Abstract
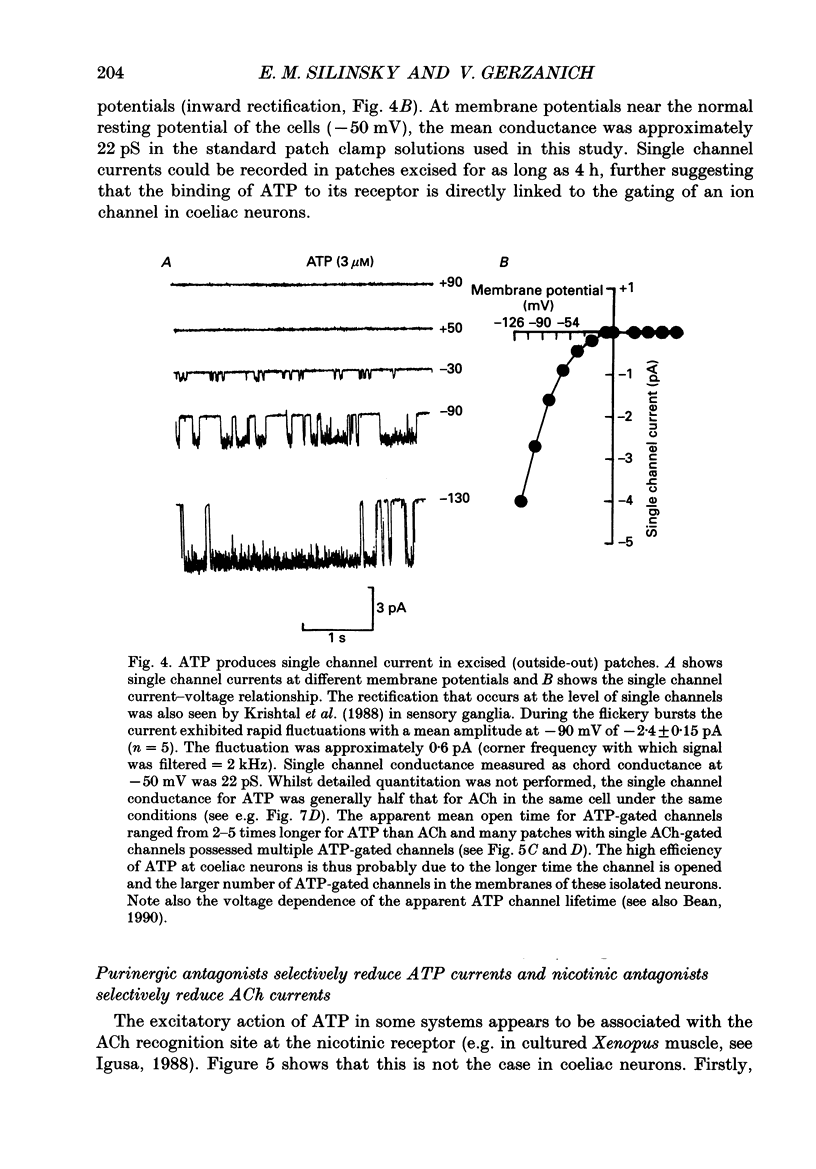
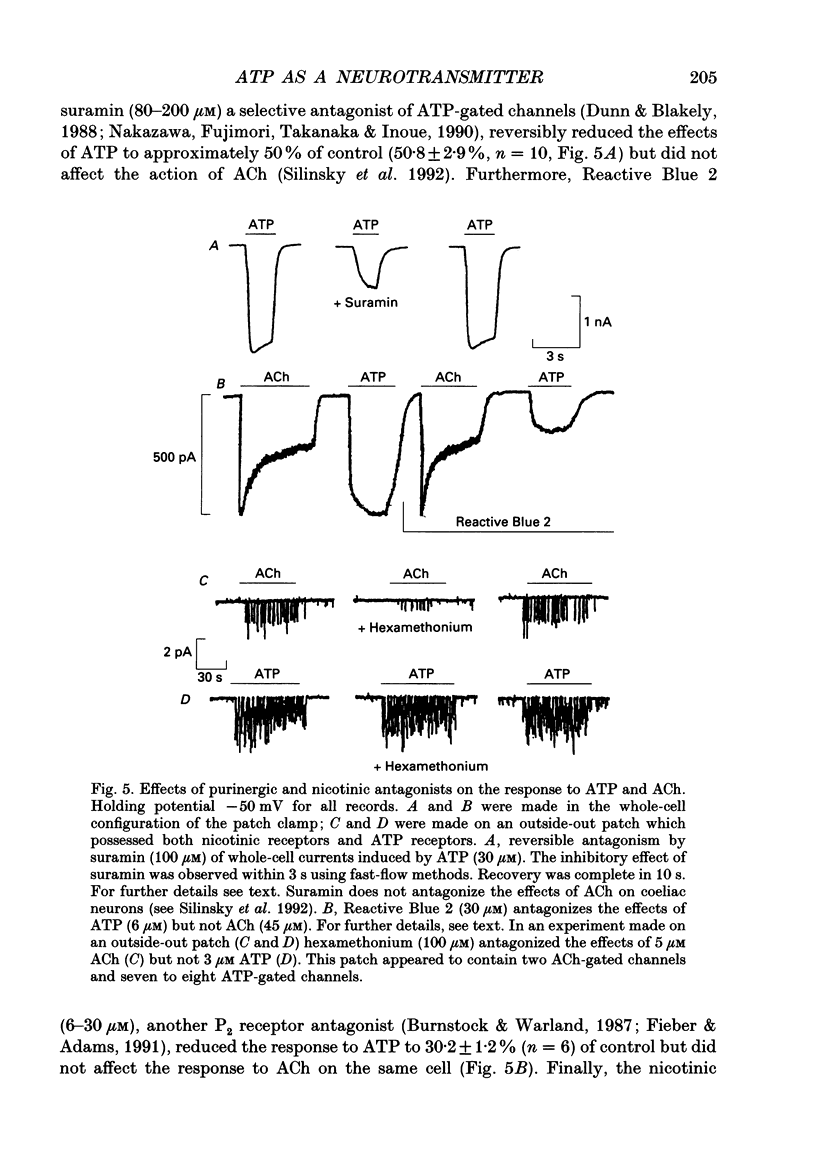
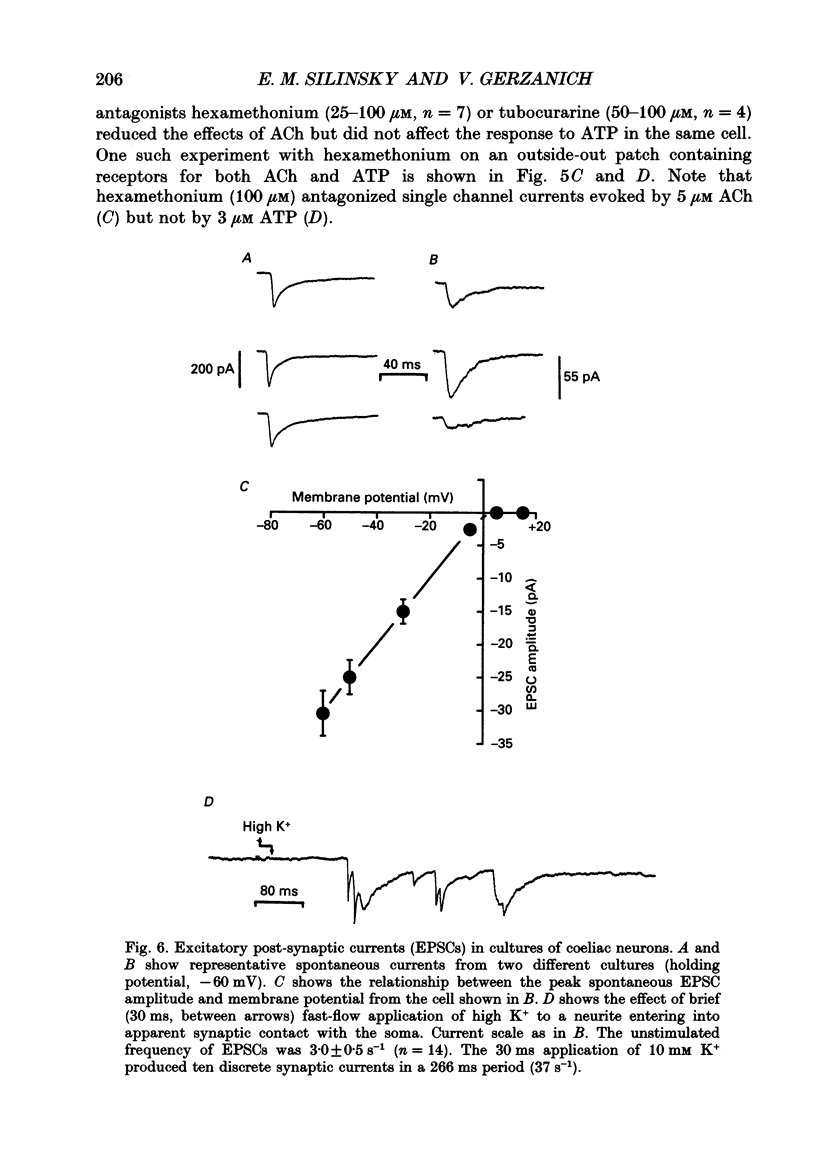
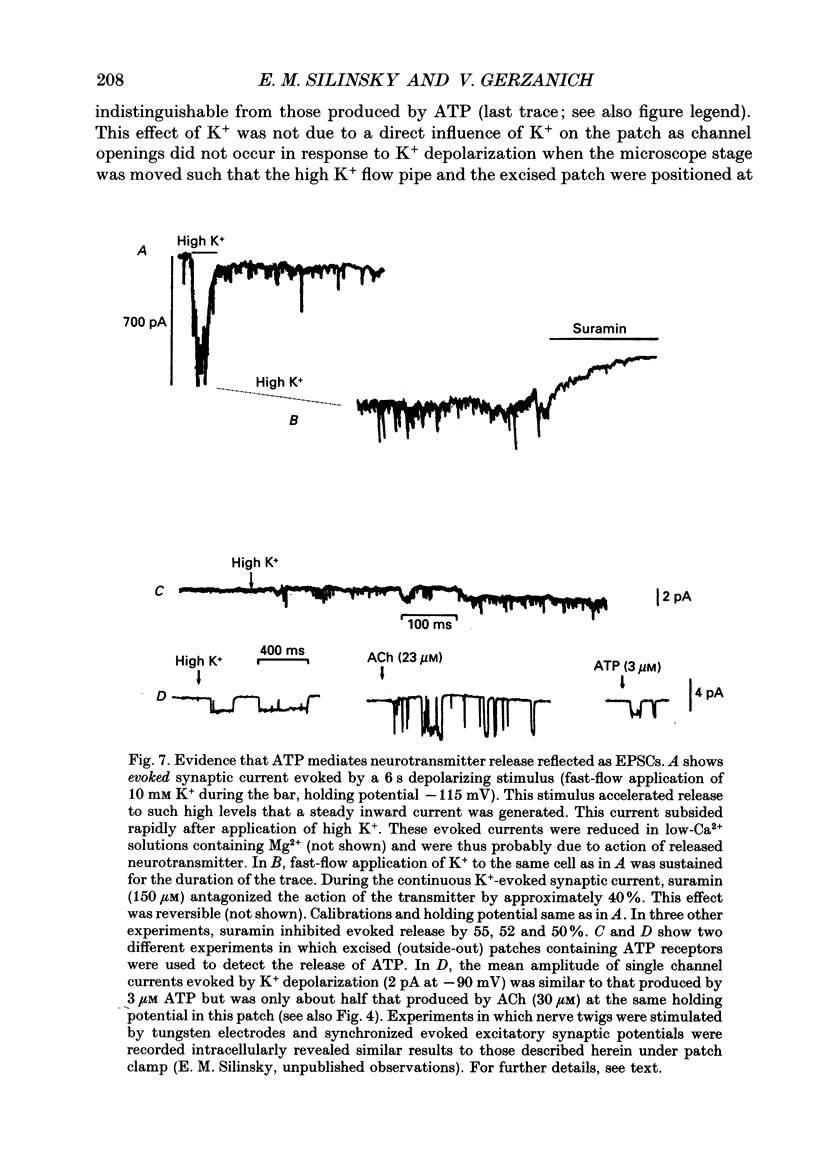
1. The effects of ATP on neurons from guinea-pig coeliac ganglia were studied to evaluate the possibility that this nucleotide acts as an excitatory neurotransmitter substance. 2. In experiments with intracellular microelectrodes, ATP (> or = 10 nM) depolarized coeliac neurons from the resting potential and produced an increase in the membrane conductance. These excitatory effects of ATP were observed in isolated coeliac ganglia, in acutely dissociated neurons or in cultured neurons. ATP also produced membrane conductance increases in neurons clamped at the resting potential using a single electrode voltage clamp. 3. When studied in the whole-cell configuration of the patch clamp (intracellular Cs+ to block K+ currents; -50 mV holding potential), ATP evoked inward currents in a manner more potent and efficacious than acetylcholine (ACh). 4. Whole-cell currents induced by ATP were inwardly rectifying and reversed at -13 mV in normal Na+ solutions. Changes in extracellular Na+ concentration altered the reversal potential in a manner predicted by the Goldman-Hodgkin-Katz bi-ionic equation with a ratio of Na+ to Cs+ permeability (PNa/PCs) = 0.6. 