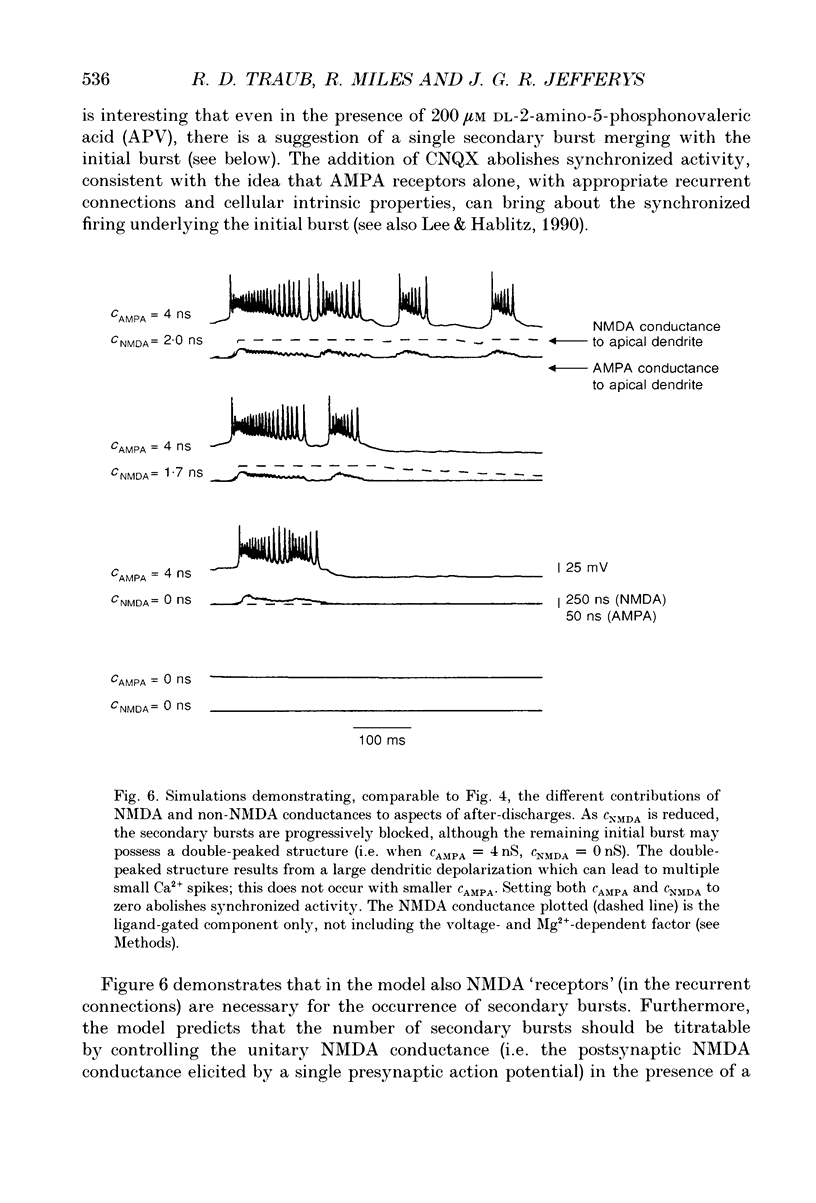
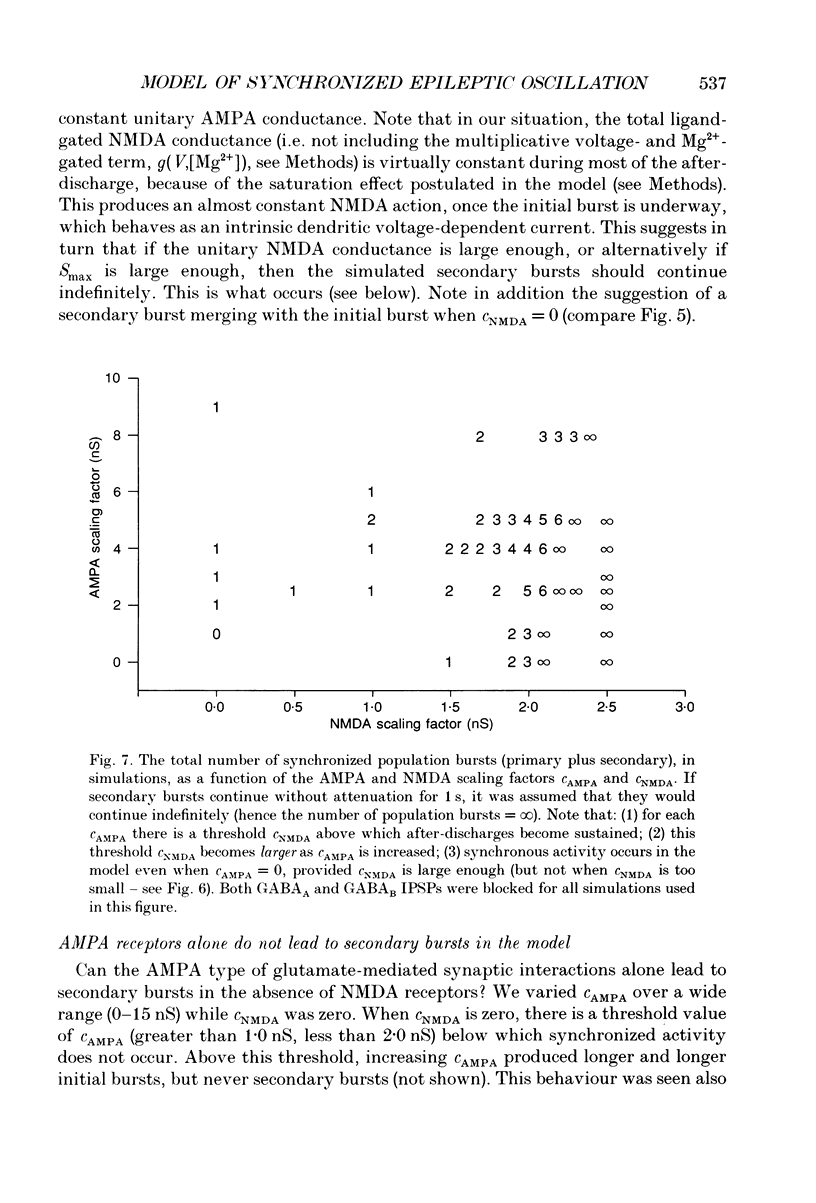
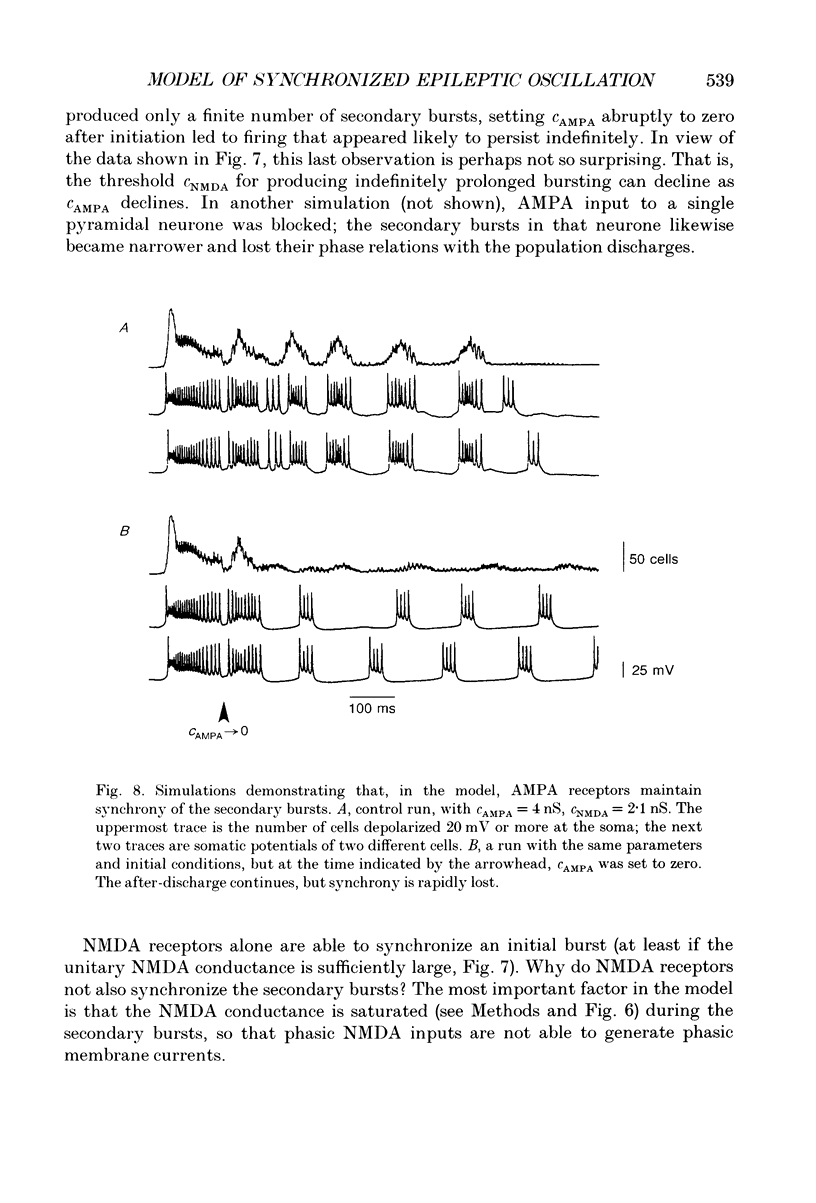
Abstract
1. A computer model was constructed of the guinea-pig hippocampal region in vitro, containing 100 pyramidal neurones. This approach has contributed to the understanding of brief (usually less than 100 ms) epileptic events known as 'interictal spikes'. The present study addresses the cellular mechanisms of more prolonged epileptic events, lasting 200 ms and more, that may represent short-duration seizures. Each neurone was simulated with a nineteen-compartment model using six voltage-dependent ionic conductances. The neurones were randomly interconnected with excitatory synapses, each synapse exerting a fast voltage-independent alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) component and a slower voltage-dependent N-methyl-D-aspartate (NMDA) component. Each neurone received input from twenty other neurones. 2. This model was able to generate, in response to synaptic noise or to stimulation of one neurone, a series of synchronized population bursts, the initial (primary) burst being longer than later (secondary) bursts, terminating in a prolonged after-hyperpolarization. The simulated after-discharge potentials resemble those recorded experimentally from pyramidal neurones during perfusion of the hippocampal slice with media containing picrotoxin, a blocker of synaptic inhibition mediated by GABAA receptors. 3. Simulated after-discharges agree with the following experiments: over a certain range of total NMDA conductance, blockade of AMPA receptors will prevent the occurrence of synchronized firing, whereas, blockade of NMDA receptors will, in contrast, abolish the secondary bursts, leaving a shortened and somewhat smaller primary burst. Dendritic potential oscillations occur in phase with somatic oscillations. When interneurones (some generating GABAA-mediated IPSPs, others generating GABAB IPSPS) are included in the model, the occurrence of synchronized events was suppressed, the most significant suppressant effect coming from GABAA IPSPS. 4. The model predicts that: a dendritic calcium spike occurs during each secondary burst; AMPA receptors serve to maintain the synchrony of secondary bursts, as well as to initiate the primary burst; and that with sufficient total NMDA conductance, synchronized firing can occur even with AMPA receptors blocked. 