Abstract
1. Excitatory inputs to amacrine cells in the salamander retinal slice preparation were examined using whole-cell patch pipette voltage-clamp techniques. In strychnine (500 nM) and bicuculline (100 microM), two types of amacrine cell were easily distinguished by their light-evoked excitatory responses: transient and sustained. 2. In transient amacrine cells the current-voltage (I-V) relation for the peak light-evoked current was non-linear with a negative slope region between -50 and -70 mV. Responses reversed near +10 mV and were prolonged at more positive holding potentials. 3. In DL-2-amino-phosphonoheptanoate (AP7, 30 microM), a selective N-methyl-D-aspartate (NMDA) receptor antagonist, both the negatively sloped region of the light I-V relation and the prolongation of the response at positive potentials were eliminated. In 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 2 microM), a selective non-NMDA receptor antagonist, light-evoked currents at the most hyperpolarized holding potentials were eliminated. At potentials positive to -85 mV the light-evoked currents lacked a fast onset. The light I-V relation in CNQX had a negative slope region between -35 and -80 mV. 4. With synaptic transmission blocked, kainate evoked responses in transient cells with a resultant I-V relation that was nearly linear, whereas glutamate and NMDA elicited responses with non-linear I-V relations. 5. Light-evoked currents in sustained amacrine cells had a nearly linear I-V relation and reversed near +10 mV. AP7 at a concentration of 30 microM did not affect the light-evoked currents in sustained cells, but 2 microM-CNQX eliminated all light-evoked currents in these cells. 6. With synaptic transmission blocked, sustained amacrine cells responded only to glutamate and kainate, not NMDA. The resultant I-V relations were linear. 7. We conclude that the light-evoked responses of transient amacrine cells are mediated by concomitant activation of both non-NMDA and NMDA receptors whereas the responses of sustained amacrine cells are mediated only by non-NMDA receptors. Furthermore, these data provide supportive evidence that the primary light-evoked excitatory neurotransmitter activating amacrine cells is glutamate.
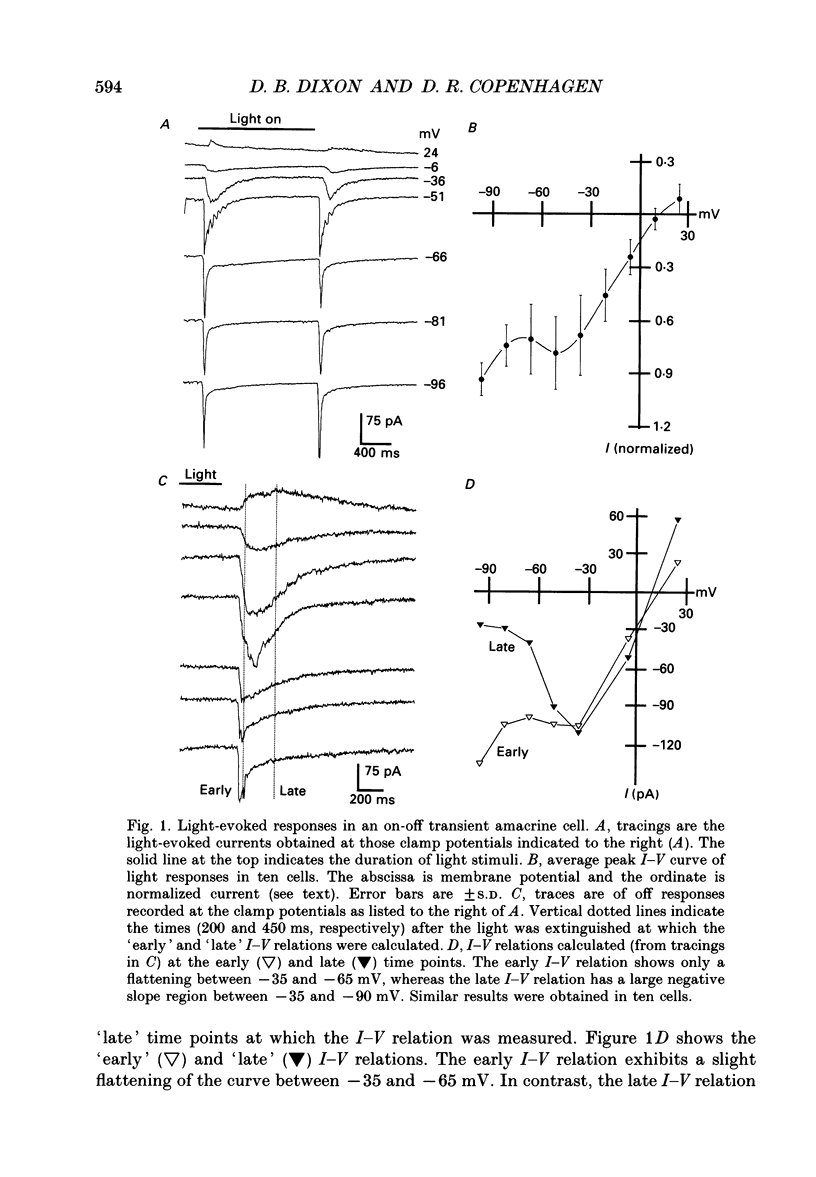
Full text
PDF















Selected References
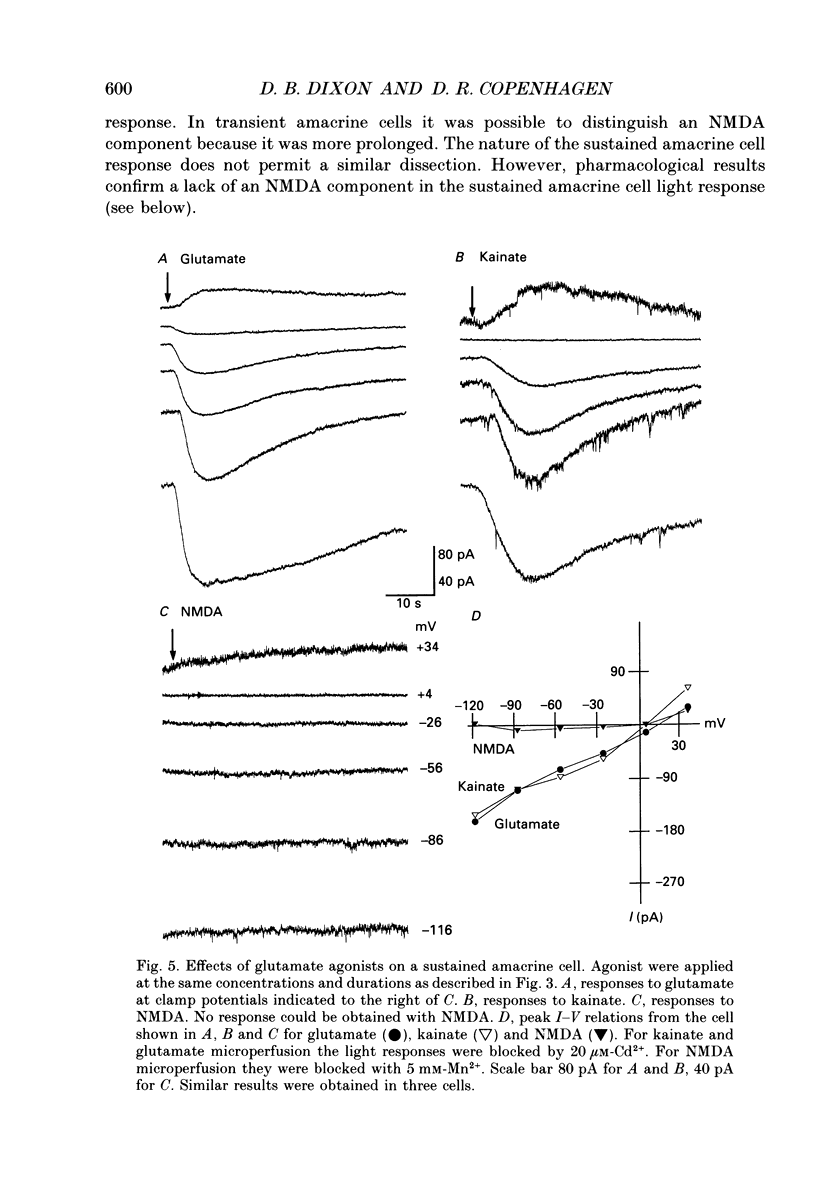
These references are in PubMed. This may not be the complete list of references from this article.