5. Single channel currents were evoked by ATP in excised (outside-out) patches. Current-voltage relationships for single channel currents exhibited inward rectification. The mean single channel conductance was 22 pS at -50 mV. 6. Antagonists of ATP-gated channels (suramin, Reactive Blue 2) reduced the effects of ATP but not ACh. 7. Antagonists at nicotinic receptors/ion channels (hexamethonium or tubocurarine) reduced the effects of ACh but not ATP. 8. Excitatory synaptic currents were observed in cultures of coeliac neurons. Synaptic currents possessed similar current-voltage relationships to currents produced by ATP, were increased in frequency by K+ depolarization in a Ca(2+)-dependent manner, and were selectively antagonized by ATP antagonists. 9. Local K+ depolarization of the ends of neurites evoked single channel currents characteristic of ATP in outside-out patches when patches were positioned near the region of apparent synaptic contact but not when patches were positioned at remote regions. 10. The results suggest that ATP receptors are linked to ion channels and mediate excitatory synaptic transmission between coeliac neurons.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. G., Burnstock G. The actions of adenosine 5'-triphosphate on guinea-pig intracardiac neurones in culture. Br J Pharmacol. 1990 Jun;100(2):269–276. doi: 10.1111/j.1476-5381.1990.tb15794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Rampart M., Verbeuren T. J., Herman A. G., Van Maercke Y. M. Evidence against ATP being the inhibitory transmitter released by nonadrenergic noncholinergic nerves in the canine ileocolonic junction. J Pharmacol Exp Ther. 1990 Aug;254(2):659–663. [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Jahr C. E. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989 Oct 12;341(6242):536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. Subtypes of P2-purinoceptors. Studies using analogues of ATP. Ann N Y Acad Sci. 1990;603:172–181. doi: 10.1111/j.1749-6632.1990.tb37671.x. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. Theory and operation of a single microelectrode voltage clamp. J Neurosci Methods. 1984 Jun;11(2):101–127. doi: 10.1016/0165-0270(84)90029-3. [DOI] [PubMed] [Google Scholar]
- Fyffe R. E., Perl E. R. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proc Natl Acad Sci U S A. 1984 Nov;81(21):6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Role L. W., Fischbach G. D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983 Oct 13;305(5935):632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- Igusa Y. Adenosine 5'-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. J Physiol. 1988 Nov;405:169–185. doi: 10.1113/jphysiol.1988.sp017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreulen D. L., Szurszewski J. H. Nerve pathways in celiac plexus of the guinea pig. Am J Physiol. 1979 Jul;237(1):E90–E97. doi: 10.1152/ajpendo.1979.237.1.E90. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Meckler R. L. Characteristics of synaptic input to three classes of sympathetic neurone in the coeliac ganglion of the guinea-pig. J Physiol. 1989 Aug;415:109–129. doi: 10.1113/jphysiol.1989.sp017714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Comparison of adenosine triphosphate- and nicotine-activated inward currents in rat phaeochromocytoma cells. J Physiol. 1991 Mar;434:647–660. doi: 10.1113/jphysiol.1991.sp018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Reversible and selective antagonism by suramin of ATP-activated inward current in PC12 phaeochromocytoma cells. Br J Pharmacol. 1990 Sep;101(1):224–226. doi: 10.1111/j.1476-5381.1990.tb12117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Sources of adenosine released during neuromuscular transmission in the rat. J Physiol. 1991 Jan;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. A., Zawisa M. J., Lin X., Hume R. I. A receptor that is highly specific for extracellular ATP in developing chick skeletal muscle in vitro. Br J Pharmacol. 1991 Aug;103(4):1963–1969. doi: 10.1111/j.1476-5381.1991.tb12360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983 Oct 13;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]