5. The model suggests, in addition, that the duration of the initial burst is determined in part by the experimentally observed delay between Ca2+ entry and peaking of the after-hyperpolarization (AHP) conductance, and hence reflects properties of the individual pyramidal neurones. Specifically, a pattern of a long initial burst followed by brief secondary bursts is elicited in single-cell simulations by injection of a steady depolarizing current into the apical dendrite. The same pattern is produced when the single-cell model includes only calcium and calcium-dependent conductances.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aicardi G., Schwartzkroin P. A. Suppression of epileptiform burst discharges in CA3 neurons of rat hippocampal slices by the organic calcium channel blocker, verapamil. Exp Brain Res. 1990;81(2):288–296. doi: 10.1007/BF00228118. [DOI] [PubMed] [Google Scholar]
- Avoli M., Drapeau C., Louvel J., Pumain R., Olivier A., Villemure J. G. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991 Oct;30(4):589–596. doi: 10.1002/ana.410300412. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Brady R. J., Swann J. W. Ketamine selectively suppresses synchronized afterdischarges in immature hippocampus. Neurosci Lett. 1986 Aug 29;69(2):143–149. doi: 10.1016/0304-3940(86)90593-8. [DOI] [PubMed] [Google Scholar]
- Chamberlin N. L., Traub R. D., Dingledine R. Role of EPSPs in initiation of spontaneous synchronized burst firing in rat hippocampal neurons bathed in high potassium. J Neurophysiol. 1990 Sep;64(3):1000–1008. doi: 10.1152/jn.1990.64.3.1000. [DOI] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Dichter M., Spencer W. A. Penicillin-induced interictal discharges from the cat hippocampus. I. Characteristics and topographical features. J Neurophysiol. 1969 Sep;32(5):649–662. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Grafe P., ten Bruggencate G. Convulsant actions of 4-aminopyridine on the guinea-pig olfactory cortex slice. Brain Res. 1982 Jun 3;241(1):75–86. doi: 10.1016/0006-8993(82)91230-6. [DOI] [PubMed] [Google Scholar]