- Ball A. K., Dickson D. H. Displaced amacrine and ganglion cells in the newt retina. Exp Eye Res. 1983 Feb;36(2):199–213. doi: 10.1016/0014-4835(83)90006-4. [DOI] [PubMed] [Google Scholar]
- Barnes S., Werblin F. Gated currents generate single spike activity in amacrine cells of the tiger salamander retina. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1509–1512. doi: 10.1073/pnas.83.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Coleman P. A., Miller R. F. Do N-methyl-D-aspartate receptors mediate synaptic responses in the mudpuppy retina? J Neurosci. 1988 Dec;8(12):4728–4733. doi: 10.1523/JNEUROSCI.08-12-04728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann J. F. Cholinergic amacrine cells in the developing cat retina. J Comp Neurol. 1989 Nov 1;289(1):143–155. doi: 10.1002/cne.902890112. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Ottersen O. P., Storm-Mathisen J., Dowling J. E. Bipolar cells in the turtle retina are strongly immunoreactive for glutamate. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8321–8325. doi: 10.1073/pnas.85.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P. D., McReynolds J. S. Synaptic transmission at N-methyl-D-aspartate receptors in the proximal retina of the mudpuppy. J Physiol. 1985 Oct;367:99–115. doi: 10.1113/jphysiol.1985.sp015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G., Lukasiewicz P., Werblin F. Synaptic and voltage-gated currents in interplexiform cells of the tiger salamander retina. J Gen Physiol. 1990 Apr;95(4):755–770. doi: 10.1085/jgp.95.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G., Maple B., Lukasiewicz P., Werblin F. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10144–10147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Liu W. L., Kalloniatis M., Raiguel S. F., van Haesendonck E. Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci. 1990 Dec;10(12):4006–4034. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey S. C., Miller R. F. N-methyl-D-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol. 1990 Jan;63(1):16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Millar T. J., Winder C., Ishimoto I., Morgan I. G. Putative serotonergic bipolar and amacrine cells in the chicken retina. Brain Res. 1988 Jan 26;439(1-2):77–87. doi: 10.1016/0006-8993(88)91463-1. [DOI] [PubMed] [Google Scholar]
- Mittman S., Taylor W. R., Copenhagen D. R. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol. 1990 Sep;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S., Copenhagen D. R. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987 Jan 1;325(6099):56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Nielsen E. O., Drejer J., Cha J. H., Young A. B., Honoré T. Autoradiographic characterization and localization of quisqualate binding sites in rat brain using the antagonist [3H]6-cyano-7-nitroquinoxaline-2,3-dione: comparison with (R,S)-[3H]alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid binding sites. J Neurochem. 1990 Feb;54(2):686–695. doi: 10.1111/j.1471-4159.1990.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Perkel D. J., Hestrin S., Sah P., Nicoll R. A. Excitatory synaptic currents in Purkinje cells. Proc Biol Sci. 1990 Aug 22;241(1301):116–121. doi: 10.1098/rspb.1990.0074. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Vernier P., Garrigues A. M., Brault E. Properties of the kainate channel in rat brain mRNA injected Xenopus oocytes: ionic selectivity and blockage. Mol Cell Biochem. 1988 Mar-Apr;80(1-2):121–132. doi: 10.1007/BF00231010. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- Yamada K. A., Dubinsky J. M., Rothman S. M. Quantitative physiological characterization of a quinoxalinedione non-NMDA receptor antagonist. J Neurosci. 1989 Sep;9(9):3230–3236. doi: 10.1523/JNEUROSCI.09-09-03230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Y., Yazulla S. Light microscopic localization of putative glycinergic neurons in the larval tiger salamander retina by immunocytochemical and autoradiographical methods. J Comp Neurol. 1988 Jun 15;272(3):343–357. doi: 10.1002/cne.902720305. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Yazulla S. Localization of putative GABAergic neurons in the larval tiger salamander retina by immunocytochemical and autoradiographic methods. J Comp Neurol. 1988 Nov 1;277(1):96–108. doi: 10.1002/cne.902770107. [DOI] [PubMed] [Google Scholar]