- Gean P. W., Chou S. M. Suppression of 4-aminopyridine-induced paroxysmal depolarizing shift in rat amygdaloid neurons by diltiazem. Brain Res. 1991 Sep 27;560(1-2):306–310. doi: 10.1016/0006-8993(91)91248-y. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J Neurophysiol. 1984 May;51(5):1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Thalmann R. H. Conductance changes underlying a late synaptic hyperpolarization in hippocampal CA3 neurons. J Neurophysiol. 1987 Jul;58(1):160–179. doi: 10.1152/jn.1987.58.1.160. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W. H., Haberly L. B. Bursting induces persistent all-or-none EPSPs by an NMDA-dependent process in piriform cortex. J Neurosci. 1989 Jan;9(1):206–215. doi: 10.1523/JNEUROSCI.09-01-00206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa G. G., Avoli M., Oliver A., Villemure J. G. Bicuculline-induced epileptogenesis in the human neocortex maintained in vitro. Exp Brain Res. 1991;83(2):329–339. doi: 10.1007/BF00231156. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990 Sep;10(9):3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys J. G. Chronic epileptic foci in vitro in hippocampal slices from rats with the tetanus toxin epileptic syndrome. J Neurophysiol. 1989 Aug;62(2):458–468. doi: 10.1152/jn.1989.62.2.458. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981 Jan 16;211(4479):294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Heinemann U. Synaptic and intrinsic responses of medical entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol. 1988 May;59(5):1476–1496. doi: 10.1152/jn.1988.59.5.1476. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Lambert J. D. Synchronous discharges in the rat entorhinal cortex in vitro: site of initiation and the role of excitatory amino acid receptors. Neuroscience. 1990;34(3):657–670. doi: 10.1016/0306-4522(90)90172-z. [DOI] [PubMed] [Google Scholar]
- Jordan S. J., Jefferys J. G. Sustained and selective block of IPSPs in brain slices from rats made epileptic by intrahippocampal tetanus toxin. Epilepsy Res. 1992 Apr;11(2):119–129. doi: 10.1016/0920-1211(92)90046-v. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Calcium current activation kinetics in isolated pyramidal neurones of the Ca1 region of the mature guinea-pig hippocampus. J Physiol. 1987 Nov;392:603–616. doi: 10.1113/jphysiol.1987.sp016799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Giacchino J. L., Chamberlin N. L., Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987 Jan;57(1):325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Köhler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986 Apr 8;246(2):149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lee W. L., Hablitz J. J. Effect of APV and ketamine on epileptiform activity in the CA1 and CA3 regions of the hippocampus. Epilepsy Res. 1990 Jul;6(2):87–94. doi: 10.1016/0920-1211(90)90082-7. [DOI] [PubMed] [Google Scholar]
- Lee W. L., Hablitz J. J. Involvement of non-NMDA receptors in picrotoxin-induced epileptiform activity in the hippocampus. Neurosci Lett. 1989 Dec 15;107(1-3):129–134. doi: 10.1016/0304-3940(89)90804-5. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMOTO H., MARSAN C. A. CORTICAL CELLULAR PHENOMENA IN EXPERIMENTAL EPILEPSY: ICTAL MANIFESTATIONS. Exp Neurol. 1964 Apr;9:305–326. doi: 10.1016/0014-4886(64)90026-3. [DOI] [PubMed] [Google Scholar]
- Mason A., Nicoll A., Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci. 1991 Jan;11(1):72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre D. C., Wong R. K. Cellular and synaptic properties of amygdala-kindled pyriform cortex in vitro. J Neurophysiol. 1986 Jun;55(6):1295–1307. doi: 10.1152/jn.1986.55.6.1295. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990 Sep;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987 Oct 22;329(6141):724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983 Nov 24;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K., Traub R. D. Synchronized afterdischarges in the hippocampus: contribution of local synaptic interactions. Neuroscience. 1984 Aug;12(4):1179–1189. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- Neuman R. S., Cherubini E., Ben-Ari Y. Endogenous and network bursts induced by N-methyl-D-aspartate and magnesium free medium in the CA3 region of the hippocampal slice. Neuroscience. 1989;28(2):393–399. doi: 10.1016/0306-4522(89)90186-3. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Alger B. E. Single calcium channels in rat and guinea-pig hippocampal neurons. J Physiol. 1991 May;436:739–767. doi: 10.1113/jphysiol.1991.sp018577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. J Physiol. 1990 Nov;430:605–616. doi: 10.1113/jphysiol.1990.sp018310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M., Gähwiler B. H., Thompson S. M. Paroxysmal inhibitory potentials mediated by GABAB receptors in partially disinhibited rat hippocampal slice cultures. J Physiol. 1991 Dec;444:375–396. doi: 10.1113/jphysiol.1991.sp018884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Penicillin-induced epileptiform activity in the hippocampal in vitro prepatation. Ann Neurol. 1977 May;1(5):463–469. doi: 10.1002/ana.410010510. [DOI] [PubMed] [Google Scholar]
- Swann J. W., Brady R. J., Friedman R. J., Smith E. J. The dendritic origins of penicillin-induced epileptogenesis in CA3 hippocampal pyramidal cells. J Neurophysiol. 1986 Dec;56(6):1718–1738. doi: 10.1152/jn.1986.56.6.1718. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Wong R. K. Development of calcium current subtypes in isolated rat hippocampal pyramidal cells. J Physiol. 1991 Aug;439:671–689. doi: 10.1113/jphysiol.1991.sp018687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., Radpour S. Excitatory Connections Between CA1 Pyramidal Cells Revealed by Spike Triggered Averaging in Slices of Rat Hippocampus are Partially NMDA Receptor Mediated. Eur J Neurosci. 1991;3(6):587–601. doi: 10.1111/j.1460-9568.1991.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Knowles W. D., Miles R., Wong R. K. Synchronized afterdischarges in the hippocampus: simulation studies of the cellular mechanism. Neuroscience. 1984 Aug;12(4):1191–1200. doi: 10.1016/0306-4522(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Buzsáki G. Computer simulation of carbachol-driven rhythmic population oscillations in the CA3 region of the in vitro rat hippocampus. J Physiol. 1992;451:653–672. doi: 10.1113/jphysiol.1992.sp019184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Wong R. K. Models of synchronized hippocampal bursts in the presence of inhibition. I. Single population events. J Neurophysiol. 1987 Oct;58(4):739–751. doi: 10.1152/jn.1987.58.4.739. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K., Miles R., Michelson H. A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J Neurophysiol. 1991 Aug;66(2):635–650. doi: 10.1152/jn.1991.66.2.635. [DOI] [PubMed] [Google Scholar]
- Williams S. H., Johnston D. Kinetic properties of two anatomically distinct excitatory synapses in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1991 Sep;66(3):1010–1020. doi: 10.1152/jn.1991.66.3.1010. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Thio L. L., Clark G. D., Clifford D. B. Calcium influx through N-methyl-D-aspartate channels activates a potassium current in postnatal rat hippocampal neurons. Neurosci Lett. 1989 May 8;99(3):293–299. doi: 10.1016/0304-3940(89)90462-x. [DOI] [PubMed] [Google Scholar]


